Abstract
Background. There is a growing consensus that similar neural mechanisms are involved in the reinforcing properties of natural rewards, like food and sex, and drugs of abuse. Rat lines selectively bred for high and low oral alcohol intake and preference have been useful for understanding factors contributing to excessive alcohol intake and may constitute proper animal models for investigating the neurobiological basis of natural rewarding stimuli.
Methods. The present study evaluated copulatory behavior in alcohol and sexually naïve Sardinian alcohol-preferring (sP) and -nonpreferring (sNP) male rats in three consecutive copulatory behavior tests.
Results. The main finding was that, under the conditions used in this study, sNP rats were sexually inactive relative to sP rats. To gain more information about the sexual behavior in sP rats, Wistar rats were included as an external reference strain. Only minor differences between sP and Wistar rats were revealed.
Conclusions. The reason behind the low copulatory activity of sNP rats remains to be elucidated, but may in part be mediated by innate differences in brain transmitter systems. The comparison between sP and Wistar rats may also suggest that the inherent proclivity to excessive alcohol drinking in sP rats may mainly be dependent on its anxiolytic properties, as previously proposed, and not changes in the reward system.
Introduction
There is a growing consensus that similar mechanisms are involved in the reinforcing properties of natural rewards, like food and sex, and drugs of abuse (Citation1-5). The demand for an improved understanding of the basal functioning of the brain reward system, especially with regard to motivational aspects and choice of reinforcer, has recently been emphasized (Citation1,4,5).
The mesocorticolimbic dopamine system with cell bodies in the ventral tegmental area and projections to areas including the nucleus accumbens, amygdala, and the prefrontal cortex is highly conserved in evolution, a natural constituent of vital life sustaining behaviors (Citation6), and a key component in reward and addiction processes (Citation2,3,7). It is well acknowledged that sexual activity (Citation8) and intake of palatable food (Citation9) and drugs of abuse including alcohol (Citation10) result in elevated levels of extracellular dopamine in areas associated with the mesocorticolimbic dopamine system, including the nucleus accumbens. In studies of basic mechanisms of eliciting reward-driven behavior it is important to differentiate the appetitive phase (seeking the reinforcer) from the act of consumption. Natural and sexual selection processes are likely to be involved in the development of appetitive and consummatory activity with some key neuronal trajectories shared between species.
The development of rat lines selectively bred for high and low oral alcohol intake and preference has been a useful strategy for understanding factors contributing to excessive alcohol intake (Citation11-16). These lines were bred for the same phenotypes, i.e. high or low voluntary alcohol intake and preference under the standard, home-cage, two-bottle free-choice paradigm with continuous access to alcohol, water, and food (Citation17-19). One common aim of these selective breeding programs has been to determine behavioral (Citation20), neurobiological (Citation21), and genetic (Citation22) characteristics associated with selection for high and low voluntary alcohol intake and preference. Considering the role of the brain reward system in reinforcing properties of natural rewards as well as drugs of abuse it is possible that selective breeding for high alcohol intake affects also other reward-related behaviors. The selectively bred alcohol-preferring and non-preferring lines may therefore represent possible models for studies of similarities between natural rewarding stimuli and drugs of abuse (Citation23-25). Here the Sardinian alcohol-preferring (sP) and -nonpreferring (sNP) rats (Citation16) were used to study some aspects of this fairly unexplored topic. The aim of the present study was to investigate if selective breeding of sP and sNP rats has also resulted in differences in copulatory behavior. Wistar rats were included as an external reference strain.
