Abstract
Background. Weight loss and depletion of fat-free mass are common problems in patients with chronic obstructive pulmonary disease (COPD) and are related to muscular weakness and exercise intolerance. Physical training of COPD patients has good effect on exercise tolerance and quality of life. The aim of this study was to examine factors that affect change in fat-free mass after physical training, in patients with COPD.
Patients. Patients were examined before and after a 4-month exercise period. Weight and height were measured, and bioelectrical impedance was performed. Fat-free mass (FFM) was calculated, by a three-compartment model, and fat-free mass index (FFMI) was calculated as FFM kg/m2 and body mass index (BMI) as kg/m2. A symptom-limited ramp ergometer test and 12-minute walk test (12MWT) were performed. Dyspnoea score of daily activities was determined by Chronic Respiratory Disease Questionnaire (CRDQ). Blood was taken for analyses of C-reactive protein (CRP) and fibrinogen. Patients with a BMI <21 kg/m2 were given nutritional support during the training period.
Results. A total of 27 patients completed the training (64 years, FEV1 31% of predicted). Patients with low FFMI gained 1.2 kg, whereas those with normal FFMI lost 0.7 kg (p = 0.04). In multivariate analyses high age (p = 0.03), low FEV1 (p = 0.02), and a high level of dyspnoea (p = 0.01) at baseline were found to be negative predictors for increase in FFM.
Conclusions. Difficulties in increasing the fat-free mass in COPD patients by physical training seem to be associated with dyspnoea in daily life and impaired lung function (FEV1).
Introduction
There is growing evidence that chronic obstructive pulmonary disease (COPD) is a multi-organ systemic disease. Skeletal muscle weakness, muscle wasting, and impaired exercise performance, which are poorly related to air flow limitation, have commonly been reported in COPD patients (Citation1).
Exercise-limiting factors include muscular weakness (Citation2), dynamic hyperinflation resulting in increased work of breathing (Citation3), increased load on the respiratory muscles (Citation4), and an intensified perception of respiratory discomfort. In addition, symptoms perceived by the patients with anxiety and depression have impact on training tolerance and physical activity (Citation5).
Loss of body weight is a common and serious finding in patients with COPD (Citation6) and is associated with increased mortality and morbidity independent of gender, smoking, and lung function (Citation7). Patients with low fat-free mass (FFM) often have muscular weakness, especially in the lower limbs (Citation8). Low weight is also related to low health-related quality of life and worsening dyspnoea (Citation9).
Pulmonary rehabilitation is an important component in the management of subjects with COPD (Citation10) and is effective in improving exercise performance and health status (Citation10). Exercise training is a core component of pulmonary rehabilitation and has been shown to be the best available means of improving muscle function, exercise capacity, and cardiovascular function (Citation10). Exercise training has also been shown to result in less dyspnoea in daily life (Citation11) and to less mood disturbance. Before training start, an adequate assessment including evaluation of body composition, i.e. FFM, needs to be performed in order to optimize intervention strategies (Citation10). Body mass index (BMI) is a simple way to assess body composition. However, it is possible to have normal BMI and still be nutritionally depleted (Citation12). FFM reduction is a better predictor of peak exercise performance than BMI (Citation13), and it has been suggested that FFM is the best parameter to assess body composition.
A few studies have investigated the efficacy of multimodal intervention, including nutrition and anabolic steroids integrated into pulmonary rehabilitation for advanced COPD patients (Citation14-16). Although the intervention was successful in improving body weight, FFM, and exercise tolerance, the influence of the various components could not be determined.
The underlying mechanisms contributing to depletion of skeletal muscle still remain unclear. Associations between various circulating markers of inflammation and the loss of muscle mass have been made in chronic inflammatory disease conditions, including COPD.
The aim of this study was to examine factors that affect change in fat-free mass after a period of physical training, in patients with COPD.
Material and methods
The study population was recruited from patients included in an exercise study comparing different training methods (Citation17). Patients with moderate to severe COPD, according to the Global Initiative for Obstructive Lung Disease (GOLD) criteria, were consecutively invited to participate in the exercise study (Citation1). Inclusion criteria were COPD with a post-bronchodilator FEV1/FVC ratio <0.7 and a FEV1 <60% of the predicted value. All patients (n = 51) included in the training study were asked if they wanted to participate in the current study. Forty-nine patients (96%) agreed to participate, of whom 27 patients completed the 4-month training period. Patients were considered drop-outs if they attended less than 75% of the training sessions. Reasons for drop-out were exacerbations (n = 16), other diseases (n = 2), and lack of motivation or transportation problems (n = 4).
