Abstract
Point-of-care testing (POCT) refers to any diagnostic test administered outside the central laboratory at or near the location of the patient. By performing the sample collection and data analysis steps in the same location POCT cuts down on transport and processing delays, resulting in the rapid feedback of test results to medical decision-makers. Over the past decades the availability and use of POCT have steadily increased in Europe and throughout the international community. However, concerns about overall utility and the reliability of benefits to patient care have impeded the growth of POCT in some areas. While there is no agreed-upon standard for how success should be judged, the increases in speed and mobility provided by POCT can lead to substantial advantages over traditional laboratory testing. When properly utilized, POCT has been shown to yield measurable improvements in patient care, workflow efficiency, and even provide significant financial benefits. However, important organizational and quality assurance challenges must be addressed with the implementation of POCT in any health care environment. To ensure maximal benefits it may be necessary to evaluate critically and restructure existing clinical pathways to capitalize better on the rapid test turnaround times provided by POCT.
Introduction
The term point-of-care testing (POCT) refers to any diagnostic test performed at or near the location of the patient. This distinguishes POCT from the traditional practice of extracting specimens from the patient and physically transporting samples to the central laboratory for analysis. In the past, the establishment and use of a central laboratory was necessary due to the size and complexity of equipment required to perform many common medical tests. However, as technology advances it is becoming increasingly possible to perform some of these tests outside of the traditional laboratory setting at the point of care. POCT has the ability to provide drastic improvements in the turnaround time (TAT) of test results. When utilized effectively this decrease in TAT can help expedite medical decision-making and translate into measurable improvements in clinical care and throughput, benefiting both the patient and the health care institution.
In Europe, POCT devices are regulated under the 1998 European Directive 98/79/EC on in vitro diagnostic medical devices (Citation1), which became operational in 2001, although POCT devices are not specifically mentioned or referred to in this directive. The directive provides the member states of the European Union (EU), the European Free Trade Association (EFTA), together with Switzerland and Turkey with a single unified regulation for in vitro diagnostics (IVD). After passing a conformity assessment procedure, medical devices receive a CE mark and can then be introduced into the European market. There have been several amendments to the directive, the latest in 2011 (2011/100/EU), as well as standards based on the framework of the directive. Recently, an international standard (ISO 22870:2006) giving specific requirements applicable to POCT was introduced (Citation2), and this is intended to be used in conjunction with ISO 15189 (Citation3), which describes the general requirements for competence and quality for medical laboratories. Patient self-testing in a home or community setting is not covered by these ISO standards.
Over the past decades the prevalence and functionality of POCT devices have expanded greatly. The use of POCT is steadily increasing in the United States, with growth rates expected to average >15% in the coming years (Citation4). The goal of this review is to describe, from a European perspective, the current state of POCT, highlighting the potential benefits and challenges associated with its implementation and use.
Overview of POCT devices
POCT encompasses a wide variety of procedures and technologies. Current POCT exists for hemoglobin concentrations, five-part differential complete blood count (CBC), pregnancy testing, blood glucose concentrations, cardiac biomarkers, coagulation testing, platelet function, group A streptococcus, HIV testing, malaria screening, and numerous additional applications. POCT devices are used in a wide variety of health care settings () and are generally divided into two broad categories depending on their size and portability: bedside, and near-bedside. Bedside POCT devices are smaller, usually hand-held, and offer the greatest mobility. Due to their compact nature they are often more specialized and limited in overall functionally. Near-bedside devices are larger and usually confined to a designated testing area. These larger devices may exhibit a greater degree of quality control and calibration functionality and will often perform more complex diagnostic tests, or a wider range of tests, than the smaller bedside POCT devices. Together, these devices offer health care professionals the ability to obtain vital care management information at near instantaneous speeds, potentially resulting in numerous benefits to downstream clinical efficiency.
Table I. Non-exhaustive list illustrating the variety of locations where POCT is used.
Implications for patient care
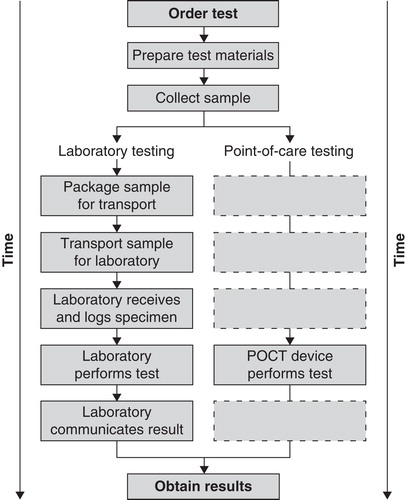
The primary advantages of POCT are rapid TAT for test results and increased mobility. By removing transport and laboratory processing times, POCT allows for near immediate access to test results, compared with the 1 to 2 h (or more) delays that occur if sent to a central laboratory (see for a schematic depiction of the differences between POCT and laboratory testing). These delays are often too long for non-emergency patients to wait in the doctor’s office, and this forces health care providers to spend additional time following up with patients over the phone or during a subsequent visit, further delaying treatment decisions. The critical factor in determining if POCT can positively influence patient outcomes is whether shorter TATs can properly be taken advantage of to guide patient care. A randomized controlled trial in the accident and emergency department of a UK teaching hospital compared the amount of time taken to reach health management decisions between patients where POCT was utilized and patients evaluated through traditional means. In the POCT treatment group physicians reached patient management decisions an average of 74 min faster (Citation5). When utilized as part of an overall health management strategy the ability to expedite decision-making and patient management can result in a number of benefits in the quality and efficiency of care.
