Abstract
Background. Our aim was to investigate cerebral and cardiac tissue injury subsequent to use of vasopressin and adrenaline in combination compared with vasopressin alone during cardiopulmonary resuscitation (CPR).
Methods. In a randomized, prospective, laboratory animal study 28 anesthetized piglets were subject to a 12-min untreated cardiac arrest and subsequent CPR. After 1 min of CPR, 10 of the piglets received 0.4 U/kg of arg8-vasopressin (V group), and 10 piglets received 0.4 U/kg of arg8-vasopressin, 1 min later followed by 20 µg/kg body weight of adrenaline, and another 1 min later continuous administration (10 µg/kg/min) of adrenaline (VA group). After 8 min of CPR, the piglets were defibrillated and monitored for another 3 h. Then they were killed and the brain immediately removed pending histological analysis.
Results. During CPR, the VA group had higher mean blood pressure and cerebral cortical blood flow (CCBF) but similar coronary perfusion pressure. After restoration of spontaneous circulation there was no difference in the pressure variables, but CCBF tended to be (36% ± 16%) higher in the V group. Neuronal injury and signs of a disrupted blood–brain barrier (BBB) were greater, 20% ± 4% and 21% ± 4%, respectively, in the VA group. In a background study of repeated single doses of adrenaline every third minute after 5 min arrest but otherwise the same protocol, histological measurements showed even worse neural injury and disruption of the BBB.
Conclusion. Combined use of vasopressin and adrenaline caused greater signs of cerebral and cardiac injury than use of vasopressin alone during experimental cardiopulmonary resuscitation.
Introduction
Since the very first application of cardiopulmonary resuscitation (CPR) over a century ago, adrenaline (epinephrine) has been recommended as an adjunct therapy during resuscitation from circulatory arrest (CA). This recommendation was first based on animal experiments (Citation1-3), then on case reports, and later on evidence of increased coronary perfusion pressure (Citation4), cerebral blood flow (Citation5-7), and subsequent increased rate of restoration of spontaneous circulation (ROSC) (Citation4).The last-mentioned, however, was contradicted by a clinical registry study (Citation8,9). The clinical dose of adrenaline has generally been recommended to be 20 µg/kg body weight (b.w.) or 1 mg to a grown-up patient (Citation10). Larger doses have been investigated but found to decrease porcine cerebral blood flow (Citation11) and not to increase clinical survival (Citation12-14). Vasopressin has been investigated as an alternative to adrenaline and was demonstrated to be experimentally superior to adrenaline in regard to organ perfusion (Citation15,16), cerebral oxygenation (Citation17), incidence of ROSC, and survival (Citation18,19). However, the largest randomized placebo-controlled clinical study (Citation20) failed to establish vasopressin as a better clinical alternative to adrenaline, as survival was no better than when adrenaline alone was used during CPR. In that clinical study vasopressin was often, actually in 60% of the patients, combined with adrenaline, since the combination had been shown to increase coronary blood flow in an asphyxial cardiac arrest model (Citation21), despite experimental results also showing that the combination of vasopressin and adrenaline caused a lower cerebral blood flow than vasopressin alone (Citation22). In a similar study of in-hospital CA, Stiell et al. (Citation23) reported addition of adrenaline in no less than 87% of the vasopressin group of patients where no benefit was found for vasopressin. A recent meta-analysis has confirmed no survival benefit for vasopressin over adrenaline (Citation24). Ristagno et al. (Citation16,25) pointed out that adrenaline administration in experimental CPR reduces cerebral microcirculatory perfusion, and Olasveengen et al. (Citation26) have in a large controlled clinical study demonstrated that survival to hospital discharge after CA and CPR with use of intravenous drugs (mainly adrenaline) was no better than without. A post hoc subgroup analysis of the same patient material, comparing those actually given versus not given adrenaline, revealed that receiving adrenaline was associated with improved short-term survival but decreased survival to hospital discharge (Citation27). It has been confirmed that adrenaline administrated during CPR causes higher rates of restoration of spontaneous circulation (ROSC) but did not improve long-term survival (Citation28).
Due to these circumstances, with alternatives to adrenaline urgently required especially for CA of long duration, we considered that further mechanistic studies on the vasopressors adrenaline and vasopressin were required to elucidate causes for these differences and more in-detail effects of their combined use during CPR. As such studies need to include histopathology and other highly invasive methods, they were made in experimental animals. Our porcine model (Citation29,30) then seemed to be well suited for the purpose. There are no previous similar studies including early cerebral injuries.