Material and methods
Animals
Adult alcohol and sexually naïve sP and sNP male rats (kept under specific pathogen and opportunistic free conditions at Charles River Laboratories, Calco, Italy; generation S65; n = 15 rats per group) and age-matched male Wistar rats (Sca:WI; Scanbur BK AB, Sollentuna, Sweden; n = 10) were studied. This study was initially designed to compare copulatory activity in sP and sNP rats; however, the large degree of sexual inactivity observed in sNP rats in several preliminary experiments forced us to include a set of Wistar rats as reference strain. Wistar rats were preferred over other strains of outbred rats as they constituted the foundation stock from which the selective breeding of sP and sNP rats was started (Citation16). It should, however, be noted that the Wistar rats used herein were from a different supplier, and vendor-dependent differences between Wistar rats exist (Citation26-29). Twenty-four female Wistar rats (HanTac:WH; Taconic, Ejby, Denmark), not related to the male Wistar rats, weighing approximately 300 g, were used as stimulus females. All rats were housed three per cage in transparent cages (59 × 38 × 20 cm) containing wood-chip bedding material and paper sheets as enrichment. The cages were placed in temperature-controlled (22 ± 1°C) and humidity-controlled (50% ± 10%) housing cabinets with a reversed 12-hour light/dark cycle (lights off between 7.00 a.m. and 7.00 p.m.). The rats were maintained on rat chow (R36; Lantmännen, Kimstad, Sweden) and water ad libitum.
The females used as stimuli in the copulatory behavior test were brought into estrus by hormone treatment. They were ovariectomized under isoflurane anesthesia and allowed to recover for 10 days. The hormone treatment consisted of subcutaneous administrations of 25 μg/kg of estradiol benzoate (Sigma-Aldrich, St. Louis, MO, USA) in olive oil 48 h before progesterone, and 1 mg/rat of progesterone (Sigma-Aldrich) in olive oil 4–6 h before use. Prior to the assessment of copulatory behavior, all male rats had undergone behavioral testing (Citation30), which was completed at approximately 20 weeks of age. The sexual behavior test was initiated at 30 weeks of age.
All animal experiments were approved by the Uppsala Animal Ethical Committee and followed the guidelines of the Swedish Legislation on Animal Experimentation (Animal Welfare Act SFS1998:56) and the European Communities Council Directive (86/609/EEC).
Copulatory behavior test
Male copulatory behavior with receptive females was scored in three tests. The first two tests, which were considered as learning tests, lasted for 15 min, and the animals had two test-free days between each test. The third test, which served to investigate copulatory behavior in sexually experienced rats, was performed one week after the second test. The third test lasted for a maximum of 30 min but was interrupted after scoring the post-ejaculatory interval. The tests were performed in a wooden cage (60 × 35 × 35 cm) with a transparent front, used to score copulatory behavior only, under dim illumination during the dark phase of the light/dark cycle. The male rat was allowed to habituate to the cage for 5 min before the receptive female was introduced. Each female was used for two to three males, and the females were alternated between the three copulatory tests. Based on established protocols (Citation31-33), parameters according to the ethogram in were scored by direct observation by an experienced, blinded observer or calculated based on the scored behaviors.
Table I. Ethogram of the behaviors scored by direct observation or calculated during the copulatory activity tests.
Statistical analyses
Data were tested for normality using the Shapiro–Wilk’s W test. Copulatory behavior data were not normally distributed, and therefore non-parametric statistical methods were used. The learning effect was evaluated by comparing latency and frequency measures over the three tests using the Friedman test followed by the Wilcoxon signed rank test when appropriate. For these analyses all values were included, and latency measures were set at 900 s if the behavior was not performed. The number of animals performing mounts, intromissions, and ejaculations was evaluated with the chi-square test. The third test was analyzed for performed behaviors, and thus if a behavior did not occur this was considered a missing value. Group comparisons of performance in the third test were performed using the Kruskal–Wallis test and/or the Mann–Whitney U test. Differences were considered statistically significant at p ≤ 0.05. Statistica 10.0 (StatSoft Inc., Tulsa, OK, USA) was used for the statistical analyses.
Results
Acknowledging that copulatory activity is a product of two individuals interacting, the behavior of the females was not scored since, after hormonal priming, all females were sexually proceptive and displayed receptive behaviors including lordosis.
