Abstract
Background. Pancreatic or islet fibrosis is often associated with activated pancreatic stellate cells (PSCs). PSCs are considered not only to promote fibrosis, but also to be associated with glucose intolerance in some diseases. We therefore evaluated morphological and functional relationships between islets and PSCs in the normal mouse pancreas and transplanted islets.
Methods. Immunohistochemistry was used to map the presence of PSCs in the normal mouse pancreas and islets implanted under the renal capsule. We isolated and cultured mouse PSCs and characterized them morphologically by immunofluorescence staining. Furthermore, we measured their cytokine production and determined their effects on insulin release from simultaneously cultured islets.
Results. PSCs were scattered throughout the pancreas, with occasional cells within the islets, particularly in the islet capsule. In islet transplants they were found mainly in the graft periphery. Cultured PSCs became functionally activated and produced several cytokines. Throughout the culture period they linearly increased their production of interleukin-6 and mammalian keratinocyte-derived chemokine. PSC cytokine production was not affected by acute hyperglycemia. Syngeneic islets co-cultured with PSCs for 24–48 h increased their insulin release and lowered their insulin content. However, short-term insulin release in batch-type incubations was unaffected after 48 h of co-culture. Increased islet cell caspase-3 activation and a decreased islet cell replication were consistently observed after co-culture for 2 or 7 days.
Conclusion. Activated PSCs may contribute to impaired islet endocrine function seen in exocrine pancreatitis and in islet fibrosis associated with some cases of type 2 diabetes.
Introduction
Pancreatic islets constitute complex organs distributed within the pancreas of almost all vertebrates. Most interest has been directed towards endocrine cells in general and beta-cells in particular. However, islets also contain a complex stroma, with many cells contributing to the unique microenvironment needed for optimal endocrine function. The connective tissue within the islets includes a capsule delineating it from the exocrine tissue, as well as an extracellular matrix (Citation1).
Pancreatic stellate cells (PSCs) are matrix-producers distributed throughout the endocrine and exocrine parts of the gland (Citation2). Stellate cells occur in many organs in the body (Citation3), and they have been intensively studied in the liver where they can be identified by the presence of the intermediary filaments vimentin and desmin, and possess vitamin-A-containing lipid droplets (Citation4). Upon stimulation by e.g. cytokines, stellate cells become activated and express nestin and in particular α-smooth muscle actin (α-SMA) (Citation5). Activated PSCs have morphological characteristics of myofibroblasts and pericytes and contribute to fibrogenesis in chronic pancreatitis as well as to the desmoplastic reactions in pancreatic cancer (Citation6-9), both of which have been implicated in impaired glucose metabolism (Citation10,11).
Despite the importance of PSCs in pancreatic pathology their role in impaired glucose tolerance and type 2 diabetes is not well understood. Interestingly, there is islet fibrosis, without any significant exocrine engagement in type 2 diabetes (Citation12). It has been suggested that local cytokine production in the islets from macrophages/lymphocytes is responsible for this (Citation12). It may therefore be that PSCs contribute to impaired islet function, not only in exocrine pancreatic disease but also in certain types of diabetes. Furthermore, PSCs are likely to contribute to the frequently seen graft fibrosis occurring after islet transplantation, which is of importance for islet graft dysfunction (Citation13). However, PSCs may to some extent be advantageous in this context, since they are known to modulate immune reactions and to improve islet transplant survival, maybe by stimulating graft revascularization (Citation7,14). It has also been suggested that they may constitute a progenitor pool of endocrine cells (Citation15).
Thus, in view of the possible and ambiguous effects of PSCs on islet function, we performed this study to evaluate in more detail the distribution of these cells in the normal mouse pancreas and in transplanted islets and to characterize functional interactions between beta-cells and culture-activated PSCs in vitro.
Material and methods
Chemicals
All chemicals and reagents were from Sigma-Aldrich (St. Louis, MO, USA) unless given otherwise.
Animals and cells
All animal experiments were approved and performed according to the guidelines and regulations of the local committees for animal care at Uppsala University. Male C57BL/6 or C57BL/6 (nu/nu) mice (Taconic, Ry, Denmark) were used in all experiments. The animals were housed in the Animal Department at the Biomedical Centre (Uppsala, Sweden) and had free access to pelleted food and tap water. Mouse pancreatic β-TC6 cells (ATCC; Manassas, VA, USA) were maintained in Dulbecco’s Modified Eagle Medium (DMEM; Gibco, Grand Island, NY, USA) with 10% fetal calf serum, 2 mM L-glutamine, 100 U/mL benzylpenicillin, 0.1 mg/mL streptomycin (Roche Diagnostics Scandinavia, Bromma, Sweden) in humidified air and 5% CO2 at 37°C. Medium was changed every third day, and the cells were used in passage 3–8.
Islet isolation, culture, and transplantation
C57BL/6 mouse pancreatic islets were isolated and transplanted as previously described in detail (Citation16(Citation17)). Pancreatic islets were prepared by collagenase digestion, as previously described, subsequently handpicked with braking pipettes under a stereo microscope (Leica M50, Leica Microsystems, Germany), and maintained free-floating in groups of 150 islets at 37°C (air/CO2, 95:5) in 5 mL RPMI 1640 culture medium (Sigma-Aldrich, St. Louis, MO, USA) supplemented with 2 mM L-glutamine, 11 mM glucose, 10% (vol/vol) fetal calf serum, and 0.1 mg/mL streptomycin.
Syngeneic, adult male, C57BL/6 mice were used as recipients. Before transplantation the animals were anesthetized with an intraperitoneal injection of avertin. The left kidney was exposed through a flank incision, and 250 islets were implanted under the renal capsule (Citation17). The animals were then observed until fully recovered from anesthesia, and were kept in separate cages for 2 or 4 weeks. Maintenance of the animals and all experiments were approved by and performed according to the guidelines and regulations of the Uppsala Ethical Committees for Animal Research (Permit number: C107/11).