In accordance with previous experimental and clinical results, the hypothesis was that the combined vasopressin-adrenaline treatment results in higher coronary perfusion pressure and rate of ROSC and less cardiac and cerebral injuries than the use of vasopressin alone during CPR.
Materials and methods
The Uppsala Institutional Review Board for Animal Experimentation approved this prospective, randomized, laboratory animal study in accordance with national law and EU directives. All animals included in the main study groups (n = 18) and the background study (n = 10) were 12–14-wk old triple breed piglets with no apparent pre-existing disease. Allocation to the different study groups was made by the same integrated randomization procedure. Details of animal preparation and the experimental model have been described previously (Citation29,30). Briefly, after administration of ketamine and morphine, an intravenous infusion of 8 mg·kg-1·h-1 sodium pentobarbital (Apoteket, Sweden), morphine (Morphine®, Pharmacia ,Umeå, Uppsala, Sweden) 0.5 mg·kg-1·h-1, and 0.25 mg·kg-1·h-1 pancuronium bromide (MSD, Solna , Sweden) dissolved in 2.5% glucose solution was administered and used for maintenance of anesthesia. The piglets were tracheotomized and mechanically ventilated (Servo-i V3.1, Siemens Medical, Solna, Sweden) with 30% oxygen in air, and a minute volume set to maintain arterial PaCO2 at an average of 5–5.5 kPa. The capnogram (CO2SMO Plus-8100, Novametrix, Wallingford, Connecticut, USA), saturation, and electrocardiogram (ECG) were displayed continuously until the end of the experiment. An 18-G arterial catheter was advanced into the aortic arch via a branch of the right carotid artery, for withdrawal of blood samples and measurement of blood pressure. A 14-G saline-filled double lumen catheter was placed into the right atrium via a cut down of the right external jugular vein. In addition, a pulmonary artery catheter was inserted to monitor pulmonary artery pressure (PAP), core temperature, cardiac output, and capillary wedge pressure (PCWP). Hemodynamic parameters including heart rate, systemic arterial blood pressure, right atrial pressure, and PAP were continuously displayed and recorded until the end of the experiment. Cardiac output, PCWP, and coronary perfusion pressure (CPP) were recorded and measured as previously described (Citation31,32). Samples of arterial and mixed venous blood were taken for blood gas analysis and acid–base balance (ABL 300, Radiometer, Copenhagen, Denmark) at regular intervals. Oxygen saturation and hemoglobin concentration were determined simultaneously on an OSM3 Hemoximeter (Radiometer, Copenhagen, Denmark) at the same time points.
Experimental protocol
After preparation, the piglets were stabilized for 1 hour. Ventricular fibrillation was induced by an alternating transthoracic current. After 12 min of untreated CA, closed-chest CPR was performed with a pneumatically driven automatic piston device for CPR (Lucas®, Jolife AB, Lund, Sweden), and mechanical ventilation with 100% oxygen was resumed with the same ventilatory settings as before induction of CA. One minute after commencement of CPR, all animals in the vasopressin and combined vasopressin-adrenaline groups (V and VA, respectively; n = 10 in both groups) received a bolus of 0.4 U·kg-1 of vasopressin (Arg8-vasopressin, Sigma Chemicals Co, St Louis, MO, USA). Fifteen minutes after CA, i.e. 2 min after commencement of CPR, the vasopressin-adrenaline (VA) group was given 20 µg/kg b.w. of adrenaline, and another 1 min later a continuous administration (10 µg/kg/min) of the same drug (Citation7,33,34) was started and maintained until ROSC or resuscitation failed and the experiment was terminated. The adrenaline group (group A; n = 10) was given the same adrenaline dosing as the VA group, but in this case administration began 1 min after commencement of CPR. This way of administrating adrenaline provides sustained greater cerebral cortical blood flow and lower cerebral oxygen extraction (Citation34), at equal total dose compared with repeated bolus doses of 20 μg/kg every 3 min and makes this study group independent of the time when ROSC occurs. After 8 min of external chest compressions, a monophasic countershock was delivered through defibrillation electrode pads (Medtronic Physio-Control Corp., Seattle, WA, USA) at an energy level of 200 J (). If ROSC was not successful, another two defibrillatory shocks (200 J, 360 J) were given. During continued CPR, DC shocks were applied at the same energy level of 360 J for a maximum period of 5 min. CPR was discontinued if ROSC was not achieved during this time. If arterial pH was <7.20 or base deficit >10 mmol/L by 5 min after ROSC, acidosis was corrected with a tris buffer mixture (Tribonat®, Fresenius Kabi, Stockholm, Sweden), 1 mmol·kg-1, and by increasing minute ventilation. Dobutamine (Dobutrex®, Eli Lilly, Solna) was administrated in a solution of 12.5 mg/mL starting at 5 µg·kg-1·min-1 if the systolic blood pressure was <70 mmHg. After completion of the study, all animals received an injection of 10 mL of 20 mmol/mL potassium chloride and were killed. The skull was opened before final CA in the prone position, and after administration of potassium the brain was rapidly, within 2 min, removed pending immunohistochemical and histopathological analyses.