Quality of care
The medical utility of POCT depends on whether decreased TATs and time to decision can translate into significant improvements in quality of care and clinical outcome. Perhaps unsurprisingly, the patient groups who often benefit the most from POCT are those for whom delays in treatment initiation can have pronounced negative effects on outcome. The rapid TAT provided by POCT allows for accelerated identification and classification of patients into high-risk and low-risk groups, improving quality of care and increasing clinical throughput. Acute cases such as chest pain sufferers usually account for only a minority of patients passing through an emergency department (ED). POCT systems with a wider test repertoire than cardiac markers, including inflammation/infection markers, hematology, and a metabolic panel, could be used for the majority of patients in the ED and would have a greater overall impact on the efficiency and quality of care in the ED. A great deal of attention has been given to the use of POCT in emergency settings for screening patients who present with symptoms of acute coronary syndromes (ACS). The rapid identification and treatment of ACS patients is critical. Current recommendations dictate testing troponin levels at least 10–12 h after symptom onset (Citation6), with adverse outcomes increasing as a function of the delay in treatment initiation (Citation7-10). Recent publications strongly indicate that with the new sensitive troponin tests the testing intervals can be reduced (Citation11,12). Due to the time-sensitive nature of this condition, the decreased TATs provided by POCT can offer a clear advantage. POCT has been shown to increase the speed at which positive cases of ACS are accurately identified (Citation13,14), allowing physicians the ability to admit and initiate treatment at a faster rate than previously possible (Citation13). Decreased TATs also can result in the earlier identification of negative cases of ACS, thereby increasing the number of successful discharges, and allowing for more efficient use of hospital resources (Citation11).
Medical benefits with POCT have also been found outside of the ED. Individuals suffering from chronic illness often require regular office check-ins with their primary care physician to evaluate and monitor disease progression. Decisions to adjust or change treatment options are often based on the results of clinical testing. POCT gives health care professionals the ability to perform and review test results during the same office visit, and can result in significant improvements in patient attitude and compliance. In an Australian study evaluating patient satisfaction with POCT in a general practice setting, Laurence et al. found that patients reported significantly greater levels of confidence in their doctor and a greater motivation to look after their own condition when POCT was used (Citation15). Supporting this result, Gialamas et al. found similar or greater levels of self-reported medical adherence in patients undergoing long-term treatment for diabetes or coagulation disorders when POCT was performed at regular office visits (Citation16). Significant improvements in glycemic control were also found in diabetes patients with POCT, demonstrating that these subjective benefits in patient motivation can translate into quantifiable improvements in disease management (Citation12).
Despite these benefits, concerns over the trueness of results and general usefulness in non-emergency settings have slowed the adoption of POCT in general practice. Questions regarding the accuracy and bias of POCT results are valid, and non-inferiority to conventional methods should always be demonstrated before implementation of any significant health care change.
The method by which sample specimens are collected with POCT also provides significant advantages over traditional laboratory analysis. Hematology POCT devices generally obtain blood samples using the finger prick (or heel prick in newborns) method. These devices not only require less overall volume than traditional laboratory tests, but are significantly less invasive than obtaining a venous blood sample. A reduction in the number of venous blood draws is preferable from a patient’s perspective and can result in greater self-reported levels of patient satisfaction (Citation15). Decreased sample sizes can be advantageous in patient groups where obtaining substantial amounts of blood might be difficult or potentially harmful, for example in children, the elderly, or those in intensive care. The use of POCT in neonatal intensive care units can reduce blood loss and lower the probability of transfusions, greatly improving overall quality of care (Citation17-22).
Efficiency of care
Due to its distributed nature, the cost-per-test of POCT is usually greater than tests performed in a central laboratory (Citation23), although this is not universally true (Citation24). Ideally, any added cost at the per-test level can be substantially offset by indirect financial gains from increased resource management and greater efficiency in patient care (Citation25). As a percentage of total health care costs, laboratory expenditures have been reported to be approximately 2%–3% in Sweden and 4% in the United Kingdom (Citation26,27). However, it has been estimated that diagnostic laboratory testing influences approximately two-thirds of total health care costs (Citation28). Therefore, while clinical testing itself may not heavily contribute to the cost of health care, its ability to influence overall expenditure can be quite significant.
ED overcrowding is an international problem, with departments often challenged to meet government wait time targets (Citation29,30). France, Sweden, UK, New Zealand, and Canada have all passed explicit length-of-stay time targets, requiring that patients must leave the emergency department within 4–8 h.