Performance over time
The main finding in the present study is a very low copulatory activity in male sNP rats (). Thus, fewer sNP than sP rats engaged in copulatory behavior in all three tests. In the first and second test, only 2 out of 15 sNP males mounted, and in the third test this number was increased to 3 males, which was significantly fewer (chi-square = 8.89, df = 1, p < 0.01; chi-square = 13.39, df = 1, p < 0.001; chi-square = 13.39, df = 1, p < 0.001, respectively) when compared with sP rats. In the second and third test, no sNP rats achieved intromission (chi-square = 9.13, df = 1, p < 0.01; chi-square = 17.37, df = 1, p < 0.0001) or ejaculation (chi-square = 10.91, df = 1, p < 0.001). Based on this sexual inactivity, sNP rats were excluded from further detailed comparison with the sP line. To get information about the copulatory behavior in sP rats, a detailed statistical analysis using Wistar rats as reference strain was performed.
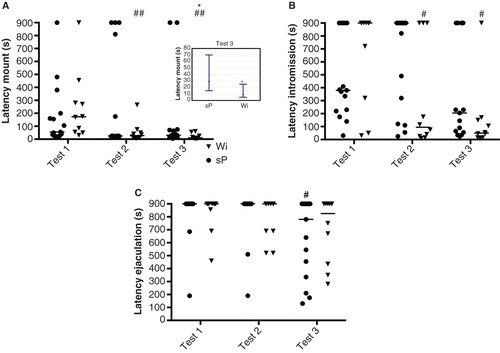
There were significant differences over time in sP and Wistar rats in latency to first mount (sP Friedman ANOVA (n = 15, df = 2) = 5.71, p = 0.058; Wistar Friedman ANOVA (n = 10, df = 2) = 15.08, p < 0.001), latency to first intromission (sP Friedman ANOVA n.s.; Wistar Friedman ANOVA (n = 10, df = 2) = 6.59, p < 0.05) and latency to first ejaculation (sP Friedman ANOVA (n = 15, df = 2) = 11.38, p < 0.01; Wistar Friedman ANOVA n.s.) (). In Wistar rats, the latency to first mount and intromission, respectively, was significantly shorter in the second (mount Z = 2.29, p < 0.05; intromission Z = 2.02, p < 0.05) and third (mount Z = 2.80, p < 0.01; intromission Z = 2.55, p < 0.05) test relative to the first test. Likewise, in the third test, the latency to ejaculation was shorter (Z = 2.52, p < 0.05) when compared with the first test in sP rats. Moreover, when comparing sP and Wistar males, Wistar rats had shorter latency to the first mount in the third test (U = 37.5, p < 0.05; ).
Figure 2. Latency to first mount (A), intromission (B), and ejaculation (C) in the three copulatory behavior tests in male sP (n = 15) and Wistar (n = 10) rats. The latency to first mount (A) in the third test is also shown in the insert for a better illustration of the difference between sP and Wistar rats. Values represent individual data points with the median value marked as a line, and in the insert values represent median and quartile range. * p < 0.05 comparing sP and Wistar rats (Mann–Whitney U test); # p < 0.05, ## p < 0.01 compared to the first copulatory behavior test within the respective group (Wilcoxon signed rank test).
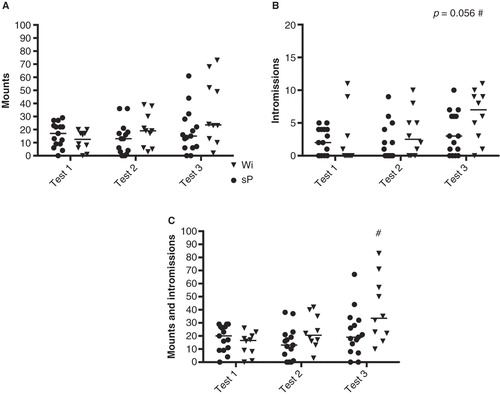
The number of intromissions was higher (Z = 2.24, p < 0.05) in the third test relative to the first test in Wistar rats, with a trend close to statistical significance also for sP rats (Z = 1.91, p = 0.056) (). The sum of mounts and intromissions was higher (Z = 2.40, p < 0.05) in the third test compared with the first test in Wistar rats, while no such difference was found in sP rats.