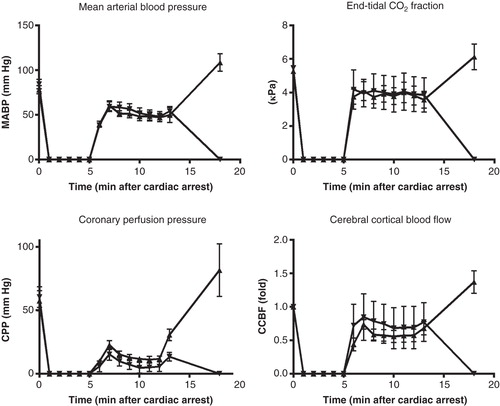
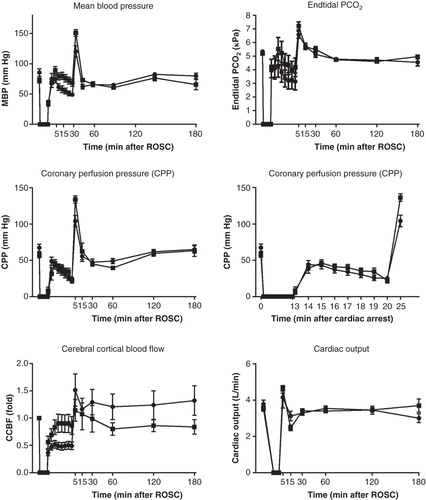
Figure 1. Systemic circulatory variables: mean blood pressure, end-tidal PCO2, coronary perfusion pressure, cerebral cortical blood flow, and cardiac output. Coronary perfusion pressure is presented both for the whole experiments and for the CPR period only. Vasopressin group •; Vasopressin-adrenaline group ▪.
Experimental groups
This open study was planned to include three study groups: the vasopressin group (V group), the vasopressin-adrenaline group (VA group), and an adrenaline group (A group). These three groups had 12 min of CA followed by 8 min of CPR. The V and VA groups received a single dose of vasopressin (0.4 U/kg) after 1 min of CPR. In addition the VA group 2 min later also received a single dose of adrenaline (20 µg/kg), followed another 1 min later by a constant infusion of 10 µg/kg/min of adrenaline maintained until CPR was successful or terminated. The A group received the same dosing of adrenaline as the VA group 1 min after initiation of CPR. Furthermore, in order to elucidate the effects of administration of single doses of adrenaline we also studied an additional group of piglets of the same characteristics, integrated into the main study, in the following called the background study. These piglets underwent 5 min of CA only, followed by 8 min of CPR during which period a single dose of 20 µg/kg adrenaline was administered 1 min after application of CPR and repeated every third minute until CPR was successful or terminated.
Blood-borne tissue injury indicators
Before CA and during reperfusion (15, 30, 60, 120, and 180 min after ROSC) blood specimens were collected from the arterial catheter for analyses of the cardiac tissue injury indicator troponin I; and from the jugular venous catheter samples for measurements of the cerebral tissue astroglial protein S-100β and, the indicator of oxidative injury, 8-iso-dihydro-PGF2α as indicators of cerebral injury. Analytical methods of these have been published previously (Citation32,35).