Overcrowding and prolonged wait times have been linked to adverse clinical outcomes and decreased patient satisfaction. While no one factor can be identified as the root cause of this issue, decrease in delays between sample collection and test results can provide health care professionals with the opportunity to arrive at faster care management decisions, resulting in increased patient throughput and decreasing average wait times. A Swiss study evaluated the addition of POCT for B-type natriuretic peptide levels for patients presenting to the emergency department with acute dyspnea as their primary symptom. As expected, POCT was associated with significant decreases in the time to treatment initiation. Importantly, the group that received POCT also exhibited a shorter length of stay and a 26% reduction in the total treatment costs to the department (Citation31). Another study looked at the effects of implementing a POCT cardiac marker screening stage in six hospitals within the UK. The investigators found that POCT increased the percentage of successful home discharges and reduced overall mean length of stay (Citation11). A follow-up report, however, showed that the effect of POCT on average cost per patient varied greatly between hospitals (Citation32), highlighting the importance of optimizing clinical pathways to benefit maximally from decreased TATs provided by POCT. Finally, several reports have evaluated the accuracy and bias of POCT for pregnancy markers in the ED, a commonly performed diagnostic test in women of reproductive age. Several studies have shown that POCT options for pregnancy testing (hCG immunoassay kit) can yield sufficiently sensitive results, with TATs much faster than central laboratory testing (Citation33,34).
Efficiency gains from POCT can also be found outside the ED. Decreased wait times were observed when POCT testing was performed during the initial evaluation of patients in a UK hematology clinic. In this study POCT enabled the clinic to meet patient-defined standards of a 30-min maximal wait time. Interestingly, testing from the central hematology laboratory was not able to meet this standard, even when samples were delivered via a vacuum tube system in an attempt to decrease transit times (Citation35).
Patients visiting a primary care facility often show symptoms that may indicate anemia or infectious diseases. As a result, laboratory tests for hemoglobin and complete blood count (CBC) are among the most frequently requested diagnostic tests. In Uppsala County in Sweden, for example, there are approximately 160 CBC and 220 hemoglobin (which include a CBC) test requests from primary care facilities per 1,000 inhabitants. Initially, POCT for hemoglobin was the only POCT option at primary care centers. In cases where additional hematology assays were needed (e.g. for anemia patients) the lack of a POCT option for CBC resulted in prolonged delays in test results, forcing doctors to perform an additional patient consultation at a later time, either in person or remotely (by mail or telephone). This follow-up requires physicians to spend more time reviewing patient files and trying to get in contact with patients than would be necessary if the test results were available at the initial visit. The cost for this time can readily be eliminated by POCT. Today, many primary care centers in Uppsala County now utilize POCT CBC technology to reduce the time to diagnosis and treatment.
Benefits in health management decisions for patients with possible deep venous thromboembolism (DVT) have also been observed. Point-of-care D-dimer testing is useful in risk stratification for low-risk DVT patients (Citation36). For patients with a low Wells score, a negative D-dimer test can exclude a diagnosis of DVT, while an ultrasound is necessary for patients with a positive D-dimer test. The addition of POCT for D-dimer significantly reduced the number of admissions and the mean length of stay in the ED (Citation37).
In patients undergoing oral coagulation therapy with vitamin K antagonists, regular testing is required to ensure that patients remain within the therapeutic range. The risk to patients for thrombosis or hemorrhage can drastically increase when coagulation measurements drift into subtherapeutic or supratherapeutic levels, respectively (Citation38). Accurate and regular monitoring of coagulation in these patients is therefore of critical importance, and patients are often kept under hospital supervision solely for the purpose of coagulation management. POCT could be utilized to increase the number of patients eligible for self-monitoring, reducing the necessity for prolonged hospital stays (Citation39,40). A German study, comparing the cost of self-management to monitoring therapy with a physician, estimated a 35% savings with patient home care (Citation41). In Ireland, a prospective audit of inpatients receiving anticoagulation therapy at Cork University Hospital found that patients spent an average of three potentially unnecessary nights under hospital care (Citation42). The average cost for a non-intensive care bed in this hospital was estimated at €638 per night, highlighting a substantial opportunity to reduce costs and overcrowding with the implementation of a patient self-monitoring POCT system.
Excessive delays in receiving test results can also contribute to greater inefficiencies in the use of hospital resources. In these cases, POCT has the potential to reduce lost revenue due to delays or disruptions in the workflow of test-dependent medical procedures. One example of this type of inefficiency is the lost revenue from idle scanner time when disruptions occur in the computer-assisted tomography (CT) or magnetic resonance imaging (MRI) queue. As a precaution against acute kidney injury, blood creatinine concentrations are routinely measured prior to contrast injection. One study found 5.3% of patients were delivered to the radiology department without recent blood creatinine measurements and therefore were unable to undergo any procedure until new measurements could be carried out and results obtained. Using POCT, nurses were able to measure and verify acceptable levels of creatinine-eGFR for the majority of patients, avoiding substantial disruptions to scanner efficiency. The results of CT or MRI scans are often critical factors in care management decisions. Delays in radiology testing can therefore affect overall hospital efficiency and have been shown to extend total length of stay in the ED (Citation43).