Measurements were made before and after the 4-month training period. Information on smoking history was obtained using a structured interview. Informed consent was obtained from all the participants.
Exercise training
Exercise sessions were performed twice a week. Each session included cycle ergometer training at an intensity ≥65% of baseline peak exercise capacity (Wpeak) for 27 min. For warming up (6 min) and cooling down (6 min) the patients cycled at 30%–40% of baseline Wpeak. After the cycling, once a week the session proceeded with flexibility and relaxation training, while the other session of the week proceeded with resistance training for upper and lower limbs as well as abdominal muscles (10 repetitions, two sets at about 70% of 1 repetition maximum). Exercise load was kept as high as tolerated at all times, above the target value when possible. Patients who desaturated on exercise (SpO2 <90%) were given supplemental oxygen during training to keep SpO2 ≥90%. All patients with BMI <21 kg/m2 were given nutritional support ad libitum. The threshold of 21 kg/m2 was chosen because mortality has been shown to be increased below this value in COPD populations (Citation18).
Body composition
Body height was measured to the nearest 0.5 cm, and weight was measured on a balance scale to the nearest 0.1 kg. Bioelectrical impedance analysis was performed in the morning with patients in a fasting state using a Hydra EFC/ICF, model 4200, Xitron Tech (San Diego, CA, USA). FFM was calculated by a three-compartment model, and the fat-free mass index (FFMI) was calculated as FFM kg/body height m2 and BMI as kg/m2. Limits for depleted FFM were employed: FFMI ≤16 kg/m2 was used for men and ≤15 kg/m2 for women. The two outcome variables used were change in FFM, and FFM in per cent of baseline values (change in FFM of baseline).
Physical capacity
Peak exercise capacity in watt (Wpeak) was determined by a progressive symptom-limited cycle ergometer test (Case 8000 Exercise Testing System, GE Medical Systems, Milwaukee, WI, USA).
Functional exercise capacity was measured by the self-paced 12-min walk test (12MWT) (Citation19). The walking tests were performed in a level corridor, and the total distance walked (in meters) was measured. Two tests were performed both before and after the training period. The second test was repeated on a different day within one week. The better of the two tests was used.
Dyspnoea
For evaluation of dyspnoea during activities in daily life the dyspnoea scale from the Chronic Respiratory Disease Questionnaire (CRDQ) was used (Citation20). Patients scored dyspnoea experienced during five self-chosen activities of daily living. The scale for each of the five activities is 7-graded, and a higher score indicates less dyspnoea. The score from the five activities were combined, and the combined dyspnoea score thereby ranged from 5 to 35.
Systemic inflammation
Blood samples were taken for analyses of C-reactive protein (CRP) and fibrinogen. High-sensitivity CRP was measured with a two-point nephelometry method with a ProSpec instrument (Dade Behring, Marburg, Germany) using monoclonal mouse antibodies (Dade Behring, Marburg, Germany). The total analytical imprecision was 1.4% at 1.23 and 5.49 mg/L, with a lowest detectable level of 0.17 mg/L.
Measurement of fibrinogen was performed using a ProSpec instrument (Dade Behring, Marburg, Germany) with a rabbit antibody against fibrinogen (Dade Behring, Marburg, Germany). The total analytical imprecision was 5.7% at 2.2 g/L and 4.4% at 4.7 g/L.
Analyses were carried out at the Department of Clinical Chemistry at Uppsala University Hospital, Sweden.
Lung function
Forced expiratory volume in one second (FEV1) was measured with a Jaeger master scope Spirometer (Jaeger, Höchberg, Germany). The best of three acceptable manoeuvres was used in accordance with the American Thoracic Society guidelines for standardization of spirometry (Citation21). FEV1 was expressed as a percentage of the predicted value using Swedish reference values (Citation22,23).
The Ethics Committee at the Medical Faculty at Uppsala University approved the study (2002-07-02, Dnr 02-307).