Figure 3. The number of mounts (A), intromissions (B), and sum of mounts and intromissions (C) in the three copulatory behavior tests in male sP (n = 15) and Wistar (n = 10) rats. Values represent individual data points with the median value marked as a line. # p < 0.05 compared to the first copulatory behavior test within the respective group (Wilcoxon signed rank test).
Assessment of copulatory behavior in sexually experienced sP and Wistar rats
Eighty-seven percent of the sP rats and 100% of the Wistar rats mounted, while the corresponding values for intromissions were 73% (sP) and 90% (Wistar), and for ejaculations 53% (sP) and 50% (Wistar) (). More detailed studies of performed behaviors in the third copulatory behavior test in sP and Wistar rats revealed a difference in inter-intromission rate (U = 6.0, p < 0.05) (). Moreover, there were trends toward longer latency to first mount (p = 0.088) and fewer mounts and intromissions per minute (p = 0.067), and longer post-ejaculatory interval (p = 0.065) in sP rats relative to Wistar rats ().
Figure 1. The percentage number of sNP (n = 15) and sP (n = 15) rats mounting (A), intromitting (B), and achieving ejaculation (C) over the three copulatory behavior tests. Due to the low number of sNP rats engaging in copulatory activity also in the third test, they were excluded from further detailed statistical analyses of copulatory behavior. Wistar (n = 10) rats were included as a reference strain. **p < 0.01, *** p < 0.001, **** p < 0.0001 compared to sP rats (chi-square test).
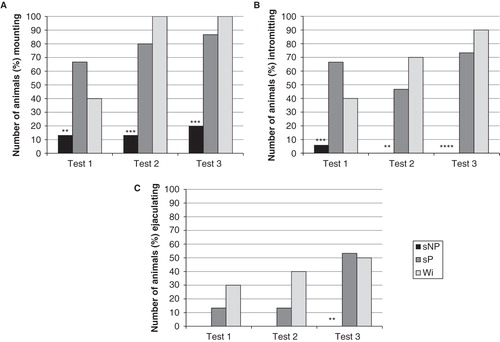
Table II. Copulatory behavior in sexually experienced sP and Wistar rats.
Discussion
The aim of this study was to determine if the response to a natural rewarding stimulus, i.e. copulatory activity, differed in rats selectively bred for opposite alcohol preference and consumption. The main finding was an almost absence of copulatory activity in the alcohol-nonpreferring sNP rats compared with -preferring sP rats. Even after three tests, no sNP rat had intromissions or achieved ejaculation, and based on this finding they are considered sexually inactive (Citation33), also termed non-copulators (Citation34).
Copulatory behavior, like drug-taking behavior, is comprised of appetitive and consummatory acts (Citation35). The mount and intromission latencies are considered measures of appetitive acts or sexual motivation (Citation33,36). Ejaculation represents a consummatory act, while the interpretation of post-ejaculatory interval is less clear (Citation33). Non-copulators are males that do not mate even after they are tested repeatedly with sexually receptive females, and are found in several species including rats and mice (Citation33,34,37). The finding that sNP rats do not display sexual motivation or engage in consummatory sexual behavior after three tests may suggest characteristics of non-copulators. Since the females used were primed and therefore displayed proceptive and receptive behaviors it is considered unlikely that the behavior of the female rats is the cause for the low copulatory activity observed in the sNP rats. In addition, since the sNP line is maintained over generations, these rats obviously copulate under other circumstances; the mating technique adopted in the breeding procedure includes indeed long periods of time (up to one week) during which male and female sNP rats are housed together in the same cage.