Challenges to optimizing benefits from POCT
The implementation of POCT greatly increases the number of testing devices and potential device operators. POCT users are often nurses and clinical staff members with existing patient care responsibilities and a limited technical background. These issues create several important oversight and quality challenges that health care providers must address prior to implementing any new POCT system. In this section we will identify and address several challenges in the management of POCT and discuss practical solutions.
It should again be stressed that the primary advantage provided by POCT is the pronounced decrease in TATs. POCT results will be available within minutes, yet the ability of clinical pathways to take advantage of these decreased TATs is what will ultimately determine the effects POCT has on workflow and patient care. Improvements in length of stay, throughput, and outcomes are all secondary results, and are highly dependent on several factors which will vary across health care settings. Emphasizing this point, the effect on average treatment costs per patient was found to vary greatly across six different hospitals in the UK when POCT systems were implemented for measuring cardiac biomarkers (Citation32).
Another prerequisite for obtaining benefits from any individual POC test is that this test is sufficient for medical decision-making in the clinical setting–and should not necessitate additional confirmatory tests from the central laboratory.
Prior to the adoption of POCT, it is recommended that a needs analysis and critical evaluation of clinical pathways be performed (Citation44). Any area in which test results are a potential limiting factor in medical decision-making will readily benefit from a shorter TAT; however, significant changes to clinical pathways may be necessary to achieve maximal benefits.
Pecoraro et al. (Citation45) have conducted a systematic review of 84 studies for five POCT instruments (neonatal bilirubin, procalcitonin, intra-operative parathyroid hormone, troponin, and blood gas analysis). They found that 50% of the papers reported correlations between POCT and laboratory instruments, but only 13% looked at the impact of POCT on clinical practice. They commented that, although POCT decreases the time it takes to make a patient management decision, the final clinical outcomes have not been evaluated in detail. We agree that only systematic surveys (such as might be undertaken in a health technology assessment) are likely to provide definitive results with respect to the final clinical outcomes from patient management programs utilizing POCT compared with central labs for a particular hospital, health care system, or country.
The structure and usage of POCT in Europe is highly irregular and has been found to differ greatly between institutions and countries (Citation46). While variable operating procedures are a necessary product of institutional differences, certain challenges with respect to oversight and quality control will need to be universally addressed wherever POCT is used. Several published guidelines provide recommendations on how to develop, regulate, and maintain POCT services successfully. The International Council for Standardization in Haematology (ICSH) (Citation47), the ISO EN 22870:2006 (Citation2), and the British Committee for Standards in Haematology (Citation48) highlight four fundamental challenges relevant to all institutions in which POCT is used: management and oversight, training, quality assurance, and documentation.
Management and oversight
Due to the highly distributed nature of POCT, it is recommended that oversight responsibilities be centralized under the position of POC coordinator (Citation47-49). This individual should be responsible for all aspects of POCT performed within an institution and maintain an active awareness of their responsibility for clinical governance (Citation50). Clinical governance is defined as the ‘framework through which organizations are accountable for continually improving the quality of their services and safeguarding high standards of care by creating an environment in which excellence in clinical care will flourish’ (Citation51,52). In operational terms, the POC coordinator’s main responsibilities are to educate users regarding good laboratory practice and proper operation of all POCT devices, maintain and optimize documentation of all POCT-related materials, and develop and enforce adequate quality control procedures as are appropriate to the types of POCT performed. The following sections will discuss these responsibilities in greater detail, highlighting their necessity and identifying possible organizational and technological solutions.
Training
Large laboratory instruments are highly automated compared with most POCT devices. This automation contributes to the increased speed and ease-of-use of these devices, but can lead to challenges in educating a larger audience in the proper usage. With significantly increased numbers of device users, it is likely that POCT will often be performed by individuals with limited technical backgrounds, strengthening the need for adequate and continuous training (Citation53). Training protocols should establish standard operating procedures for all potential point-of-care devices, and users should be required to verify competency before operating any POCT device. It is recommended that records of certification be kept for all POCT systems within an institution, and that users be required to renew their training on an annual or semi-annual basis (Citation54,55). Operation by inadequately trained users can drastically increase the probability of inaccurate results due to human error. In particular, mixing up patient data is probably the most common reason for erroneous POCT results. It is the job of the POC coordinator to enact measures that ensure that POCT may only be performed by staff whose training and competence are up to date. Several bedside testing devices include barcode scanning functionality, requiring operators to input their identification credentials prior to use. This gives POC coordinators the ability electronically to ‘lock-out’ personnel with inadequate or expired training credentials, preventing operation of POCT by undertrained users. Some devices also support remote capability, allowing POC coordinators to update and enforce ‘lock-out’ lists in real time from a central database. Web-based training resources can be very valuable as an aid to optimizing continuous and individualized training.