Statistics
Results are expressed as mean ± SD. Spearman rank correlation and multiple linear regression analysis were used when analysing the relation between change in FFM expressed in absolute values and per cent of the baseline values and baseline characteristics. Spearman rank correlation was also used when analysing association between change in FFM and change in 12MWT and Wpeak. Variables associated with change in FFM at p < 0.10 in bivariate analyses were included in the multiple linear regression model. CRP was log-transformed before being entered into the analyses. Statistically significant difference was assumed when p < 0.05.
Results
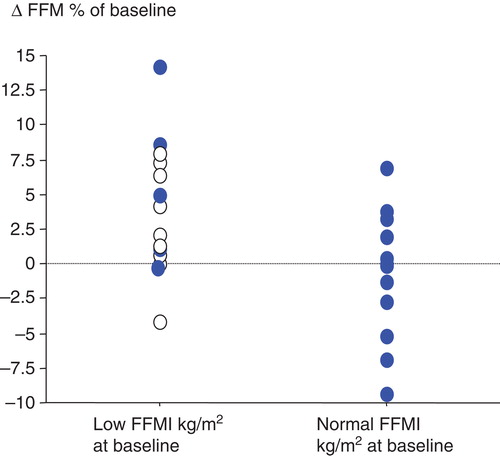
The study included 27 patients who completed the 4-month physical training period. The attendance rate was 29 ± 3 of 32 possible sessions. Patients with low FFMI at training start were more likely to gain weight than patients with normal FFMI (). Nutritional supplementation during the exercise training period was given to all patients with BMI below 21 kg/m2 at baseline; this meant that most patients with low FFMI received supplementation, whereas none of the patients with normal FFMI received supplementation ().
Table I. Baseline characteristics and changes in body composition and physical capacity, for patients with low or normal fat-free mass index (FFMI). Low was defined as FFMI ≤16 kg/m2 for men and ≤15 kg/m2 for women.
Figure 1. Changes in fat-free mass (FFM) in patients with low fat-free mass index (FFMI) and normal FFMI between baseline and four months of training. Low FFMI is defined as FFMI ≤16 kg/m2 for men and ≤15 kg/m2 for women. Filled-symbols = patients given no supplementation; open symbols = patients given supplementation.
The median change in FFM was 0.6 kg, while the median change in 12MWT and Wpeak was 54 m and 10 W, respectively (). Statistically significant correlations were found between changes in FFM and FEV1, FFMI, dyspnoea score, and fibrinogen at baseline (). There were no significant associations between change in FFM and change in 12MWT or change in Wpeak.
Figure 2. Distribution of changes in fat-free mass (FFM) between baseline and four months of training.
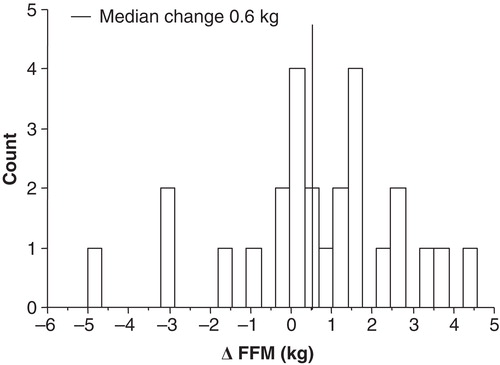
Table II. Associations between changes in fat-free mass (FFM), and variables showing lung function, body composition, systemic inflammation, physical capacity, and dyspnoea at baseline.
In the multivariate analyses approximately 70% of the variation in FFM change was accounted for when age, sex, FEV1, dyspnoea, and fibrinogen were combined in a multiple regression model (). Change in FFM was negatively correlated with age and positively with FEV1 and a higher dyspnoea score indicating less dyspnoea at the start of the training period.
Table III. Associations between changes in fat-free mass (FFM), and age, sex, lung function, dyspnoea, and fibrinogen. Estimates (beta coefficients) are adjusted for all the variables in the table.
Discussion
Our main finding is that patients with lower age, higher FEV1, and a lower level of dyspnoea were more likely to increase in muscle mass during a 4-month physical training period.