Isolation and culture of mouse pancreatic stellate cells
PSCs were isolated by a modification of the method described by Apte et al. (Citation18). Briefly, pancreatic tissue from mice was minced and digested with 0.1% Collagenase A (Sigma-Aldrich, St. Louis, MO, USA) and 0.1% DNAse in Hanks’s balanced salt solution (HBSS; SVA, Uppsala, Sweden) for 10 min. Digested tissue was then filtered through a 100-µm nylon cell strainer (BD Falcon, Franklin Lakes, NJ, USA). Cells were washed and resuspended in HBSS. The cell suspension was centrifuged into a 30% (wt/vol) solution of Nycodenz (Axis-Shield, Oslo, Norway) at 1,400 g for 20 min. PSCs separated into a grainy band just above the interface of the Nycodenz cushion and the HBSS. This band was harvested, and the cells were washed and resuspended in DMEM containing 10% FBS, 4 mM glutamine, and antibiotics (penicillin 100 U/mL and streptomycin 100 µg/mL). Cells were maintained at 37°C in a humidified atmosphere of 5% CO2/95% air. The culture medium was replaced the day after initial seeding and subsequently each third day. The purity of the isolated PSCs was determined by staining for desmin, vimentin, glial fibrillary acidic protein (GFAP), and SMA. Only isolations with purity >95% were used for further experiments.
Staining of cells and sections
The following antibodies and dilutions were used: PDX-1 primary antibody (sc-14664, Santa Cruz Biotechnology, Santa Cruz, CA, USA; 1:100, goat polyclonal), cleaved caspase-3 primary antibody (9661, Cell Signaling Technology Inc., Danvers, MA, USA; 1:200, rabbit polyclonal), desmin (CM036, Biocare Medical, Concord, CA, USA; 1:100, for immunohistochemistry, mouse monoclonal), desmin primary antibody (5332, Cell Signaling Technology Inc.; 1:50, for immunofluorescence, rabbit monoclonal), secondary antibody FITC-conjugated donkey anti-rabbit IgG (H+L) (711-095-152, Jackson ImmunoResearch Lab., Bar Harbor, ME, USA; 1:500), vimentin (5741, Cell Signaling Technology Inc.; 1:100, rabbit monoclonal), secondary antibody FITC-conjugated donkey anti-rabbit IgG (H+L) (711-095-152, Jackson ImmunoResearch Lab.; 1:500), anti-α-SMA primary antibody (sc-32251 Santa Cruz Biotechnology; 1:100, mouse monoclonal), secondary antibody Alexa Fluor 594 donkey anti-mouse IgG (H+L) (Invitrogen, Eugene, OR, USA; 1:500).
β-TC6 cells, islets, paraffin-embedded pancreas, and islet-graft containing kidneys were stained as previously described (Citation19).
For quantification of PSCs we counted the fraction of the area occupied by desmin-positive cells in pancreatic sections or islets implanted under the renal capsule. A square grid (121 intersections) was randomly placed over the sections, and the number of intersections located over desmin-positive cells in both endocrine and exocrine pancreas as well as in islet grafts was estimated. A minimum of 1,210 intersections were counted in each sample.
For morphologic characterization, isolated PSCs were seeded and cultured in Culture Slides (BD Biosciences, Erembodegem, Belgium) for 2 or 10 days, washed in PBS, fixed in ice-cold acetone for 15 min at room temperature (RT), and subsequently blocked in PBS supplemented with 3% BSA for 20 min at RT, then incubated with primary antibodies in blocking solution for 16 h at 4°C. Thereafter the slides were washed in PBS and incubated with secondary antibodies in PBS 1% BSA for 1 h at RT. Nuclear staining was performed by incubation with Hoechst 33258 (Invitrogen), 1 g/mL, for 30 min at RT. For lipid droplet determination, slides were further incubated for 30 min at RT with Nile red (Sigma-Aldrich, St. Louis, MO, USA) solution at a final concentration of 10 g/mL. Cells were washed in PBS and analyzed using fluorescence microscopy (Zeiss Axioplan 2 microscope; Carl Zeiss, Göttingen, Germany), using an Axiocam HRm camera and an Axiovision imaging software.
Co-culture of PSCs and islets
Following isolation, islets were cultured for 24 h before they were included in any experiments. Islets were cultured with or without culture-activated PSCs on cover slips. A total of 1 × 105 PSCs were seeded in a six-well plate (cover slip Ø 25 mm) and 40 islets, pre-incubated for 24 h in medium RPMI 1640, were added 24 h later. All co-culture experiments were performed in medium RPMI 1640 as outlined above for islet cultures. The islets were harvested after 2 or 7 days.
In some experiments the removed islets were fixed in methanol for 2 h. They were then blocked with 0.5% PBS, 0.5% FCS, 0.2% Triton-X followed by applying a primary antibody against caspase-3 at 4°C overnight. The islets were then washed with PBS, incubated with goat anti-rabbit secondary antibody (A11008, Invitrogen; dilution 1:500) for 1 h, washed and mounted with DAPI.
To study cell proliferation a total of 1 × 105 PSCs or 5 × 104 β-TC6 cells were seeded in six-well plates with Ø 25 mm cover slips as given above and cultured for 24 h. After this, β-TC6 cells on cover slips or islets were further cultured, with or without PSCs, for 24 or 72 h. EdU (5-ethynyl-2´-deoxyuridine; 10 µM; Invitrogen) was added to the medium for 2 h (β-TC6 cells) or overnight (islets). After this, cells were fixed in 4% formaldehyde for 15 min and permeabilized in 0.5% Triton X solution for 20 min. Islets were stained for PDX-1 at 4°C overnight, washed with PBS, and further incubated with rabbit anti-goat secondary antibody (A11080, Invitrogen; dilution 1:500) for 1 h. Finally, they were treated for 30 min with Click-iT reaction cocktail, mounted with immunofluorescence mounting, as outlined in the description of Click-iT EdU Imaging Kits (C10339, Invitrogen). EdU incorporation is shown as the percentage of positive nuclei of the total, for β-TC6 cells, and as percentage of positive nuclei of the PDX-1-positive cells, for islets.