Quality assurance
Issues of compliance and quality assurance increase in complexity as clinical testing becomes more decentralized. With so many additional users and devices in operation, maintaining acceptable quality levels can be a challenging task. POCT devices for measuring the CBC and clotting times, the most commonly ordered hematology tests, have been shown to produce accurate and reliable results when used under ideal conditions (Citation54-58). A previous Swedish study found no differences in bleeding complications between Swedish primary health care centers (which primarily use POCT) and specialized anticoagulation clinics (Citation59). However, like any tool, POCT can only be as accurate as its operator is competent. Therefore, a continued commitment to quality assurance is an essential component of POCT, and the principles of quality management should be followed by all members of the health care community. Ensuring that POCT is performed only by sufficiently trained staff is a good first step toward reducing the incidence of human error. Certified operators must remain aware of institutional principles of quality management at all stages of testing.
Internal quality control (IQC) and external quality assessment (EQA) are two methods that help ensure reliable results from POCT devices. IQC methods are used primarily to ensure that devices are producing accurate and consistent results. The most common way in which this is accomplished is by requiring operators to analyze and document the output of a control sample prior to the performance of any patient test. Various types of control samples are available, and device manufacturers will often provide the necessary control materials. Consistent control measurements are essential to ensure that devices are functioning properly, and should be conducted at a sufficiently high frequency. The results of IQC testing should be documented each time the device is used and recorded along with the test results and patient information. Records of IQC testing should be kept for each POCT device. This is necessary to identify any changes in device sensitivity, or bias, that may exist between batches of testing materials. Additionally, chronological IQC records will allow POCT users to identify gradual drifts in performance that may be symptomatic of underlying technical problems. Many devices now offer increased quality control functionality and will require IQC checks to be performed and documented before releasing patient results. Additionally, POCT devices with network access will be able to integrate quality control measurements into a central data management system, thereby reducing paperwork, the burdens of which would otherwise be placed on operators.
Several guidelines recommend that POCT users participate in an external quality assessment (EQA) scheme on a regular basis (Citation2,47,48). EQA is performed by testing samples containing an undisclosed value received from some external source. These external samples can be obtained from an accredited EQA program and are critical to maintaining the trueness of POCT results. Device manufacturers may also operate EQA schemes, and POC coordinators should consult with hospital laboratories for recommendations of EQA schemes that are appropriate for institutional needs. Parallel testing of patient samples by the central laboratory can provide additional confirmation of device accuracy and bias, and should be integrated into EQA practice when laboratory resources are available.
Documentation
Proper documentation of all test results and testing materials is vital to ensuring quality control in POCT. In addition to patient identification (full name, date of birth, sex), information identifying the requesting physician, test administrator, reagent batch number, and internal quality control results should be documented for each test. Many POCT devices are able to store this information electronically and include barcode scan functionality to ensure accurate input. It is increasingly common for devices to connect with the hospital’s local network and upload test information directly into electronic medical records. This eliminates the need to enter manual results into a separate laboratory or hospital information system, reducing staff workloads and lowering the incidence of clerical errors. All result records should be properly identified and clearly distinguish POCT results from those performed at the central laboratory (Citation2).
Future developments for POCT
Given the rate of technological advancement and the potential benefits to efficiency and quality of care offered by POCT, it seems likely that the prevalence of POCT in health care will continue to grow in the future. Government initiatives, along with a high incidence of time-sensitive medical conditions, already provide strong incentives for the expansion of POCT in hospitals and emergency departments. In the surrounding community, financial incentives and trends toward increased patient involvement in their own care (empowerment of the patient) will likely continue to drive the expansion of POCT outside hospital centers. When POCT enters the community, issues concerning management and oversight, training, quality assurance, and documentation are all greatly amplified. In the UK there are various pressures, not least from government, to move pathology testing closer to the patient (i.e. general practices, pharmacies, supermarkets, etc.). This initiative raises many questions about clinical governance, clinician ‘buy-in’, and patient confidence/participation that have yet to be addressed. Indeed, the purported benefits versus risks have not substantively been assessed. A comprehensive needs analysis is warranted to assess what POCT is required in the community by clinicians and the public, and how best to meet those needs. Pathology and POCT staff are best positioned to assist in this process and should work closely and sensitively with their counterparts in the surrounding community–general practitioners, nurses, pharmacists, health care assistants–to ensure a quality level consistent with hospital-based centralized laboratories.