When compared with Wistar rats, sP rats displayed low sexual behavior in initial tests but increased their copulatory activity over successive testing. This pattern of increasing copulatory activity over time has been attributed to distraction or fear generated by the novelty of the test situation (Citation34). Beside inherent proclivity to excessive alcohol drinking, sP rats display more anxiety-related behaviors compared with sNP rats and, when tested, sometimes also compared with Wistar rats (Citation20,30,38-41). In a more complex setting, i.e. the multivariate concentric square field™ test, sP rats are characterized by low general activity and exploratory drive, and low risk-taking behavior compared with sNP rats (Citation20,30) as well as other selectively bred alcohol-preferring rat lines (Citation20). Since voluntarily consumed alcohol ameliorated different anxiety-related behaviors (Citation41,42), it has been proposed that sP rats consume alcohol for its anxiolytic properties (Citation16,41). When sexually experienced, i.e. in the third test, the copulatory behavior of sP rats was similar to that of Wistar rats. Thus, explorative strategies and copulatory activity in sP rats display differences as the copulatory behavior in the third test was similar to that of the Wistar rats while no attenuation of anxiety-related behaviors in sP rats occurs with repeated testing [(Citation30), and Roman and Colombo, unpublished observation].
A number of brain transmitter systems and specific brain areas have been linked to sexual function. The sP and sNP rats differ in a number of brain transmitter systems; these differences have been suggested to underlie their opposite alcohol-drinking phenotypes (Citation21). For example, basal dopamine levels were higher in the nucleus accumbens shell in sP than in sNP and Wistar rats, and in the medial prefrontal cortex in sP rats relative to Wistar but not sNP rats (Citation39). Dopamine denervation of the nucleus accumbens and infusion of the dopamine D2 antagonist raclopride both affect sexual behavior by delaying the initiation of mounting and intromitting without affecting the number of mounts and intromissions (Citation43). Also the endocannabinoid system seems to play a role in the regulation of sexual behavior as the endocannabinoid anandamide was shown to induce copulatory behavior in non-copulating animals (Citation44). The sP rats have higher density of the cannabinoid receptor CB1 and higher levels of the endocannabinoids anandamide and 2-arachidonoylglycerol compared with sNP rats (Citation45). These innate differences between sP and sNP rats could explain, at least in part, the differences in copulatory behavior observed herein.
In previous investigations of responses to a natural reward stimulus, such as palatable food, there were no differences in intake and in some cases also preference of intake of a chocolate-flavored drink (Citation46), sucrose (Citation46,47), or saccharin solutions (Citation48) between sP and sNP rats. However, in these studies, the high reinforcing properties of these fluids might have overwhelmed any possible existing difference in reward sensitivity between sP and sNP rats. In addition, both sP and sNP rats initiated and maintained operant self-administration of sucrose (Citation49). Therefore, on the basis of the relatively scarce amount of data available, at present it cannot be ruled out that sNP rats are characterized by a generally lower motivational drive (not limited solely to alcohol and sexual activity) than sP rats.
The results in the present study differ markedly from those of a previous experiment where only modest differences between sP and sNP rats were found (Citation38). However, the study by Cagiano et al. (Citation38) was designed to study the impact of alcohol exposure during the perinatal period on subsequent behavioral development, and the animals had undergone perinatal treatment with alcohol or sucrose from gestational day 15 to postnatal day 7, which may explain the different outcome. Perinatal exposure to both alcohol and sugar-rich diets affect responses to drugs of abuse later in life (Citation50,51), likely through adaptations in the reward-related mesostriatal dopaminergic system that also may impact on copulatory behavior.