Insulin release from islets co-cultured with PSCs
As given above, 40 islets were co-cultured with PSCs on cover slips. The RPMI 1640 medium was collected after 12, 24, 36, and 48 h, and its insulin content analyzed by application of the protocol for Mouse/Rat Insulin Assay Kit (K152BZC-1; Meso Scale Diagnostics, Gaithersburg, MD, USA) measured on a Meso Scale Diagnostics instrument. For measurement of total insulin content, the islets were harvested and homogenized by sonicating 20 islets in 100 µL redistilled H2O and subsequently mixed with 135 µL 95% acid ethanol. This solution was incubated at –20°C overnight and then centrifuged for 5 min at 10,000 g. The insulin concentration of the supernatant was analyzed following the same protocol as given above.
To evaluate possible effects of substances released from culture-activated PSCs into the medium we performed studies in which conditioned medium from 48-h cultured PSCs was added (5% [vol/vol]) to islet culture medium. Islets were further incubated in this mixed medium for 24 h. Medium was then removed, and both this and the islets were analyzed for their insulin content as given above.
Further, isolated mouse islets were cultured alone, pre-cultured for 48 h with culture-activated PSCs, and then incubated in KRBH during 2 consecutive h at 1.7 and 16.7 mM glucose, respectively. Insulin release into the medium during each of these hours was then measured.
Detection of proinflammatory cytokines released from cultured PSCs
To determine the effects of the inflammatory reactions present in the diabetic pancreas on the PSC’s cytokine expression, isolated PSCs in passage 3–8 were either untreated or cultured for 24 h in the presence of one of the following cytokines: 50 U/mL interleukin-1β (IL-1β; 200-01; PeproTech, Rocky Hill, NJ, USA), 20 ng/mL tumor necrosis factor-α (TNF-α; 310-01A, PeproTech), 10 ng/mL interleukin-6 (IL-6; 216-16, PeproTech), 1,000 U/mL interferon-γ (IFN-γ; 300-02, PeproTech). Then the culture medium was collected, and its cytokine contents were analyzed according to the manufacturer’s instructions for Mouse ProInflammatory 7-Plex Assay Ultra-Sensitive Kit (K15012C-1, Meso Scale Diagnostics). This allowed us to assess the concentrations of interleukin-1β (IL-1β), interleukin-6 (IL-6), interleukin-10 (IL-10), interleukin 12p70 (IL-12p70), interferon-γ (IFN-γ), tumor necrosis factor-α (TNF-α), and mammalian keratinocyte chemoattractant (mKC).
Cytokines in PSCs measured with quantitative real-time PCR
Cultured PSCs were treated with 1.67 mM for 1 h and then for another hour with 16.7 mM glucose in KRBH buffer. In separate experiments other PSCs were incubated with 11 mM, 25 mM, or 50 mM glucose in the normal culture medium for 96 h.
Total RNA of PSCs was extracted according to the procedure of the RNeasy mini kit (74104; Qiagen, Hilden, Germany) using on-column DNase digestion with RNase-Free DNase set (79254, Qiagen). One-step quantitative real-time RT-PCR was performed with QuantiTect™ SYBR®Green RT-PCR-kit (204243, Qiagen) on a LightCycler™ real-time PCR machine (lightcycler 2.0; Roche). Primer sequences are shown in . Cycle threshold (CT) values were determined with the LightCycler Software v3.5 (Qiagen). Primers for β-actin were used as internal standards. Products were analyzed using melting-curve analysis and gel separation.
Table I. Primer sequences used in the semi-quantitative real-time PCR studies.
Statistical calculations
All values are given as means ± SEM. Probabilities (P) of chance differences between the groups were calculated with ANOVA variance analysis or Student’s unpaired t test as given in the text.
Results
Stellate cells in pancreas and islet grafts
Desmin-positive PSCs were found throughout the mouse pancreas, associated with intra- and interlobular connective tissue in the exocrine parenchyma but also within and surrounding the islets (). In islets syngeneically transplanted under the renal capsule, scattered desmin-positive cells were found, especially in the periphery of the grafts. They were mainly located in the connective tissue capsule overlying the endocrine cell aggregates ().
Figure 1. Desmin staining (brown) of endogenous pancreas and implanted mouse pancreatic islets. Transplants were implanted under the renal capsule 4 weeks before study. A: There are stellate cells within the exocrine parenchyma and within the islets in the endogenous gland, with especially prominent cells seen in the islet capsule. B: Stellate cells are also associated with the capsule overlying the transplant. A few scattered cells are also seen within the graft. Scale bars 100 μm. C: The fractional area of desmin-positive cells in exocrine pancreas and within (intra-islet) or around (peri-insular) endogenous islets and transplanted islets. Values are means ± SEM for 5–6 experiments.
** P < 0.01, and *** P < 0.001 compared with the value for endogenous islets (Student’s t test).
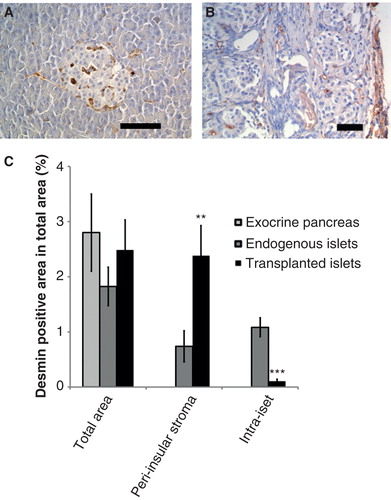
There were no differences between the total fractional area occupied by stellate cells in the exocrine pancreas or endogenous and transplanted islets (). We separately counted this fraction in the peri- or intra-insular parts of endogenous or transplanted islets. The peri-insular region was defined as being associated with the capsule surrounding the islets, whereas the intra-islet region was totally within the islets. In endogenous islets, PSCs were present to the same degree in these regions (). In islet grafts, however, there was a marked enhancement of the area of peri-insular PSCs, whilst these cells were very few within the transplanted islets ().