Conclusion
Technological advances have made it possible to conduct many laboratory tests at, or near, the point of care. These POCT devices give physicians rapid access to test results, allowing for greater quality and efficiency in medical care. Increased availability of POCT devices for more commonly performed routine tests would improve efficiency further. In the absence of any immediate health risk, physicians will likely wait to review all results before reaching any patient management decision. Without universal POCT, prolonged turnarounds for laboratory transit and processing will continue to be the rate-limiting step in medical decision-making. As technological innovation provides more comprehensive POCT options for CBC, pregnancy testing, infectious disease, cancer screening, and other frequently ordered tests, near-patient testing will become increasingly integrated into the traditional health care structure. The role and responsibilities of laboratory personnel will likely need reviewing and reworking as testing migrates away from the lab bench and closer to the bedside. Coordination with the central laboratory regarding quality assurance and regulatory matters will be crucial as technology allows for more efficient allocation of testing resources. Outside the hospital setting, POCT provides laboratory quality services to underserviced areas and general practitioners. Near-immediate test results allow patients and doctors to evaluate progress, review results, and establish treatment regimens in a single visit. This simple change can result in improved disease management, treatment adherence, and patient satisfaction. However, without the presence of an in-house laboratory, having in place the measures to ensure adequate levels of quality assurance in POCT becomes critically important. As POCT continues to become an integral part of health care management, expansive quality assurance and training protocols should be established to ensure maximal benefits to patient care and efficiency in any setting.
Acknowledgements
Medical writing and editorial support for preparation of this manuscript were provided by Cory Hussar, PhD, and Robin Smith, PhD, of The Curry Rockefeller Group, LLC, Tarrytown, NY. Funding for this support was provided by Abbott Point of Care. However, the opinions expressed are solely those of the authors, and the authors confirm independence from the funding source. The authors received no payment for their work.
Declaration of interest: Roman Greig-Pylypczuk has received a travel grant and an honorarium from Vox Medica within the last 18 months. Anders Larsson and Albert Huisman have no disclosures or conflicts of interest to report.
References
- European Commission. Directive 98/79/EC of the European Parliament and of the Council of 27 October 1998 on in vitro diagnostic medical devices. Brussels, Belgium: European Commission 1998. Available from http://ec.europa.eu/enterprise/policies/european-standards/harmonised-standards/iv-diagnostic-medical-devices/. Last accessed 7 December 1998.
- International Organization for Standardization. Point of care (POC): Requirements for quality and competence (ISO 22870:2006). Geneva, Switzerland: International Organization for Standardization 2006. Available from http://www.iso.org/iso/catalogue_detail.htm?csnumber=35173. Last accessed 01 February 2006.
- International Organization for Standardization. Medical laboratories: Particular requirements for quality and competence (ISO 15189:2012). Geneva, Switzerland: International Organization for Standardization 2012. Available from http://www.iso.org/iso/home/store/catalogue_ics/catalogue_detail_ics.htm?csnumber=56115. Last accessed 01 November 2012.
- Scalise D. Poised for growth. point-of-care testing. Hosp Health Netw. 2006;80:77–83.
- Kendall J, Reeves B, Clancy M. Point of care testing: randomised controlled trial of clinical outcome. BMJ. 1998;316:1052–7.
- Reichlin T, Hochholzer W, Bassetti S, Steuer S, Stelzig C, Hartwiger S, et al. Early diagnosis of myocardial infarction with sensitive cardiac troponin assays. N Engl J Med. 2009;361:858–67.
- Luepker RV, Raczynski JM, Osganian S, Goldberg RJ, Finnegan JRJr, Hedges JR, et al. Effect of a community intervention on patient delay and emergency medical service use in acute coronary heart disease: The Rapid Early Action for Coronary Treatment (REACT) Trial. JAMA. 2000;284:60–7.
- Neumann FJ, Kastrati A, Pogatsa-Murray G, Mehilli J, Bollwein H, Bestehorn HP, et al. Evaluation of prolonged antithrombotic pretreatment ("cooling-off" strategy) before intervention in patients with unstable coronary syndromes: a randomized controlled trial. JAMA. 2003;290:1593–9.
- Tricoci P. Time to coronary angiography in patients with non-ST-segment elevation acute coronary syndrome: how fast should patients go to the catheterization laboratory? Curr Opin Cardiol. 2008;23:585–90.
- Tricoci P, Lokhnygina Y, Berdan LG, Steinhubl SR, Gulba DC, White HD, et al. Time to coronary angiography and outcomes among patients with high-risk non ST-segment elevation acute coronary syndromes: results from the SYNERGY trial. Circulation. 2007;116:2669–77.
- Goodacre S, Bradburn M, Fitzgerald P, Cross E, Collinson P, Gray A, et al. The RATPAC (Randomised Assessment of Treatment using Panel Assay of Cardiac markers) trial: a randomised controlled trial of point-of-care cardiac markers in the emergency department. Health Technol Assess. 2011;15:1–102.
- Rust G, Gailor M, Daniels E, McMillan-Persaud B, Strothers H, Mayberry R. Point of care testing to improve glycemic control. Int J Health Care Qual Assur. 2008;21:325–35.
- Renaud B, Maison P, Ngako A, Cunin P, Santin A, Hervé J, et al. Impact of point-of-care testing in the emergency department evaluation and treatment of patients with suspected acute coronary syndromes. Acad Emerg Med. 2008;15:216–24.
- Storrow AB, Zhou C, Gaddis G, Han JH, Miller K, Klubert D, et al. Decreasing lab turnaround time improves emergency department throughput and decreases emergency medical services diversion: a simulation model. Acad Emerg Med. 2008;15:1130–5.