Moreover, patients with a low FEV1 were less likely to increase in FFM. It has been shown that muscle strength and FEV1 are factors limiting exercise capacity (Citation2), and that expiratory airflow obstruction is a limiting factor to maximal ventilation and thereby limits exercise tolerance (Citation24). There are multiple possibilities for the association between airflow obstruction and exercise limitation, for example dynamic hyperinflation (Citation3) resulting in increased work of breathing, increased load on the respiratory muscles (Citation4), and the intensified perception of respiratory discomfort. Another possible reason is that a low FEV1 is related to hypoxic muscles due to insufficient oxygen caused by insufficient blood supply or hypoxemia (Citation25).
We found that dyspnoea was a risk factor for not increasing FFM during an exercise programme. One recent study reported that dyspnoea on exertion is correlated with general anxiety, indicating that exercise training may be influenced negatively by anxiety-worsened dyspnoea (Citation26). As dyspnoea has been shown to be related to all subscores of St Georges Respiratory Questionnaire, a low health-related quality of life is obvious in subjects with dyspnoea (Citation27). Dyspnoea can of course have many different explanations, and some of them can probably be treated. In malnourished patients with COPD nutritional interventions can improve quality of life (Citation28). Strength training results in less dyspnoea during exercise, thereby making this strategy easier to tolerate than aerobic training (Citation29).
Our finding that age is correlated with difficulties to increase FFM is consistent with the fact that cachexia is more common in older age (Citation30). CRP in our patients with low and normal FFMI was within the normal range, and there was no significant correlation in the bivariate analysis between CRP at baseline and change in FFMI after training. This is in accordance with results of Eagan et al. who found, in a large COPD population, that CRP was positively associated with FFMI (Citation31), thus CRP was not elevated in patients having lower FFMI. They concluded that CRP is not elevated in cachectic COPD patients. Our results and the results from Eagan et al. are in contrast to a prominent theory suggesting that pathological weight loss is at least partly explained by an increase in systemic inflammation reflected in enhanced plasma or serum concentrations of inflammatory markers (Citation32). A probable explanation for increased CRP in COPD patients might instead be that active fat mass tissue in obese patients is associated to higher systemic levels of CRP (Citation33).
There was a training response in our patients, which was seen in both increased maximal capacity (Wpeak) and 12-min walk distance (Citation34-36), and there was no significant difference in this response between the low FFMI group and the normal FFMI group. We found that FFM decreased slightly in the low FFMI group and increased in the normal FFMI group, but the difference between the two groups did not attain statistical significance. In accordance with our results, Berton et al. reported that both their depleted patients (who were given nutritional supplementation and endurance and strength training for 12 weeks) and their non-depleted patients (who just got the 12 weeks of training) slightly increased FFM and that there was no statistically significant difference between the groups (Citation34). Two recent studies have shown improvements in FFM in depleted patients as a result of exercise training and nutritional supplementation, whereas depleted control patients who just received exercise decreased in FFM (Citation36,37).
Our depleted patients received one oral nutritional supplementation after training, i.e. twice a week, whereas in the study by Berton et al. patients received a daily polysaccharide supplementation if caloric ingestion was judged inadequate (Citation34). In the two studies that improved FFM, one study gave essential amino acid supplementation twice a day for 12 weeks (Citation37), and in the other study the patients were given three oral liquid supplements per 24 hours for four months (Citation36). Thus, the amount and type of supplement may be an important factor to consider. As exercise capacity increases as a result of exercise training independently of BMI (Citation38), we suggest that to improve FFM in depleted patients nutritional supplementation has to be extensive.
While muscle mass can be readily increased by progressive strength training, this would not be expected from an aerobic training programme such as ours. Strength training has been shown to have greater potential to improve muscle mass and strength than endurance training (Citation39). In addition, it was recently shown that cachectic patients can retain the potential for skeletal muscle remodelling as a result of strength training (Citation40).
It was not possible to study the effectiveness of supplementation during exercise training in patients with low FFMI because of our small number of patients. Another limitation is that potential confounding factors including physical activity and metabolic syndrome features were not measured. Our training programme was not primarily aimed at improving muscle mass but rather endurance capacity. Another limitation is that we did not control for daily caloric intake, i.e. patients might have dropped in the normal dietary intake.
Our conclusion is that in COPD old age, low FEV1, and high level of dyspnoea in daily life were, independently of each other, related to less chance of increasing fat-free mass by physical training.