In conclusion, the present investigation reveals profound differences in copulatory activity between the selectively bred alcohol-preferring sP and -nonpreferring sNP rats. sP rats, with an innate proclivity for high voluntary alcohol intake and high levels of anxiety-related behaviors, display sexual behavior similar to that of outbred Wistar rats when sexually experienced; sNP rats were considered sexually inactive, with characteristics of non-copulating males, suggesting a lower motivational drive. With regard to natural rewards, our results imply different responses following access to palatable food reward and a sexually receptive female in male sP and sNP rats. Considering the known differences between the different lines of rats selectively bred for high and low alcohol intake and preference it would be of interest in future studies to compare sexual behavior also in other lines.
Acknowledgements
The authors are grateful for the excellent technical assistance provided by Ms Marita Berg and for valuable comments by Professor Bengt J Meyersonduring the preparation of the manuscript. This study was supported by grants (E.R.) from the Alcohol Research Council of the Swedish Alcohol Retailing Monopoly, the Swedish Society for Medical Research (SSMF), the Swedish Brain Foundation, and the Facias, Magnus Bergvall, Fredrik och Ingrid Thuring and Åke Wiberg Foundations.
Declaration of interest: The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162:1403–13.
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–38.
- Volkow ND, Wang GJ, Tomasi D, Baler RD. Obesity and addiction: neurobiological overlaps. Obes Rev. 2013;14:2–18.
- Olsen CM. Natural rewards, neuroplasticity, and non-drug addictions. Neuropharmacology. 2011;61:1109–22.
- Leeman RF, Potenza MN. A targeted review of the neurobiology and genetics of behavioural addictions: an emerging area of research. Can J Psychiatry. 2013;58:260–73.
- Alcaro A, Panksepp J. The SEEKING mind: primal neuro-affective substrates for appetitive incentive states and their pathological dynamics in addictions and depression. Neurosci Biobehav Rev. 2011;35:1805–20.
- Everitt BJ, Belin D, Economidou D, Pelloux Y, Dalley JW, Robbins TW. Review. Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Philos Trans R SocLond B Biol Sci. 2008;363:3125–35.
- Pfaus JG, Damsma G, Nomikos GG, Wenkstern DG, Blaha CD, Phillips AG, et al. Sexual behavior enhances central dopamine transmission in the male rat. Brain Res. 1990;530:345–8.
- Bassareo V, Di Chiara G. Differential influence of associative and nonassociative learning mechanisms on the responsiveness of prefrontal and accumbal dopamine transmission to food stimuli in rats fed ad libitum. J Neurosci. 1997;17:851–61.
- Imperato A, Di Chiara G. Preferential stimulation of dopamine release in the nucleus accumbens of freely moving rats by ethanol. J Pharmacol ExpTher. 1986;239:219–28.
- Bell RL, Rodd ZA, Lumeng L, Murphy JM, McBride WJ. The alcohol-preferring P rat and animal models of excessive alcohol drinking. Addict Biol. 2006;11:270–88.
- Dyr W, Kostowski W. Warsaw high-preferring (WHP) and Warsaw low-preferring (WLP) lines of rats selectively bred for high and low voluntary ethanol intake: preliminary phenotypic characterization. Alcohol. 2008;42:161–70.
- Murphy JM, Stewart RB, Bell RL, Badia-Elder NE, Carr LG, McBride WJ, et al. Phenotypic and genotypic characterization of the Indiana University rat lines selectively bred for high and low alcohol preference. Behav Genet. 2002;32:363–88.
- Quintanilla ME, Israel Y, Sapag A, Tampier L. The UChA and UChB rat lines: metabolic and genetic differences influencing ethanol intake. Addict Biol. 2006;11:310–23.
- Sommer W, Hyytiä P, Kiianmaa K. The alcohol-preferring AA and alcohol-avoiding ANA rats: neurobiology of the regulation of alcohol drinking. Addict Biol. 2006;11:289–309.