- Laurence CO, Gialamas A, Bubner T, Yelland L, Willson K, Ryan P, et al. Patient satisfaction with point-of-care testing in general practice. Br J Gen Pract. 2010;60:e98–104.
- Gialamas A, Yelland LN, Ryan P, Willson K, Laurence CO, Bubner TK, et al. Does point-of-care testing lead to the same or better adherence to medication? A randomised controlled trial: the PoCT in General Practice Trial. Med J Aust. 2009;191:487–91.
- Bubner TK, Laurence CO, Gialamas A, Yelland LN, Ryan P, Willson KJ, et al. Effectiveness of point-of-care testing for therapeutic control of chronic conditions: results from the PoCT in General Practice Trial. Med J Aust. 2009;190:624–6.
- Hinds LE, Brown CL, Clark SJ. Point of care estimation of haemoglobin in neonates. Arch Dis Child Fetal Neonatal Ed. 2007;92:F378–80.
- Huisman A, Van Solinge WW. Point-of-care testing in hematology. In Price C, St John A, Hicks JM, editors. Point-of-care testing. 2nd ed Washington DC: AACC Press; 2004.
- Madan A, Kumar R, Adams MM, Benitz WE, Geaghan SM, Widness JA. Reduction in red blood cell transfusions using a bedside analyzer in extremely low birth weight infants. J Perinatol. 2005;25:21–5.
- Mahieu L, Marien A, De Dooy J, Mahieu M, Mahieu H, Van Hoof V. Implementation of a multi-parameter Point-of-Care-blood test analyzer reduces central laboratory testing and need for blood transfusions in very low birth weight infants. Clin Chim Acta. 2012;413:325–30.
- Widness JA, Madan A, Grindeanu LA, Zimmerman MB, Wong DK, Stevenson DK. Reduction in red blood cell transfusions among preterm infants: results of a randomized trial with an in-line blood gas and chemistry monitor. Pediatrics. 2005;115:1299–306.
- Asha SE, Chan AC, Walter E, Kelly PJ, Morton RL, Ajami A, et al. Impact from point-of-care devices on emergency department patient processing times compared with central laboratory testing of blood samples: a randomised controlled trial and cost-effectiveness analysis. Emerg Med J. 2014;31:714–19.
- Chin CD, Cheung YK, Laksanasopin T, Modena MM, Chin SY, Sridhara AA, et al. Mobile device for disease diagnosis and data tracking in resource-limited settings. Clin Chem. 2013;59:629–40.
- Weber CF, Zacharowski K. Perioperative point of care coagulation testing. Dtsch Arztebl Int. 2012;109:369–75.
- Plebani M. Appropriateness in programs for continuous quality improvement in clinical laboratories. Clin Chim Acta. 2003;333:131–9.
- Mindemark M, Larsson A. Longitudinal trends in laboratory test utilization at a large tertiary care university hospital in Sweden. Ups J Med Sci. 2011;166:34–8.
- Lee-Lewandrowski E, Lewandrowski K. Implementing point-of-care testing to improve outcomes. J Hosp Admin. 2013;2:125–32.
- Boyle A, Beniuk K, Higginson I, Atkinson P. Emergency department crowding: time for interventions and policy evaluations. Emerg Med Int. 2012;2012:838610.
- Coughlan M, Corry M. The experiences of patients and relatives/significant others of overcrowding in accident and emergency in Ireland: a qualitative descriptive study. Accid Emerg Nurs. 2007;15:201–9.
- Mueller C, Scholer A, Laule-Kilian K, Martina B, Schindler C, Buser P, et al. Use of B-type natriuretic peptide in the evaluation and management of acute dyspnea. N Engl J Med. 2004;350:647–54.
- Bradburn M, Goodacre SW, Fitzgerald P, Coats T, Gray A, Hassan T, et al. RATPAC Research Team. Interhospital variation in the RATPAC trial (Randomised Assessment of Treatment using Panel Assay of Cardiac markers). Emerg Med J. 2012;29:233–8.
- Cole LA. Human chorionic gonadotropin tests. Expert Rev Mol Diagn. 2009;9:721–47.
- Fromm C, Likourezos A, Haines L, Khan AN, Williams J, Berezow J. Substituting whole blood for urine in a bedside pregnancy test. J Emerg Med. 2012;43:478–82.
- Galloway MJ, Woods RS, Nicholson SL, Foggin JJ, Elliott L. An audit of waiting times in a haematology clinic before and after the introduction of point-of-care testing. Clin Lab Haematol. 1999;21:201–5.
- Geersing GJ, Janssen KJ, Oudega R, Bax L, Hoes AW, Reitsma JB, et al. Excluding venous thromboembolism using point of care D-dimer tests in outpatients: a diagnostic meta-analysis. BMJ. 2009;339:b2990.
- Lee-Lewandrowski E, Nichols J, Van Cott E, Grisson R, Louissaint A, Benzer T, et al. Implementation of a rapid whole blood D-dimer test in the emergency department of an urban academic medical center: impact on ED length of stay and ancillary test utilization. Am J Clin Pathol. 2009;132:326–31.