Histological analyses
Both brain hemispheres were immersed in 4% buffered formalin and stored at 4°C for 1 week before small tissue pieces (<3×5 mm) from the parietal-temporal cerebral cortex were cut and processed for histology or immunohistochemistry. Samples were embedded in low-temperature paraffin (56–58°C) according to a standard protocol (Citation36). Multiple sections approximately 3–5 μm thick were cut from cerebral cortex and stained using a commercial protocol. Immunohistochemistry for albumin was performed using a sheep polyclonal anti-rat albumin antibody (Sigma Aldrich, Stockholm, Sweden) and the streptavidin–HRP–biotin technique (Citation30,31,36). Numbers of distorted neurons in one whole section, and of albumin-positive cells, were counted in one identical area of the cortex from each animal at least three times and in a blinded fashion in order to determine leakage of the blood–brain barrier (BBB) (Citation30,31) and neuronal injuries. Median values were recorded for data analysis. Positive and negative controls from the same laboratory have been published previously (Citation31).
Statistical analysis
Circulatory data are presented as means ± SEM and indicators of tissue injury in box plots (median, 25th and 75th as well as 10th and 90th percentiles). Comparisons of circulatory data between groups have been performed by Student’s t test on the area under the curve calculated according to the trapezoidal rule. In the case of histological and immunohistochemical analyses, normal distribution of data could not be presumed. We therefore used the non-parametric Mann–Whitney U test for comparisons between groups at 180 min after CA. Pearson correlation has also been used. A P value less than 0.05 was considered as statistically significant.
Results
Survival
In the V and VA groups 8 piglets (2 pigs not included for technical/procedural reasons in both the V and VA groups) achieved ROSC and subsequently survived until the experiments were finished (n.s.). In the A group there was only 1 piglet achieving ROSC out of 8 (2 piglets not included for technical/procedural reasons). As the study was mainly designed to analyze cerebral histology after 3 h survival, this group was eliminated from further analysis. There was no statistical difference in dobutamine or alkaline buffer consumption after ROSC. In the background study using adrenaline only, 5 out of 10 piglets had ROSC and survived until the end of the experiments.
Circulatory variables
During CPR the mean blood pressure (MBP) (P = 0.027) was higher in the VA group compared with the V group, while numerical trends for higher values in end-tidal CO2 tension (P = 0.3) and CPP (P = 0.088) in VA group did not differ (). During CPR the cerebral cortical blood flow (CCBF) was higher in the VA group (P = 0.049), but after ROSC it tended to be higher (36%±16%) in the V group instead (P = 0.6). Thus, after ROSC there were no statistical differences in any of these variables ().
Figure 2. Systolic and mean blood pressures as related to cerebral cortical blood flow before cardiac arrest, during CPR, and immediately after restoration of spontaneous circulation. Vasopressin group •; Vasopressin-adrenaline group ▪.
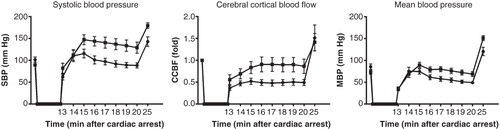
During CPR, surviving animals had somewhat higher CPP (P = 0.02), but similar MBP (P = 0.8) and CCBF (P = 0.9) compared with the animals not achieving ROSC ().
Blood-borne tissue injury indicators
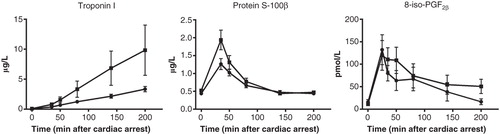
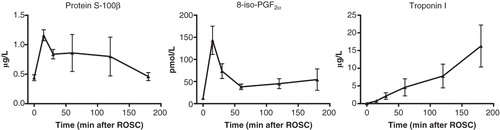
Serum concentrations of troponin I did not differ between the two groups (P = 0.18) in spite of greater numerical values in the VA group. This was due to the fact that approximately 50% of the animals had hugely high values, while the remainder of the animals had troponin I values at the same level as those of the V group. In the same group serum concentrations of protein S-100β were only transiently higher (P = 0.038) during the first hour after ROSC (). The indicator of oxidative injury, 8-iso-dihydro-PGF2α, was numerically somewhat greater in the VA group also (P = 0.5). In surviving animals of the background study, serum concentrations of troponin I and 8-iso-PGF2α peaked shortly after ROSC, while troponin I gradually increased to very high values at the end of the study ().