Funding
This paper was supported by grants from the Swedish Heart and Lung Association E011-02, The Swedish Heart-Lung Foundation 2004-10-33, and Bror Hjerpstedt’s foundation 2003-010.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Vestbo J, Hurd SS, Agusti AG, Jones PW, Vogelmeier C, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187:347–65.
- Gosselink R, Troosters T, Decramer M. Peripheral muscle weakness contributes to exercise limitation in COPD. Am J Respir Crit Care Med. 1996;153:976–80.
- O’Donnell DE, Revill SM, Webb KA. Dynamic hyperinflation and exercise intolerance in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164:770–7.
- Aliverti A, Stevenson N, Dellaca RL, Lo Mauro A, Pedotti A, Calverley PM. Regional chest wall volumes during exercise in chronic obstructive pulmonary disease. Thorax. 2004;59:210–16.
- Di Marco F, Terraneo S, Roggi MA, Repossi AC, Pellegrino GM, Veronelli A, et al. Physical activity impairment in depressed COPD subjects. Respir Care. 2014;59:726–34.
- Engelen MP, Schols AM, Lamers RJ, Wouters EF. Different patterns of chronic tissue wasting among patients with chronic obstructive pulmonary disease. Clin Nutr. 1999;18:275–80.
- Vestbo J, Prescott E, Almdal T, Dahl M, Nordestgaard BG, Andersen T, et al. Body mass, fat-free body mass, and prognosis in patients with chronic obstructive pulmonary disease from a random population sample: findings from the Copenhagen City Heart Study. Am J Respir Crit Care Med. 2006;173:79–83.
- Franssen FM, Broekhuizen R, Janssen PP, Wouters EF, Schols AM. Limb muscle dysfunction in COPD: effects of muscle wasting and exercise training. Med Sci Sports Exerc. 2005;37:2–9.
- Katsura H, Yamada K, Kida K. Both generic and disease specific health-related quality of life are deteriorated in patients with underweight COPD. Respir Med. 2005;99:624–30.
- Spruit MA, Singh SJ, Garvey C, ZuWallack R, Nici L, Rochester C, et al. An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med. 2013;188:13–64.
- O’Donnell DE, McGuire M, Samis L, Webb KA. The impact of exercise reconditioning on breathlessness in severe chronic airflow limitation. Am J Respir Crit Care Med. 1995;152:2005–13.
- Schols AM, Soeters PB, Dingemans AM, Mostert R, Frantzen PJ, Wouters EF. Prevalence and characteristics of nutritional depletion in patients with stable COPD eligible for pulmonary rehabilitation. Am Rev Respir Dis. 1993;147:1151–6.
- Baarends EM, Schols AM, Mostert R, Wouters EF. Peak exercise response in relation to tissue depletion in patients with chronic obstructive pulmonary disease. Eur Respir J. 1997;10:2807–13.
- Creutzberg EC, Wouters EF, Mostert R, Pluymers RJ, Schols AM. A role for anabolic steroids in the rehabilitation of patients with COPD? A double-blind, placebo-controlled, randomized trial. Chest. 2003;124:1733–42.
- Pison CM, Cano NJ, Cherion C, Caron F, Court-Fortune I, Antonini MT, et al. Multimodal nutritional rehabilitation improves clinical outcomes of malnourished patients with chronic respiratory failure: a randomised controlled trial. Thorax. 2011;66:953–60.
- Schols AM, Slangen J, Volovics L, Wouters EF. Weight loss is a reversible factor in the prognosis of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;157:1791–7.
- Arnardottir RH, Boman G, Larsson K, Hedenstrom H, Emtner M. Interval training compared with continuous training in patients with COPD. Respir Med. 2007;101:1196–204.
- Celli BR, Cote CG, Marin JM, Casanova C, Montes de Oca M, Mendez RA, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med. 2004;350:1005–12.
- McGavin C, Groupta S, McHarty G. Twelve-minute walking test for assessing disability in chronic bronchitis. Br Med J. 1976;1:822–3.
- Guyatt GH, Berman LB, Townsend M, Pugsley SO, Chambers LW. A measure of quality of life for clinical trials in chronic lung disease. Thorax. 1987;42:773–8.
- Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319–38.