Isolation of pancreatic stellate cells
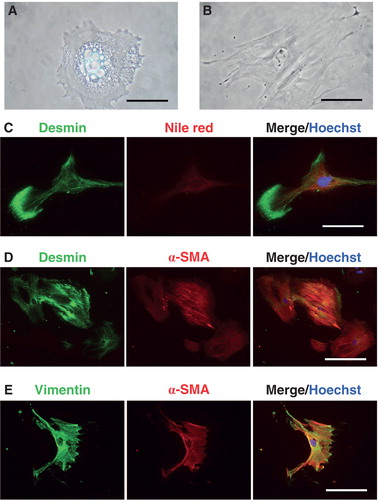
We could routinely isolate and culture PSCs with a purity of more than 95%. Immediately after isolation they contained cytoplasmic lipid droplets (). After culture for a few days the PSCs became activated, as represented by morphological changes with disappearance of lipid droplets and a more fibroblastoid appearance (). Moreover, PSCs were immuno-positive for desmin (), vimentin (), and α-SMA (). Up to 3 days after isolation, desmin expression was doubled by the peri-nuclear presence of lipid droplets in the cytoplasm, as assessed by Nile red staining (). At later stages (10–14 days of culturing) desmin and vimentin expression was paralleled by a strong -SMA immunoreactivity ().
Figure 2. Isolation and identification of mouse pancreatic stellate cells. A: Pancreatic stellate cell from mouse cultured for 24 h. There are numerous lipid droplets in the cytoplasm. B: Activated pancreatic stellate cells have lost lipid droplets and attained a fibroblastoid appearance. C–E: Isolated PSCs were cultured on culture slides, and their protein expression was analyzed as described in ‘Materials and methods’. C: Desmin (green) and Nile red (denoting lipid droplet presence) (red) fluorescence in PSCs cultured for 2 days after isolation. D: Desmin (green) and α-SMA (red) immunoreactivities in PSCs cultured for 14 days after isolation. 20× magnification. E: Vimentin (green) and -SMA (red) immunoreactivities in PSCs for 14 days after isolation. Scale bars 50 m (A and C) and 100 m (B, D, E). In all micrographs Hoechst 33258 was used for nuclear staining (blue).
Co-culture of islets and pancreatic stellate cells
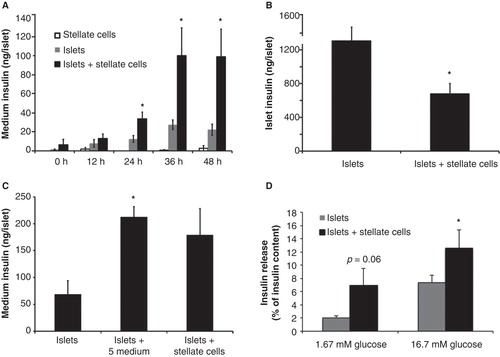
When culturing stellate cells and islets together for 2 days there were no adverse morphological effects on either cell type. There was a marked increase in medium insulin concentration after co-culture periods between 24 h and 48 h when compared with culture of the same number of islets alone (). PSCs cultured alone as expected released no insulin (). Medium insulin accumulation was unaffected when freshly isolated islets were exposed to PSCs for 12 h (). On the other hand, islet insulin content was decreased after 48 h of co-culture with PSCs (). Additional experiments were performed after 48 h of co-culture, when glucose-stimulated insulin release was measured in a batch-type incubation system. PSCs did not significantly affect insulin release in these experiments (data not shown). However, when we considered insulin secretion as percentage of the intracellular insulin content, we found a higher glucose-induced insulin release as well as elevated basal insulin secretion, although the latter did not attain statistical significance (P = 0.067) (). When islets were cultured in medium preconditioned with PSCs for 24 h there was an increase in insulin release to the culture medium similar to that seen when co-cultured with PSCs (). However, islet insulin content remained unchanged (data not shown).
Figure 3. Mouse islet insulin release after co-culture with mouse pancreatic stellate cells. A: Insulin release into the medium at different time points after co-culture of isolated mouse islets and mouse pancreatic stellate cells. B: Islet insulin content in isolated mouse pancreatic islets after 48 h of co-culture with mouse pancreatic stellate cells. C: Insulin release from isolated mouse islets cultured alone or with either 5% (vol/vol) medium conditioned by 24 h of culture with mouse stellate cells. D: Insulin release into the medium, presented as fraction of total insulin content, from isolated mouse pancreatic islets co-cultured with isolated mouse pancreatic stellate cells. A total of 40 islets and 105 pancreatic stellate cells were present in each culture dish. During the release experiments islets were incubated in KRBH 1 h each at 1.7 and 16.7 mM glucose. Insulin concentrations were measured with either Meso Scale immunoelectrodetection (A, B) or ELISA (C, D). Values are means ± SEM for 4–9 experiments. * P < 0.05 compared with islets alone.
Effects on beta-cell replication and cell death
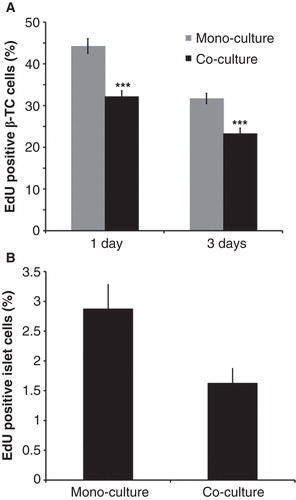
β-TC6 cells were harvested during passage 3–8 and then cultured with or without attached PSCs in the culture dish. There was a clear and consistent reduction in EdU-staining in PDX-1-positive cells (), and this was also seen when mouse islets were similarly cultured together with PSCs ().