Histological analyses
Neural injury and albumin immunostaining ( and ) were significantly more intense, 20%±4% and 21%±4%, respectively, in the VA group compared with the V group (P = 0.039 and P = 0.016, respectively). There was also a high correlation between the histological neural injury and the albumin leakage to the brain (). In the separate background study the same measurements revealed an even worse neural injury and disruption of the BBB ().
Figure 6. Leakage of albumin into the neuropil of cerebral cortex in pig brain after cardiac arrest and treatment with vasopressin and adrenaline (VA group) (a) and vasopressin alone (V group) (b). Albumin leakage in the neuropil and neurons was considerably reduced in pigs treated with vasopressin alone in comparison with the group receiving vasopressin and adrenaline. ×300.
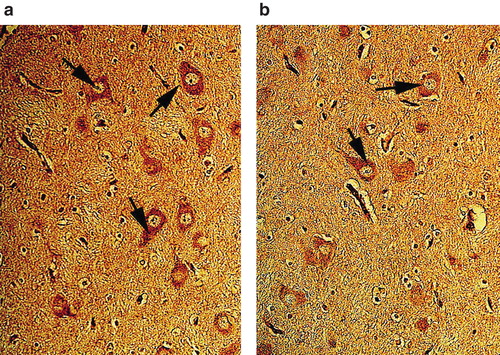
Figure 7. Nissl-stained neurons in the neuropil of cerebral cortex in pigs treated with vasopressin and adrenaline in combination (VA group) (a) or vasopressin alone (b). Vasopressin alone caused less neuronal damage, sponginess, and edema up to a certain extent in pigs after cardiac arrest, while several neurons were damaged with perineuronal edema in the group given combined treatment with vasopressin and adrenaline (VA group). ×300.
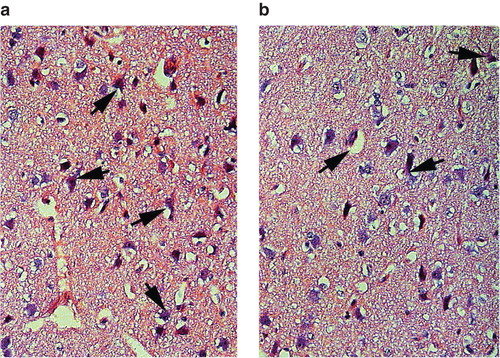
Figure 8. Box plots (median, 25th and 75th as well as 10th and 90th percentiles) of microscopical assessment of neuronal injury and albumin leakage (vasopressin group, vasopressin-adrenaline group, and background study) and correlation of these two variables for the vasopressin and vasopressin-adrenaline groups (R2 = 0.85, P < 0.0001).
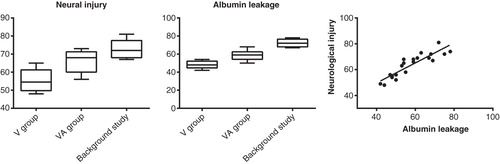
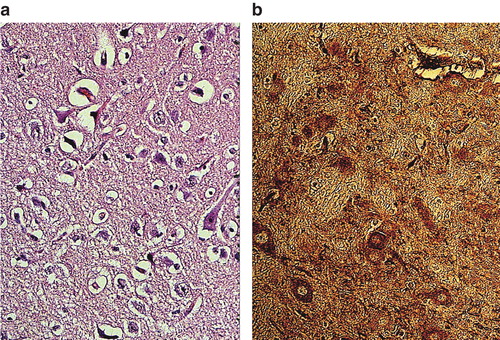
Figure 9. Neuronal damage (a) and albumin leakage (b) in the cerebral cortex of pigs treated with adrenaline only during CPR after cardiac arrest (background study). Massive neuronal damage with perineuronal edema and sponginess was evident (b). There was profound leakage of albumin in the neuropil and strong albumin-positive nerve cells as well (b). ×300.
