Abstract
Background. Somatostatin acts through five receptor subtypes (SSTRs 1–5). We aimed to investigate SSTRs mRNA expression and protein distribution in whole rat embryos, with special emphasis on the pancreas.
Material and methods. Rat embryos were collected on embryonal days 10, 11, 12, 14, 15, 17, 19, 21, and at birth. Presence of SSTRs was investigated with RT-PCR techniques and immunohistochemistry.
Results. There was no SSTR5 mRNA expression in the whole rat embryos. All SSTR1–5 proteins were observed at embryonal day 10, but the localization varied between the different subtypes. From day 11 to birth SSTRs protein presence increased with time in major structures such as skin and cartilage. It remained similar over time in the heart and liver. In the fetal pancreas mRNA expression of SSTR2 and 4 was detected at day 14, and there was an increase up to birth. Only SSTR1 protein co-localized to a higher extent with the islet hormones studied. SSTR2 was present in all islet endocrine cells except for β-cells. In contrast, the immunostaining for SSTR3–4 was co-localized with insulin and PP, and, finally, SSTR5 with glucagon and pancreatic polypeptide. In mRNA isolated from whole rat embryos SSTR1-2 and SSTR4 expression showed a peak at day 14, while SSTR3 mRNA was not present until day 15.
Conclusion. The present data suggest a role for SSTRs during the development of the rat embryo. Subsequent functional studies may elucidate regulatory roles of specific SSTRs for the growth and differentiation of the pancreas as well as other organs.
Introduction
Somatostatin is a cyclic polypeptide hormone with two biologically active forms, somatostatin-14 and the N-terminal extended version, somatostatin-28. The hormone was initially described in the brain, but is today known to exist in a variety of tissues and organs (Citation1-3). The peptide is known to inhibit secretion of hormones from endocrine cells associated with growth, development, and metabolism (Citation2,3). The inhibitory effects of somatostatin are mediated by specific G-protein coupled membrane receptors (SSTRs). These SSTRs are able to activate a variety of intracellular signalling mechanisms, leading to different cellular responses. To date five mammalian SSTRs have been cloned, studied, and named according to the order of identification (Citation4-7). They are distinguished by their pharmacological specificity for different somatostatin analogues, tissue distribution, regulation, and intracellular signalling pathways (Citation2,3,8). Inhibition of hormone secretion and growth is mainly considered to be mediated by SSTR1, SSTR2, and SSTR5 (Citation9,10). In the pancreas, decreased insulin secretion is thought to be regulated via SSTR5, while decreased glucagon secretion is mediated via SSTR2 (Citation11,12). Inhibition of growth hormone secretion is more complex and is considered to be a combined effect of signalling through both SSTR2 and SSTR5 (Citation13). Moreover, signalling via SSTR2 and SSTR3 is believed to be involved in apoptosis (Citation14,15). So far, not much is known about the functional effects mediated via SSTR4. There are reasons to believe that two or more SSTRs may operate together to elicit specific functions, which is supported by the fact that several SSTRs are expressed in the same tissues or organs (Citation2,16,17), and recent findings also support the idea of cross-talk between different SSTRs (Citation18,19).
Previous studies on SSTRs distribution have mainly focused on tissues or organs from adult animals. However, some studies have implied a critical role for somatostatin and its receptors during embryonic development in different species (Citation20-23). Regional distribution of SSTR mRNAs in the rat brain at embryonic day 17–18 has been investigated using in situ hybridization (Citation23). High expression of SSTR3 mRNA and low expression of SSTR1 and SSTR2 mRNAs were demonstrated, whereas SSTR4 and SSTR5 mRNAs were absent in distinct areas of the brain. In the adult rat brain the SSTR mRNAs were highly expressed, and it was suggested that some SSTRs might play an important role during neurogenesis (Citation23). During early neurogenesis in the rat SSTRs were present in a transient manner, with a peak around embryonal day 14, and it was suggested that the transient expression of SSTRs was associated with critical episodes in the development of a cohort of neurons and that somatostatin may play a fundamental role during neurogenesis (Citation22). Moreover, in a recent study in orange-spotted grouper, the presence of SSTR1–3 and SSTR5 mRNA expression during development was demonstrated (Citation24). However, teleost fish lack SSTR4, but they have SSTR6 instead which is believed to be lost in mammals and birds (Citation25).