Figure 4. Effects of mouse pancreatic stellate cells on beta-cell replication. A: β-TC6 cells cultured alone or co-cultured with isolated mouse pancreatic stellate cells for 24 h. B: Isolated mouse pancreatic islets cultured without or with isolated mouse pancreatic stellate cells for 72 h. At least 3,000 cells/experimental group were counted for each observation. Values are means ± SEM for 4–5 experiments.
** P < 0.01, and *** P < 0.001 when compared with mono-cultures.
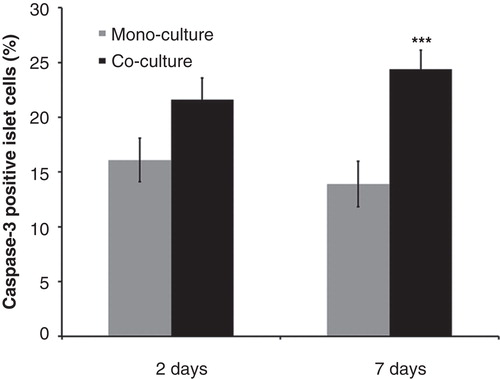
The number of caspase-3-positive islet cells was higher when islets and PSCs were co-cultured for 7 days compared with that of islets cultured alone ().
Figure 5. Effects of mouse pancreatic stellate cells on islet cell death. Fraction of caspase-3-positive cells in islets cultured alone (mono-culture) or after co-culture with isolated pancreatic stellate cells for 2 or 7 days. At least 3,000 cells were counted for each experimental group. Values are means ± SEM for 4 experiments. *** P < 0.001 when compared with the corresponding mono-culture value.
Effects of exogenously added glucose and cytokines on PSCs cytokine production
When PSCs were incubated for 1 h at a low and then for 1 h at a high glucose concentration (1.67 or 16.7 mM) we observed no differences in the cytokine expression for TNF-α, IFN-γ, IL-1β, IL-6, or IL-10 (). When similar experiments were performed but different glucose concentrations (11, 25, or 50 mM) were maintained for 96 h there was a tendency towards an increase in IL-1β in the PSCs cultured with 50 mM and a decrease in TNF-α for those cultured with either 25 or 50 mM glucose ().
Table II. Cytokine expression in PSCs as determined by semi-quantitative real-time PCR. PSCs were either incubated for 2 h in KRBH buffer (1 h 1.67 mM followed by 1 h 16.7 mM glucose concentration or without any glucose at all for 2 h) or incubated for 96 h at different glucose concentrations.
PSCs produced high amounts of IL-1β, TNF-α, and mKC, and measurable quantities of IL-6 and IL-12p70 (). PSCs were exposed to different cytokines for 24 h, and addition of IL-6 mainly increased the medium concentrations of IL-1β and TNF-α (), whilst addition of IFN-γ increased IL-6 medium concentrations (). Addition of IL-1β increased IL-6, IL-10, and mKC (), whereas the combined supplementation with IFN-γ and IL-1β increased concentrations of IL-6, IL-10, TNF-α , and mKC () in the culture media.
Figure 6. Medium release of interferon-γ (IFN-γ), interleukin-10 (IL-10), interleukin 12p70 (IL-12p70), interleukin-1β (IL-1β), interleukin 6 (IL-6), tumor necrosis factor-α (TNF-α), and keratinocyte-derived chemokine (mKC) from PSCs after incubation for 24 h either alone or with added cytokines. The latter consisted of IL-6 (A: 10 ng/mL), IFN-γ (B: 1,000 U/mL), IL-1β (C: 50 U/mL), or IFN-γ + IL-1β (D: 1,000 U/mL + 50 U/mL). E: Cytokine concentrations in medium from mouse pancreatic stellate cells after different times of culture. Values are means ± SEM for 3–7 experiments. Note logarithmic scale in A–D. * P < 0.05, ** P < 0.01, and *** P < 0.001 compared with the corresponding control value. In E, values for mKC and IL-6 are higher (P < 0.01) at all times compared with their respective value at day 3.
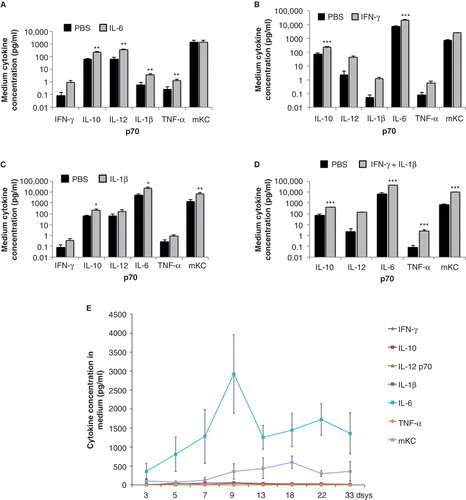
When concentrations of cytokine released into the medium by cultured PSCs were followed for 33 days we found a linear increase in IL-6 and mKC concentrations, and the increase in concentration was most pronounced in the former (). Concentrations of the other cytokines were low ().
Discussion
PSCs have been estimated to constitute nearly 4% of the total number of cells in the normal pancreas (Citation18). Their number though is markedly increased at least in animal models in which islet fibrosis seems to play a role in the development of type 2 diabetes. This assumption has been based on previous studies in which PSC inactivation was paralleled by reduced islet fibrosis and increased insulin content (Citation20).
This is, to our knowledge, the first study with primary islet cells, showing a direct effect of isolated culture-activated PSCs on the function and proliferation of insulin-producing cells. We have applied protocols enabling us to detect, isolate, and culture PSCs and have confirmed that activated PSCs produce cytokines, both during basal conditions and especially so after cytokine stimulation. Co-culture of activated PSCs and isolated islets affected insulin release in short-term batch-type experiments when insulin secretion was calculated as a percentage of insulin content, reduced insulin content, and increased accumulation of insulin in the medium during prolonged culture periods. Islet cell death increased, whereas beta-cell replication decreased. Thus, activated PSCs may play a role in the long-term impairment of glucose tolerance associated with exocrine pancreatic diseases. However, we cannot quantify how much of the negative effect of fibrosis comes from PSCs per se, but our data demonstrate that these cells, when activated, can promote beta-cell dysfunction and death, highlighting the need for further studies of these interactions. Furthermore, our results in the mouse insulinoma β-TC6 cell line confirm some of the recently published results suggesting negative functional effects of pancreatic stellate cells on other immortalized insulin-producing cell lines (Citation21,22) as well as in animal models of type 2 diabetes (Citation22).