Discussion
The present experimental study was not designed to provide statistical power for determining differences in incidence of ROSC and survival in general. Rather, it was meant as a mechanistic study elucidating circulatory and especially cerebral histologic effects of the combination vasopressin-adrenaline. In accordance with previous experimental results and clinical impression, adrenaline administrated as a reinforcement of the vasopressor effect caused by vasopressin during experimental CPR resulted in higher mean blood pressure and cerebral cortical blood flow but, surprisingly, similar coronary perfusion pressure. However, this combined therapy (i.e. VA group) also tended to cause higher troponin I concentrations in systemic blood, but the difference did not attain statistical significance as only half of the animals had very high troponin I values, and transiently greater protein S-100β levels in jugular venous blood, implying a tendency towards greater cerebral and cardiac injury. This might explain the post-resuscitation myocardial dysfunction observed after adrenaline administration during CPR (Citation37) in contrast to the beneficial visceral effects of the vasopressin-noradrenaline combination observed (Citation38) in experimental septic shock. In addition, cerebral cortical blood flow (CCBF) was numerically (36%) higher after ROSC in the V group, indicating difficulties in maintaining cerebral perfusion after ROSC in the VA group. The latter finding could possibly result from the impaired perfusion after administration of adrenaline that recently was described by Ristagno et al. (Citation25), and might explain our most obvious finding of a greater cortical neural injury and BBB disruption in the VA group after 3 h of reperfusion. Consistent with these histological results—but possibly more unanticipated—was the result indicating that adrenaline administered as the sole vasopressor as repeated bolus doses in the background study, where the untreated duration of CA was less than half of that in the main study groups, caused the greatest neural injury and BBB disruption. This result was unexpected as longer duration of CA generally is considered to cause a more extended brain injury.
Thus, we were able to confirm that adrenaline-vasopressin treatment during experimental CPR seemingly promoted systemic and cerebral circulation during CPR but the resulting tissue injury after 3 h of reperfusion in the heart and brain was much worse than after vasopressin only. These results seem to explain some aspects of the results of the clinical controlled studies of Olasveengen et al. (Citation26,27), Jacobs et al. (Citation28), and Jeung et al. (Citation12) but to be at variance with the results of combined use of vasopressin and adrenaline in the study of Wenzel et al. (Citation20) where adrenaline improved survival when used after vasopressin administration.
Our background study showed mean troponin I levels that were approximately 170% of those in the VA group and three times those in the V group, although the time for untreated cardiac arrest was less than half of that in the main study. Surprisingly again, the non-survivors, i.e. those not achieving ROSC, in the background study had somewhat higher CCBF during CPR than surviving animals. Seemingly, there was no difference between CCBF in the V and VA groups and the animals in the background study. When examining our present results together, there was no obvious difference in jugular venous protein S-100β or the jugular venous indicator of oxidative injury, 8-iso-dihydro-PGF2α, but clearly more histological injuries in the cerebrum in the animals of the background study. This leads to a rather complicated picture in the background study where a short cardiac arrest treated with adrenaline during CPR only resulted in very high levels of cardiac injury indicators in some animals, blood-borne indicators of cerebral injury being moderate, but severe histological cerebral injury occurred. Thus, the results indicate that the myocardial injury caused by adrenaline in pigs during experimental CPR is mechanistically different from that of cerebral injury, and, furthermore, there was a great inter-individual variation in the extent of especially cardiac tissue injury after combined vasopressin-adrenaline administration. Our results suggest that adrenaline administered during CPR might influence the extent of early cerebral survival negatively and possibly explain why frequent ROSC found in previous studies (Citation12-14) do not translate into better survival.
One limitation of our study was that the background animals had a shorter untreated CA. Thus, it is impossible statistically to compare this group with the two others. The animals were evaluated 3 h after CA, and conclusions regarding development of pathology thereafter cannot be made. Also, comparisons to human/clinical studies should only be made with great caution.
It can be concluded that the combined use of vasopressin and adrenaline caused greater signs of cerebral and cardiac injury than the use of vasopressin alone during experimental porcine cardiopulmonary resuscitation. In the background study, where duration of CA was less than half of that in the main study, using the same model but repeated administration of adrenaline alone every third minute during CPR, the same indicators of tissue injury indicated even greater cerebral and cardiac injury than in the main study where vasopressin was compared with the combined use of vasopressin and adrenaline.
Acknowledgements
We are grateful to Mr Anders Nordgren RN, Mrs Monica Hall, and Mrs Mari-Anne Carlsson for excellent technical assistance. Financial support from The Laerdal Foundation for Acute Medicine is gratefully acknowledged.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Gottlieb R. Ueber die wirkung der nebennieren extracte auf herz und blutdruck. Arch Exp Path Pharmacol. 1896;38:99–112.