The pancreas is a foregut derivative and develops from a dorsal and a ventral outgrowth of the primitive duodenum. In rodents early pancreatic buds become visible around embryonal day 9. Subsequently, the ventral pancreatic bud rotates around the stomach and duodenum and fuses with the dorsal pancreatic bud. During organogenesis, the pancreatic epithelium proliferates and invades the surrounding mesenchyme. Epithelial cells then differentiate into ductal, endocrine, and acinar cells. Cells containing glucagon have been detected as early as embryonic day 9.5, and one day later insulin-containing cells have been demonstrated. Cells secreting somatostatin appeared at day 15, and PP-producing cells were identified at day 18.5 (Citation26,27). In the latter context it is interesting that in previous studies of fetal murine pancreases co-expression of more than one of the four major islet hormones during development was observed, but also co-localization with other polypeptides not demonstrated in the adult pancreas (Citation28). It could be envisaged that embryonic islet cell expression of SSTRs may play a role in the further differentiation of islet cells.
The aim of this study was to follow the mRNA and protein expression profiles of the SSTRs during normal development in rat embryos, with special emphasis on the pancreas, from embryonic day 10.
Materials and methods
Animals
Embryos were obtained from pregnant Sprague Dawley rats (Bomholtgaard Research and Breeding Center, Ry, Denmark) and from a local outbred Sprague Dawley rat strain (Citation29). The animals were housed in a room with a 12-h light/dark cycle and had free access to water and pelleted food. The regional laboratory animal ethics committee in Tierp, Sweden, approved all experiments. Rats were killed by cervical dislocation at embryonic day 10, 11, 12, 14, 15, 17, 19, 21, and at postnatal day 1 (newborn). Embryos were dissected out, and deciduas and membranes were carefully removed. Further dissection of the pancreas was performed on some of the embryos. All whole embryos (n = 1–8) or pancreases isolated from embryos (n = 2–10) of one pregnant rat were pooled and considered as one observation (n = 1–3). After birth, RNA from one pancreas of one fetus was treated as one observation. Pancreases from adult animals were used as controls.
PCR measurements of SSTR mRNAs, preparation of total RNA
Expression profiles of the SSTR mRNA in both total embryos and embryonic pancreas were studied. Total RNA from embryonic pancreases from pregnant rats (n = 15) was collected from embryonic day 14 up to birth. Total RNA from either pooled whole embryos or pooled fetal pancreases was isolated using RNeasy mini kits (Qiagen AG, Basel, Switzerland) according to the manufacturer’s description. Briefly, embryos or pancreases from embryos of one pregnant rat were homogenized in buffer RLT. Next, 70% ethanol was added to the homogenates, and the samples were mixed and applied to RNeasy mini columns. Homogenates were centrifuged, the flow-through was discarded, and the columns were washed. Membrane-bound RNA was treated with DNase I to exclude chromosomal DNA. Columns were subsequently washed and RNA eluted using RNase-free water. RNA was precipitated with 0.3 M NaAc and ice-cold 99% ethanol, washed and dissolved in water at a final volume of 10 μL.
Preparation of cDNA
Synthesis of cDNA was performed using a Reversed Transcription System (Promega, Madison, WI, USA). For each reaction of 20 μL, 9 μL of total RNA, 5 mmol/L MgCl2, 1 × Reverse transcription buffer, 1 mmol/L of each dNTP, 1 U/μL Recombinant RNasin® ribonuclease inhibitor, 15 U/μg AMV Reverse Transcriptase, and 0.5 μg (dT)15 primer were used. The RNA and primers were preincubated for 5 min at 60°C, then incubated for 60 min at 42°C, followed by 5 min at 99°C, and subsequently stored at –20°C.