Immediately after isolation PSCs could be identified by their lipid droplets and positive staining for vimentin, desmin, and GFAP (Citation5,23). Isolated PSCs were negative for platelet-derived growth factor receptors (data not shown), thereby confirming that they were not pericytes (Citation24). PSCs in culture became activated as evidenced by the disappearance of lipid droplets and expression of α-SMA. All these findings are corroborative of previous studies (Citation5,18).
PSCs were found throughout the pancreas, and we, in accordance with previous investigators (Citation5,23), chose to use desmin as the staining of choice for their detection after comparing several other markers. In pancreatic islets PSCs were mainly associated but not limited to the islet capsule. The capsule composition is species-dependent (Citation25), and cells making up the capsule have been supposed to derive from fibroblasts. In view of our present findings it may be that PSCs also participate in the formation of this structure. It should be noted that PSC activation is prevented by somatostatin (Citation26) produced in islet δ-cells in rodents, which are preferentially located in the islet mantle (Citation27). It can be conceived that high local somatostatin concentrations would maintain PSCs in the islet periphery quiescent. Interestingly, the presence of a continuous interstitial matrix connection between the endocrine and exocrine pancreas has been previously suggested, which is lost due to fibrosis in rodent models and humans with type 2 diabetes mellitus (Citation28). This organized, fibrillar collagen was closely associated with pericytes, which were proposed to be able to differentiate into myofibroblasts/PSCs (Citation28). Indeed, when activated PSCs were suppressed by conophylline in GK rats, a type 2 diabetes model, islet fibrosis was reduced (Citation20), supporting a role for PSCs in this process. This interesting concept is worthy of further investigations.
After syngeneic transplantation under the renal capsule desmin-positive cells were found, mainly in the renal capsule over the graft, and their combined area was approximately the same as in the endogenous islets. This location is somewhat surprising, since a pronounced central fibrosis is usually seen in intra-renal grafts, probably due to hypoxia (Citation29). The present findings do not support a major role for PSCs in islet graft fibrosis. This is in contrast to the role played by PSCs in both experimental animals and man in fibrosis seen in chronic pancreatitis and the desmoplastic reactions associated with pancreatic adenocarcinomas (Citation2). In the latter condition PSCs contribute to the tumor-promoting and immunosuppressive environment (Citation6-8). As will be discussed in more detail below, both these conditions with pancreatic fibrosis are associated with an increased incidence of type 2 diabetes (Citation10,11), as is islet fibrosis in itself (Citation12).
Co-cultures of PSCs and islets were performed to evaluate possible direct interactions affecting endocrine function. After >12 h of co-culture a marked increase of insulin released into the medium was detected, and it was associated with a decrease in islet insulin content. Under normal physiological conditions pancreatic beta-cells maintain a remarkably stable balance between insulin secretion and insulin production. Whenever glucose stimulates insulin release, there is a rapid and corresponding increase in proinsulin biosynthesis that efficiently replenishes intracellular insulin stores (Citation30,31). However, it is possible that chronic exposure to paracrine interactions with activated PSCs evokes a continuous stimulation of insulin release, which is only partially compensated for by a sustained insulin biosynthesis. This, in turn results in a decrease of the intracellular insulin stores. That the increased medium insulin release was due to beta-cell death, with leakage of insulin, seems unlikely, since the culture conditions were optimized for islets and we could detect no increased cell necrosis with propidium iodide staining. An increased apoptosis was seen as evidenced by caspase-3 staining, but this should only marginally affect the insulin concentrations. Therefore it seems as if activated PSCs stimulate insulin release from beta-cells. This was at least partially due to a factor released from the PSCs, since PSCs-conditioned medium had similar effects. When we performed acute batch-type release experiments there were no differences in insulin release between islets cultured alone or co-cultured islets and PSCs after 48 h of pre-culture. However, it should also be considered that PSCs-mediated elevation of insulin release into the medium resulted in a significant reduction in the insulin content of pancreatic islet cells. Thus, when insulin secretion was considered as a percentage of the intracellular insulin content, we found that PSCs significantly potentiated glucose-induced insulin release as well as elevating basal insulin secretion. Viewed in this way, co-culture of beta-cells with activated PSCs over a 48-h period in vitro enhanced rather than inhibited glucose-induced insulin release, which points out a possible additional mechanism by which activated PSCs present in the fibrotic pancreas can contribute to beta-cell exhaustion in the pathogenesis or amplification of type 2 diabetes.
In the co-culture experiments we found an increased apoptotic cell death and a decreased replication rate in the islet cells. The latter was seen also in beta-cell lines. This is an interesting finding since, as mentioned above, both chronic pancreatitis and pancreatic adenocarcinomas are associated both with the presence of activated PSCs with increased matrix formation and an increased incidence of type 2 diabetes (Citation10,11). The reasons for the glucose intolerance have been suggested to be the fibrosis per se, with destruction of the pancreatic architecture, especially in chronic pancreatitis (Citation11), as well as production of various tumor-derived factors exerting diabetogenic effects in the pancreas and liver (Citation32). Our present findings open up the possibility that also an increased beta-cell death and defect replication may be involved in the type 2 diabetes pathogenesis during these conditions. This is in line with some studies in chronic pancreatitis in man (Citation33) and pancreatic cancers (Citation34). Islet fibrosis per se has been suggested to play an important role also in type 2 diabetes in man and experimental animals (Citation12,35). In a secreted proteome analysis of quiescent and activated human PSCs many pro-apoptotic proteins were expressed, whereas those stimulating proliferation mainly affected tumor cells rather than islet cells (Citation36). Interestingly, it has previously been demonstrated that activated rat PSCs produce connective tissue growth factor (CTGF), which binds to integrin 51 and exerts a profibrogenic effect (Citation37).