- Crile G, Dolley DH. An experimental research into the resuscitation of dogs killed by anesthetics and asphyxia. J Exp Med. 1906;8:713–25.
- Redding JS, Pearson JW. Evaluation of drugs for cardiac resuscitation. Anesthesiology. 1963;24:203–7.
- Michael JR, Guerci AD, Koehler RC, Shi AY, Tsitlik J, Chandra N, et al. Mechanisms by which epinephrine augments cerebral and myocardial perfusion during cardiopulmonary resuscitation in dogs. Circulation. 1984;69:822–35.
- Brown CG, Werman HA, Davis EA, Hamlin R, Hobson J, Ashton JA. Comparative effect of graded doses of epinephrine on regional brain blood flow during CPR in a swine model. Ann Emerg Med. 1986;15:1138–44.
- Hoekstra JW, Van Ligten P, Neumar R, Werman HA, Anderson J, Brown CG. Effect of high dose norepinephrine versus epinephrine on cerebral and myocardial blood flow during CPR. Resuscitation. 1990;19:227–40.
- Koehler RC, Michael JR, Guerci AD, Chandra N, Schleien CL, Dean JM, et al. Beneficial effect of epinephrine infusion on cerebral and myocardial blood flows during CPR. Ann Emerg Med. 1985;14:744–9.
- Herlitz J, Ekstrom L, Wennerblom B, Axelsson A, Bang A, Holmberg S. Adrenaline in out-of-hospital ventricular fibrillation. Does it make any difference? Resuscitation. 1995;29:195–201.
- Holmberg M, Holmberg S, Herlitz J. Low chance of survival among patients requiring adrenaline (epinephrine) or intubation after out-of-hospital cardiac arrest in Sweden. Resuscitation. 2002;54:37–45.
- Deakin CD, Nolan JP, Soar J, Sunde K, Koster RW, Smith GB, et al. European Resuscitation Council Guidelines for Resuscitation 2010 Section 4. Adult advanced life support. Resuscitation. 2010;81:1305–52.
- Gedeborg R, Silander HC, Ronne-Engstrom E, Rubertsson S, Wiklund L. Adverse effects of high-dose epinephrine on cerebral blood flow during experimental cardiopulmonary resuscitation. Crit Care Med. 2000;28:1423–30.
- Jeung KW, Ryu HH, Song KH, Lee BK, Lee HY, Heo T, et al. Variable effects of high-dose adrenaline relative to standard-dose adrenaline on resuscitation outcomes according to cardiac arrest duration. Resuscitation. 2011;82:932–6.
- Brown CG, Martin DR, Pepe PE, Stueven H, Cummins RO, Gonzalez E, et al. A comparison of standard-dose and high-dose epinephrine in cardiac arrest outside the hospital. The Multicenter High-Dose Epinephrine Study Group. N Engl J Med. 1992;327:1051–5.
- Berg RA, Otto CW, Kern KB, Sanders AB, Hilwig RW, Hansen KK, et al. High-dose epinephrine results in greater early mortality after resuscitation from prolonged cardiac arrest in pigs: a prospective, randomized study. Crit Care Med. 1994;22:282–90.
- Lindner KH, Prengel AW, Pfenninger EG, Lindner IM, Strohmenger HU, Georgieff M, et al. Vasopressin improves vital organ blood flow during closed-chest cardiopulmonary resuscitation in pigs. Circulation. 1995;91:215–21.
- Ristagno G, Sun S, Tang W, Castillo C, Weil MH. Effects of epinephrine and vasopressin on cerebral microcirculatory flows during and after cardiopulmonary resuscitation. Crit Care Med. 2007;35:2145–9.
- Prengel AW, Lindner KH, Keller A. Cerebral oxygenation during cardiopulmonary resuscitation with epinephrine and vasopressin in pigs. Stroke. 1996;27:1241–8.
- Wenzel V, Lindner KH, Krismer AC, Voelckel WG, Schocke MF, Hund W, et al. Survival with full neurologic recovery and no cerebral pathology after prolonged cardiopulmonary resuscitation with vasopressin in pigs. J Am Coll Cardiol. 2000;35:527–33.
- Babar SI, Berg RA, Hilwig RW, Kern KB, Ewy GA. Vasopressin versus epinephrine during cardiopulmonary resuscitation: a randomized swine outcome study. Resuscitation. 1999;41:185–92.