- Keeling D, Baglin T, Tait C, Watson H, Perry D, Baglin C, et al. British Committee for Standards in Haematology. Guidelines on oral anticoagulation with warfarin - fourth edition. Br J Haematol. 2011;154:311–24.
- Giles MT, Parker V, Bevan H, Wright IM. Comparing Point of Care International Normalised Ratio testing with laboratory testing methods in a cardiac inpatient population. J Clin Nurs. 2010;19:3085–91.
- Lawrie AS, Hills J, Longair I, Green L, Gardiner C, Machin SJ, et al. The clinical significance of differences between point-of-care and laboratory INR methods in over-anticoagulated patients. Thromb Res. 2012;130:110–14.
- Taborski U, Wittstamm FJ, Bernardo A. Cost-effectiveness of self-managed anticoagulant therapy in Germany. Semin Thromb Hemost. 1999;25:103–7.
- Forde D, O’Connor MB, Gilligan O. Potentially avoidable inpatient nights among warfarin receiving patients; an audit of a single university teaching hospital. BMC Res Notes. 2009;2:41.
- Miele V, Andreoli C, Grassi R. The management of emergency radiology: key facts. Eur J Radiol. 2006;59:311–14.
- Huisman A. Needs analysis and selection of point-of-care testing analyzers. In Kottke-Marchant K, Davis BH, editors. Laboratory hematology practice. Oxford, UK: Wiley-Blackwell; 2012:668–72.
- Pecoraro V, Germagnoli L, Banfi G. Point-of-care testing: where is the evidence? A systematic survey. Clin Chem Lab Med. 2014;52:313–24.
- Kitchen DP, Kitchen S, Jennings I, Woods TA, Walker ID, Preston FE. Point of care testing by health care professionals: current practice amongst the UK National External Quality Assessment Scheme Participants. Br J Heamatol. 2005;130:320–1.
- Briggs C, Carter J, Lee SH, Sandhaus L, Simon-Lopez R, Vives Corrons JL. ICSH Guideline for worldwide point-of-care testing in haematology with special reference to the complete blood count. Int J Lab Hematol. 2008;30:105–16.
- Briggs C, Guthrie D, Hyde K, Mackie I, Parker N, Popek M, et al. British Committee for Standards in Haematology General Haematology Task Force. Guidelines for point-of-care testing: haematology. Br J Heamatol. 2008;142:904–15.
- Pernet P, Goudable J, Annaix V, Vaubourdolle M, Szymanowicz A. [Guidelines for the formation of a multidisciplinary group for point-of-care testing supervision according to EN ISO 22870]. Ann Biol Clin (Paris). 2012;70:161–6.
- Clinical governance in the new NHS (Health Service Circular: (99) 065). London, United Kingdom: Department of Health, Government of the United Kingdom 1999. Available from http://webarchive.nationalarchives.gov.uk/+/www.dh.gov.uk/en/Publicationsandstatistics/Lettersandcirculars/Healthservicecirculars/DH_4004883. Last accessed 16 March 1999.
- Scally G, Donaldson LJ. The NHS’s 50 anniversary. Clinical governance and the drive for quality improvement in the new NHS in England. BMJ. 1998;317:61–5.
- Pearson J. Point-of-care-testing and clinical governance. Clin Chem Lab Med. 2006;44:765–7.
- Lewandrowski K, Gregory K, Macmillan D. Assuring quality in point-of-care testing: evolution of technologies, informatics, and program management. Arch Pathol Lab Med. 2011;135:1405–14.
- Briggs C, Kimber S, Green L. Where are we at with point-of-care testing in haematology? Br J Heamatol. 2012;158:679–90.
- Briggs C, Longair I, Kumar P, Singh D, Machin SJ. Performance evaluation of the Sysmex haematology XN modular system. J Clin Pathol. 2012;65:1024–30.
- Hollis VS, Holloway JA, Harris S, Spencer D, van Berkel C, Morgan H. Comparison of venous and capillary differential leukocyte counts using a standard hematology analyzer and a novel microfluidic impedance cytometer. PLoS ONE. 2012;7:e43702.
- Papadea C, Foster J, Grant S, Ballard SA, Cate JC, Southgate WM, et al. Evaluation of the i-STAT Portable Clinical Analyzer for point-of-care blood testing in the intensive care units of a university children’s hospital. Ann Clin Lab Sci. 2002;32:231–43.
- Rao LV, Ekberg BA, Connor D, Jakubiak F, Vallaro GM, Snyder M. Evaluation of a new point of care automated complete blood count (CBC) analyzer in various clinical settings. Clin Chim Acta. 2008;389:120–5.
- Wallvik J, Sjalander A, Johansson L, Bjuhr O, Jansson JH. Bleeding complications during warfarin treatment in primary healthcare centres compared with anticoagulation clinics. Scand J Prim Health Care. 2007;25:123–8.