The diabetic pancreas is characterized by immunological (type 1 diabetes) or inflammatory (type 2 diabetes) reactions in which cytokines are important players, facilitating functional impairment and cytotoxic effects on pancreatic islet cells. Therefore we found it of interest to evaluate PSCs’ cytokine production following a hyperglycemic challenge as well as in the presence of cytokines proven to be present around and within the diabetic endocrine pancreas. In our hands, PSCs produced large amounts of IL-1β, TNF-α , and measurable quantities of IL-6 and IL-12p70. This is in line with previous studies on cultured stellate cells from other organs. Furthermore, we demonstrate here, for the first time in stellate cells, high expression of mKC, a functional homolog of human interleukin IL-8, which is central for induction of Th1 responses. A continuous increase in IL-6 and mKC secretion was observed, whilst other cytokine concentrations did not change significantly, which is in line with previous studies (Citation38,39). The cytokine release was not affected by an acute 1-h exposure to hyperglycemia, besides a marginal effect of very high (50 mM) glucose concentrations on IL-1β. The rationale for studying this was previous observations that both hyperglycemia and hyperinsulinemia stimulate activation and proliferation of PSCs through ERK 1/2 phosphorylation in vitro (Citation40). Moreover, when PSCs were exposed to exogenously added high doses of cytokines we found that IL-6 and TNF-α had only minor effects on their cytokine secretion. Exogenous IL-1β increased release of both IL-6 and mKC, whereas a combination of IL-1β and IFN-γ increased IL-12p70, IL-6, and mKC secretion. All of these cytokines may therefore, in an autocrine fashion, act in synergy to promote pancreatic fibrosis (Citation2) and contribute to the impaired islet endocrine function and islet cell survival associated with some cases of type 2 diabetes.
To summarize we report a direct effect of culture-activated PSCs on the viability and proliferation rate of mouse islet cells in in vitro co-culture experiments, paralleled by a stimulated insulin release both at basal levels and after a glucose challenge. Our data suggest a possible additional mechanism by which activated PSCs present in the fibrotic pancreas can contribute to beta-cell exhaustion. This could be involved in the pathogenesis or amplification of type 2 diabetes and opens up for new treatment scenarios based on modulation of the activation of PSCs.
Acknowledgements
The skilled assistance of Ing-Britt Hallgren is gratefully acknowledged. Leif Jansson and Andreea Barbu contributed equally to this work.
Funding: This work was supported by The Swedish Research Council (521-2011-3777), the Juvenile Diabetes Research Foundation, an EFSD/Novo Nordisk grant, the Swedish Diabetes Association and the Family Ernfors Fund. The study was also supported by grants in the name of Michael Welsh, Uppsala University (Swedish Diabetes Association, Swedish Research Council and Swedish Cancer Association).
Declaration of interest: There are no conflicts of interest that could be perceived as prejudicing the impartiality of the research reported.
References
- Stendahl JC, Kaufman DB, Stupp SI. Extracellular matrix in pancreatic islets: relevance to scaffold design and transplantation. Cell Transplant. 2009;18:1–12.
- Omary MB, Lugea A, Lowe AW, Pandol SJ. The pancreatic stellate cell: a star on the rise in pancreatic diseases. J Clin Invest. 2007;117:50–9.
- Zhao L, Burt AD. The diffuse stellate cell system. J Mol Histol. 2007;38:53–64.
- Senoo H, Yoshikawa K, Morii M, Miura M, Imai K, Mezaki Y. Hepatic stellate cell (vitamin A-storing cell) and its relative--past, present and future. Cell Biol Int. 2010;34:1247–72.
- Bachem MG, Schneider E, Gross H, Weidenbach H, Schmid RM, Menke A, et al. Identification, culture, and characterization of pancreatic stellate cells in rats and humans. Gastroenterology. 1998;115:421–32.
- Vonlaufen A, Joshi S, Qu C, Phillips PA, Xu Z, Parker NR, et al. Pancreatic stellate cells: partners in crime with pancreatic cancer cells. Cancer Res. 2008;68:2085–93.
- Vonlaufen A, Phillips PA, Xu Z, Goldstein D, Pirola RC, Wilson JS, et al. Pancreatic stellate cells and pancreatic cancer cells: an unholy alliance. Cancer Res. 2008;68:7707–10.
- Tang D, Yuan Z, Xue X, Lu Z, Zhang Y, Wang H, et al. High expression of Galectin-1 in pancreatic stellate cells plays a role in the development and maintenance of an immunosuppressive microenvironment in pancreatic cancer. Int J Cancer. 2012;130:2337–48.
- Hwang RF, Moore T, Arumugam T, Ramachandran V, Amos KD, Rivera A, et al. Cancer-associated stromal fibroblasts promote pancreatic tumor progression. Cancer Res. 2008;68:918–26.
- Wang F, Herrington M, Larsson J, Permert J. The relationship between diabetes and pancreatic cancer. Mol Cancer. 2003;2:4.
- Malka D, Hammel P, Sauvanet A, Rufat P, O’Toole D, Bardet P, et al. Risk factors for diabetes mellitus in chronic pancreatitis. Gastroenterology. 2000;119:1324–32.
- Donath MY, Boni-Schnetzler M, Ellingsgaard H, Ehses JA. Islet inflammation impairs the pancreatic beta-cell in type 2 diabetes. Physiology (Bethesda). 2009;24:325–31.
- Jansson L, Carlsson PO. Graft vascular function after transplantation of pancreatic islets. Diabetologia. 2002;45:749–63.