- Hedenstrom H, Malmberg P, Agarwal K. Reference values for lung function tests in females. Regression equations with smoking variables. Bull Eur Physiopathol Respir. 1985;21:551–7.
- Hedenstrom H, Malmberg P, Fridriksson HV. Reference values for lung function tests in men: regression equations with smoking variables. Ups J Med Sci. 1986;91:299–10.
- Hyatt RE. Expiratory flow limitation. J Appl Physiol Respir Environ Exerc Physiol. 1983;55:1–7.
- Hoppeler H, Kleinert E, Schlegel C, Claassen H, Howald H, Kayar SR, et al. Morphological adaptations of human skeletal muscle to chronic hypoxia. Int J Sports Med. 1990;11:S3–9.
- de Voogd JN, Sanderman R, Postema K, van Sonderen E, Wempe JB. Relationship between anxiety and dyspnea on exertion in patients with chronic obstructive pulmonary disease. Anxiety Stress Coping. 2011;24:439–49.
- Mostert R, Goris A, Weling-Scheepers C, Wouters EF, Schols AM. Tissue depletion and health related quality of life in patients with chronic obstructive pulmonary disease. Respir Med. 2000;94:859–67.
- Ferreira IM, Brooks D, White J, Goldstein R. Nutritional supplementation for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;12:CD000998.
- Probst VS, Troosters T, Pitta F, Decramer M, Gosselink R. Cardiopulmonary stress during exercise training in patients with COPD. Eur Respir J. 2006;27:1110–18.
- Bosy-Westphal A, Muller MJ. Identification of skeletal muscle mass depletion across age and BMI groups in health and disease-there is need for a unified definition. Int J Obes (Lond). 2014; Epub ahead of print.
- Eagan TM, Aukrust P, Ueland T, Hardie JA, Johannessen A, Mollnes TE, et al. Body composition and plasma levels of inflammatory biomarkers in COPD. Eur Respir J. 2010;36:1027–33.
- Barnes PJ, Celli BR. Systemic manifestations and comorbidities of COPD. Eur Respir J. 2009;33:1165–85.
- Poulain M, Doucet M, Drapeau V, Fournier G, Tremblay A, Poirier P, et al. Metabolic and inflammatory profile in obese patients with chronic obstructive pulmonary disease. Chron Respir Dis. 2008;5:35–41.
- Berton DC, Silveira L, Da Costa CC, De Souza RM, Winter CD, Zimermann Teixeira PJ. Effectiveness of pulmonary rehabilitation in exercise capacity and quality of life in chronic obstructive pulmonary disease patients with and without global fat-free mass depletion. Arch Phys Med Rehabil. 2013;94:1607–14.
- Steiner MC, Barton RL, Singh SJ, Morgan MD. Nutritional enhancement of exercise performance in chronic obstructive pulmonary disease: a randomised controlled trial. Thorax. 2003;58:745–51.
- van Wetering CR, Hoogendoorn M, Broekhuizen R, Geraerts-Keeris GJ, De Munck DR, Rutten-van Molken MP, et al. Efficacy and costs of nutritional rehabilitation in muscle-wasted patients with chronic obstructive pulmonary disease in a community-based setting: a prespecified subgroup analysis of the INTERCOM trial. J Am Med Dir Assoc. 2010;11:179–87.
- Baldi S, Aquilani R, Pinna GD, Poggi P, De Martini A, Bruschi C. Fat-free mass change after nutritional rehabilitation in weight losing COPD: role of insulin, C-reactive protein and tissue hypoxia. Int J Chron Obstruct Pulmon Dis. 2010;5:29–39.
- Greening NJ, Evans RA, Williams JE, Green RH, Singh SJ, Steiner MC. Does body mass index influence the outcomes of a Waking-based pulmonary rehabilitation programme in COPD? Chron Respir Dis. 2012;9:99–106.
- O’Shea SD, Taylor NF, Paratz JD. Progressive resistance exercise improves muscle strength and may improve elements of performance of daily activities for people with COPD: a systematic review. Chest. 2009;136:1269–83.
- Vogiatzis I, Simoes DC, Stratakos G, Kourepini E, Terzis G, Manta P, et al. Effect of pulmonary rehabilitation on muscle remodelling in cachectic patients with COPD. Eur Respir J. 2010;36:301–10.