- Colombo G, Lobina C, Carai MA, Gessa GL. Phenotypic characterization of genetically selected Sardinian alcohol-preferring (sP) and -non-preferring (sNP) rats. Addict Biol. 2006;11:324–38.
- Li TK, Lumeng L, Doolittle DP. Selective breeding for alcohol preference and associated responses. Behav Genet. 1993;23:163–70.
- Lumeng L, Murphy JM, McBride WJ, Li TK. Genetic influences on alcohol preference in animals. In Bergleiter H, Kissin B, editors. The genetics of alcoholism. New York: Oxford University Press; 1995. p. 165–201.
- McBride WJ, Li TK. Animal models of alcoholism: neurobiology of high alcohol-drinking behavior in rodents. Crit Rev Neurobiol. 1998;12:339–69.
- Roman E, Stewart RB, Bertholomey ML, Jensen ML, Colombo G, Hyytiä P, et al. Behavioral profiling of multiple pairs of rats selectively bred for high and low alcohol intake using the MCSF test. Addict Biol. 2012;17:33–46.
- Bell RL, Sable HJ, Colombo G, Hyytiä P, Rodd ZA, Lumeng L. Animal models for medications development targeting alcohol abuse using selectively bred rat lines: neurobiological and pharmacological validity. Pharmacol Biochem Behav. 2012;103:119–55.
- McBride WJ, Kimpel MW, Mc Clintick JN, Ding ZM, Hyytiä P, Colombo G, et al. Gene expression in the ventral tegmental area of 5 pairs of rat lines selectively bred for high or low ethanol consumption. Pharmacol Biochem Behav. 2012;102:275–85.
- Hyytiä P, Schulteis G, Koob GF. Intravenous heroin and ethanol self-administration by alcohol-preferring AA and alcohol-avoiding ANA rats. Psychopharmacology (Berl). 1996;125:248–54.
- Hyytiä P, Sinclair JD. Oral etonitazene and cocaine consumption by AA, ANA and Wistar rats. Psychopharmacology (Berl). 1993;111:409–14.
- Le AD, Li Z, Funk D, Shram M, Li TK, Shaham Y. Increased vulnerability to nicotine self-administration and relapse in alcohol-naive offspring of rats selectively bred for high alcohol intake. J Neurosci. 2006;26:1872–9.
- Goepfrich AA, Gluch C, Friemel CM, Schneider M. Behavioral differences in three Wistar Han rat lines for emotional reactivity, cognitive processing and ethanol intake. Physiol Behav. 2013;110–111:102–8.
- Palm S, Hävermark Å, Meyerson BJ, Nylander I, Roman E. When is a Wistar a Wistar? Behavioral profiling of outbred Wistar rats from five different suppliers using the MCSF test. Appl Animal Behav Sci. 2011;135:128–37.
- Palm S, Roman E, Nylander I. Differences in voluntary ethanol consumption in Wistar rats from five different suppliers. Alcohol. 2011;45:607–14.
- Palm S, Roman E, Nylander I. Differences in basal and ethanol-induced levels of opioid peptides in Wistar rats from five different suppliers. Peptides. 2012;36:1–8.
- Roman E, Colombo G. Lower risk taking and exploratory behavior in alcohol-preferring sP rats than in alcohol nonpreferring sNP rats in the multivariate concentric square field™ (MCSF) test. Behav Brain Res. 2009;205:249–58.
- Meyerson BJ, Höglund AU. Exploratory and socio-sexual behaviour in the male laboratory rat: a methodological approach for the investigation of drug action. Acta Pharmacol Toxicol (Copenh). 1981;48:168–80.
- Sisk CL, Meek LR. Sexual and reproductive behaviors. Curr Protocol Neurosci 1997;chapter8:unit 8.2.
- Ågmo A. Male rat sexual behavior. Brain Res Brain Res Protoc. 1997;1:203–9.