- Wenzel V, Krismer AC, Arntz HR, Sitter H, Stadlbauer KH, Lindner KH. A comparison of vasopressin and epinephrine for out-of-hospital cardiopulmonary resuscitation. N Engl J Med. 2004;350:105–13.
- Mayr VD, Wenzel V, Voelckel WG, Krismer AC, Mueller T, Lurie KG, et al. Developing a vasopressor combination in a pig model of adult asphyxial cardiac arrest. Circulation. 2001;104:1651–6.
- Wenzel V, Linder KH, Augenstein S, Prengel AW, Strohmenger HU. Vasopressin combined with epinephrine decreases cerebral perfusion compared with vasopressin alone during cardiopulmonary resuscitation in pigs. Stroke. 1998;29:1462–7.
- Stiell IG, Hebert PC, Wells GA, Vandemheen KL, Tang AS, Higginson LA, et al. Vasopressin versus epinephrine for inhospital cardiac arrest: a randomised controlled trial. Lancet. 2001;358:105–9.
- Layek A, Maitra S, Pal S, Bhattacharjee S, Baidya D. Efficacy of vasopressin during cardio-pulmonary resuscitation in adult patients: a meta-analysis. Resuscitation. 2014;85:855–63.
- Ristagno G, Tang W, Huang L, Fymat A, Chang YT, Sun S, et al. Epinephrine reduces cerebral perfusion during cardiopulmonary resuscitation. Crit Care Med. 2009;37:1408–15.
- Olasveengen TM, Sunde K, Brunborg C, Thowsen J, Steen PA, Wik L. Intravenous drug administration during out-of-hospital cardiac arrest: a randomized trial. JAMA. 2009;302:2222–9.
- Olasveengen TM, Wik L, Sunde K, Steen PA. Outcome when adrenaline (epinephrine) was actually given vs. not given - post hoc analysis of a randomized clinical trial. Resuscitation. 2012;83:327–32.
- Jacobs IG, Finn JC, Jelinek GA, Oxer HF, Thompson PL. Effect of adrenaline on survival in out-of-hospital cardiac arrest: a randomised double-blind placebo-controlled trial. Resuscitation. 2011;82:1138–43.
- Wiklund L, Sharma HS, Basu S. Circulatory arrest as a model for studies of global ischemic injury and neuroprotection. Ann N Y Acad Sci. 2005;1053:205–19.
- Sharma H, Miclescu A, Wiklund L. Cardiac arrest-induced regional blood-brain barrier breakdown, edema formation and brain pathology: a light and electron microscopic study on a new model for neurodegeneration and neuroprotection in the porcine brain. J Neural Transm. 2011;118:87–114.
- Miclescu A, Sharma HS, Martijn C, Wiklund L. Methylene blue protects the cortical blood-brain barrier against ischemia/reperfusion-induced disruptions. Crit Care Med. 2010;38:2199–206.
- Miclescu A, Basu S, Wiklund L. Methylene blue added to a hypertonic-hyperoncotic solution increases short-term survival in experimental cardiac arrest. Crit Care Med. 2006;34:2806–13.
- Johansson J, Gedeborg R, Rubertsson S. Vasopressin versus continuous adrenaline during experimental cardiopulmonary resuscitation. Resuscitation. 2004;62:61–9.
- Johansson J, Gedeborg R, Basu S, Rubertsson S. Increased cortical cerebral blood flow by continuous infusion of adrenaline (epinephrine) during experimental cardiopulmonary resuscitation. Resuscitation. 2003;57:299–307.
- Basu S, Nozari A, Liu XL, Rubertsson S, Wiklund L. Development of a novel biomarker of free radical damage in reperfusion injury after cardiac arrest. FEBS Lett. 2000;470:1–6.
- Sharma HS, Cervos-Navarro J. Brain oedema and cellular changes induced by acute heat stress in young rats. Acta Neurochir Suppl (Wien). 1990;51:383–6.
- Tang W, Weil M, Sun S, Noc M, Yang L, Gazmuri RJ. Epinephrine increases the severity of postresuscitation myocardial dysfunction. Circulation. 1995;92:3089–93.
- Ji MH, Yang JJ, Wu J, Li RQ, Li GM, Fan YX, et al. Experimental sepsis in pigs--effects of vasopressin on renal, hepatic, and intestinal dysfunction. Ups J Med Sci. 2012;117:257–63.