- Yin Z, Wu W, Fung JJ, Lu L, Qian S. Cotransplanted hepatic stellate cells enhance vascularization of islet allografts. Microsurgery. 2007;27:324–7.
- Mato E, Lucas M, Petriz J, Gomis R, Novials A. Identification of a pancreatic stellate cell population with properties of progenitor cells: new role for stellate cells in the pancreas. Biochem J. 2009;421:181–91.
- Andersson A. Isolated mouse pancreatic islets in culture: effects of serum and different culture media on the insulin production of the islets. Diabetologia. 1978;14:397–404.
- Mattsson G, Jansson L, Carlsson PO. Decreased vascular density in mouse pancreatic islets after transplantation. Diabetes. 2002;51:1362–6.
- Apte MV, Haber PS, Applegate TL, Norton ID, McCaughan GW, Korsten MA, et al. Periacinar stellate shaped cells in rat pancreas: identification, isolation, and culture. Gut. 1998;43:128–33.
- Funa NS, Kriz V, Zang G, Calounova G, Akerblom B, Mares J, et al. Dysfunctional microvasculature as a consequence of shb gene inactivation causes impaired tumor growth. Cancer Res. 2009;69:2141–8.
- Saito R, Yamada S, Yamamoto Y, Kodera T, Hara A, Tanaka Y, et al. Conophylline suppresses pancreatic stellate cells and improves islet fibrosis in Goto-Kakizaki rats. Endocrinology. 2012;153:621–30.
- Kikuta K, Masamune A, Hamada S, Takikawa T, Nakano E, Shimosegawa T. Pancreatic stellate cells reduce insulin expression and induce apoptosis in pancreatic beta-cells. Biochem Biophys Res Commun. 2013;433:292–7.
- Zha M, Xu W, Zhai Q, Li F, Chen B, Sun Z. High glucose aggravates the detrimental effects of pancreatic stellate cells on Beta-cell function. Int J Endocrinol. 2014;2014:165612.
- Jaster R. Molecular regulation of pancreatic stellate cell function. Mol Cancer. 2004;3:26.
- Lindblom P, Gerhardt H, Liebner S, Abramsson A, Enge M, Hellström M, et al. Endothelial PDGF-B retention is required for proper investment of pericytes in the microvessel wall. Genes Dev. 2003;17:1835–40.
- van Deijnen JH, Hulstaert CE, Wolters GH, van Schilfgaarde R. Significance of the peri-insular extracellular matrix for islet isolation from the pancreas of rat, dog, pig, and man. Cell Tissue Res. 1992;267:139–46.
- Long D, Lu J, Luo L, Guo Y, Li C, Wu W, et al. Effects of octreotide on activated pancreatic stellate cell-induced pancreas graft fibrosis in rats. J Surg Res. 2012;176:248–59.
- Ludvigsen E, Olsson R, Stridsberg M, Janson ET, Sandler S. Expression and distribution of somatostatin receptor subtypes in the pancreatic islets of mice and rats. J Histochem Cytochem. 2004;52:391–400.
- Hayden MR, Patel K, Habibi J, Gupta D, Tekwani SS, Whaley-Connell A, et al. Attenuation of endocrine-exocrine pancreatic communication in type 2 diabetes: pancreatic extracellular matrix ultrastructural abnormalities. J Cardiometab Syndr. 2008;3:234–43.
- Parr EL, Bowen KM, Lafferty KJ. Cellular changes in cultured mouse thyroid glands and islets of Langerhans. Transplantation. 1980;30:135–41.
- Pipeleers DG, Marichal M, Malaisse WJ. The stimulus-secretion coupling of glucose-induced insulin release. XV. Participation of cations in the recognition of glucose by the beta-cell. Endocrinology. 1973;93:1012–18.
- Itoh N, Okamoto H. Translational control of proinsulin synthesis by glucose. Nature. 1980;283:100–2.
- Chen N, Unnikrishnan IR, Anjana RM, Mohan V, Pitchumoni CS. The complex exocrine-endocrine relationship and secondary diabetes in exocrine pancreatic disorders. J Clin Gastroenterol. 2011;45:850–61.
- Schrader H, Menge BA, Schneider S, Belyaev O, Tannapfel A, Uhl W, et al. Reduced pancreatic volume and beta-cell area in patients with chronic pancreatitis. Gastroenterology. 2009;136:513–22.
- Campbell-Thompson M, Dixon LR, Wasserfall C, Monroe M, McGuigan JM, Schatz D, et al. Pancreatic adenocarcinoma patients with localised chronic severe pancreatitis show an increased number of single beta cells, without alterations in fractional insulin area. Diabetologia. 2009;52:262–70.
- Kim JW, Ko SH, Cho JH, Sun C, Hong OK, Lee SH, et al. Loss of beta-cells with fibrotic islet destruction in type 2 diabetes mellitus. Front Biosci. 2008;13:6022–33.
- Wehr AY, Furth EE, Sangar V, Blair IA, Yu KH. Analysis of the human pancreatic stellate cell secreted proteome. Pancreas. 2011;40:557–66.
- Gao R, Brigstock DR. Connective tissue growth factor (CCN2) in rat pancreatic stellate cell function: integrin alpha5beta1 as a novel CCN2 receptor. Gastroenterology. 2005;129:1019–30.
- Masamune A, Kikuta K, Watanabe T, Satoh K, Hirota M, Hamada S, et al. Fibrinogen induces cytokine and collagen production in pancreatic stellate cells. Gut. 2009;58:550–9.
- Jonitz A, Fitzner B, Jaster R. Molecular determinants of the profibrogenic effects of endothelin-1 in pancreatic stellate cells. World J Gastroenterol. 2009;15:4143–9.
- Hong OK, Lee SH, Rhee M, Ko SH, Cho JH, Choi YH, et al. Hyperglycemia and hyperinsulinemia have additive effects on activation and proliferation of pancreatic stellate cells: possible explanation of islet-specific fibrosis in type 2 diabetes mellitus. J Cell Biochem. 2007;101:665–75.