- Whalen RE, Beach FA, Kuehn RE. Effects of exogenous androgen on sexually responsive and unresponsive male rats. Endocrinology. 1961;69:373–80.
- Craig W. Appetites and aversions as constituents of instincts. Biol Bull. 1918;34:91–107.
- Pfaus JG, Mendelson SD, Phillips AG. A correlational and factor analysis of anticipatory and consummatory measures of sexual behavior in the male rat. Psychoneuroendocrinology. 1990;15:329–40.
- Portillo W, Antonio-Cabrera E, Camacho FJ, Diaz NF, Paredes RG. Behavioral characterization of non-copulating male mice. Horm Behav. 2013;64:70–80.
- Cagiano R, Cassano T, Coluccia A, Gaetani S, Giustino A, Steardo L, et al. Genetic factors involved in the effects of developmental low-level alcohol induced behavioral alterations in rats. Neuropsychopharmacology. 2002;26:191–203.
- Leggio B, Masi F, Grappi S, Nanni G, Gambarana C, Colombo G, et al. Sardinian alcohol-preferring and non-preferring rats show different reactivity to aversive stimuli and a similar response to a natural reward. Brain Res. 2003;973:275–84.
- Richter RM, Zorrilla EP, Basso AM, Koob GF, Weiss F. Altered amygdalar CRF release and increased anxiety-like behavior in Sardinian alcohol-preferring rats: a microdialysis and behavioral study. Alcohol Clin Exp Res. 2000;24:1765–72.
- Colombo G, Agabio R, Lobina C, Reali R, Zocchi A, Fadda F, et al. Sardinian alcohol-preferring rats: a genetic animal model of anxiety. Physiol Behav. 1995;57:1181–5.
- Lobina C, Gessa GL, Colombo G. Anxiolytic effect of voluntarily consumed alcohol in Sardinian alcohol-preferring rats exposed to the social interaction test. J Alcoholism Drug Depend. 2013;1:132.
- Everitt BJ. Sexual motivation: a neural and behavioural analysis of the mechanisms underlying appetitive and copulatory responses of male rats. Neurosci Biobehav Rev. 1990;14:217–32.
- Canseco-Alba A, Rodriguez-Manzo G. Anandamide transforms noncopulating rats into sexually active animals. J Sex Med. 2013;10:686–93.
- Vinod KY, Maccioni P, Garcia-Gutierrez MS, Femenia T, Xie S, Carai MA, et al. Innate difference in the endocannabinoid signaling and its modulation by alcohol consumption in alcohol-preferring sP rats. Addict Biol. 2012;17:62–75.
- Colombo G, Agabio R, Diaz G, Fa M, Lobina C, Reali R, et al. Sardinian alcohol-preferring rats prefer chocolate and sucrose over ethanol. Alcohol. 1997;14:611–15.
- Wilhelm CJ, Mitchell SH. Acute ethanol does not always affect delay discounting in rats selected to prefer or avoid ethanol. Alcohol Alcohol. 2012;47:518–24.
- Agabio R, Carai MA, Lobina C, Pani M, Reali R, Bourov I, et al. Dissociation of ethanol and saccharin preference in sP and sNP rats. Alcohol ClinExp Res. 2000;24:24–9.
- Vacca G, Serra S, Brunetti G, Carai MA, Samson HH, Gessa GL, et al. Operant self-administration of ethanol in Sardinian alcohol-preferring rats. Alcohol Clin Exp Res. 2002;26:1678–85.
- Spear NE, Molina JC. Fetal or infantile exposure to ethanol promotes ethanol ingestion in adolescence and adulthood: a theoretical review. Alcohol Clin Exp Res. 2005;29:909–29.
- Bocarsly ME, Barson JR, Hauca JM, Hoebel BG, Leibowitz SF, Avena NM. Effects of perinatal exposure to palatable diets on body weight and sensitivity to drugs of abuse in rats. Physiol Behav. 2012;107:568–75.