Analysis of SSTRs mRNA expression
The cDNA synthesized was amplified using a LightCycler (Roche Diagnostics GmbH, Mannheim, Germany). Specific primers for the SSTRs were designed by TIB MolBiol (Berlin, Germany) (). Duplicates of the cDNA synthesized were amplified according to the LightCycler protocol in a final volume of 10 µL containing 5 µL FastStart DNA Master SYBR Green (Roche Molecular Biochemicals, Mannheim, Germany) and 0.5 mM of the sense and anti-sense primers. We assessed the stability of expression of various housekeeping genes, such as TATA-binding protein (TBP) (Cybergene AB, Stockholm, Sweden), glucose-6-phosphate dehydrogenase (G6PDH), and porphobilinogen deaminase haem biosynthetic enzyme (PBG) (TIB MolBiol Syntheselabor, Berlin, Germany). The TBP mRNA was found to be most stable in accordance with the total RNA concentration, in embryos at day 10–21 (data not shown) and was therefore used as housekeeping gene. The LightCycler Run version 5.32 was used with the following parameters: 1) denaturation: 95°C 10 min; and 2) cycling: 95°C 15 s, 60°C 10 s, and 72°C 15 s. Data were analysed using melting-point curves, temperature-dependent dissociation of the products, verifying that the correct product was amplified. Furthermore, the purity of the products was checked using agarose gel separation. All samples had a crossing point between 19 and 39 cycles. Melting curve analyses confirmed no primer-dimer products.
Table I. PCR primers.
Results were recalculated comparing the difference between crossing point (cp) values of the amplified sample and the housekeeping mRNA using the formula 2-Δ (cpssts-cpTBP).
Immunohistochemistry
The entire embryo or its pancreas was immediately fixed in 4% formalin (Merck, Darmstadt, Germany) for 24 h at room temperature, and then moved into 70% ethanol and embedded in paraffin. Sections of 5 µm were attached to Polysine™ glass slides (Menzel-Gläser, Braunschweig, Germany) for further treatment as below.
SSTR1–5 antibodies
All pancreases were immunostained for the five SSTRs as previously described (Citation29). The production and specificity of subtype-specific somatostatin receptor antibodies have been described and discussed earlier (Citation30,31). Briefly, the immune reaction was amplified by an avidin-biotin complex coupled to alkaline phosphates (Vectastain ABC-AP; Vector Laboratories Inc., Burlingame, CA, USA) and visualized with Vector Red (Vector Laboratories) as substrate (Citation30). When SSTR-specific antibodies were preincubated with the peptides used for immunization, the immunoreactivity for each receptor was blocked (data not shown) (Citation30).
Morphological evaluation
The expression and distribution of SSTRs were evaluated in a light microscope. The anatomical distribution of SSTRs in rat fetuses during development was noted, and the intensities of SSTR immunoreactivities were scored semi-quantitatively: ++ = marked positive immunoreactivity; + = weak positive immunoreactivity; – = not detected. The rat embryo or pancreatic specimens were evaluated with the examiner being unaware of the origin of the sections and the identity of the antibody used.
Double immunofluorescence of SSTR1–5 and islet hormones in the fetal pancreas.
To investigate the co-expression of SSTR1–5 in pancreatic islet cells of the rat fetus we used a previously described immunofluorescence method (Citation30). Fetal pancreatic specimens were collected, paraffin-embedded, and stained for SSTR1–5 in a cocktail with chicken anti-insulin (1:750, Immunsystem, Uppsala, Sweden), chicken anti-glucagon (raised against human glucagon, 1:400, a kind gift from Associate Professor Anders Larsson; Novo Nordisk, Bagsvaerd, Denmark), sheep anti-somatostatin (1:25; Guildhay, Guildford, UK), or sheep anti-PP (1:25; SeroTech, Oxford, UK) as previously described (Citation30). The immune reaction was visualized with a cocktail consisting of secondary antibodies, Cy3-conjugated donkey anti-rabbit IgG (1:100; Jackson ImmunoResearch, West Grove, PA, USA), and Cy2-conjugated donkey anti-sheep IgG (1:100; Jackson ImmunoResearch) or Cy2-conjugated donkey anti-chicken IgG (1:100; Jackson ImmunoResearch). Using different dilutions of the antibodies as well as omitting the primary antibodies, we tested the specificity of the commercial anti-hormone antibodies.
Evaluation of immunofluorescence
Pancreatic sections were examined in a Leica Leitz DMR fluorescence microscope (Leica Microsystems, Wetzlar, Germany) equipped with filters of 492 nm to 510 nm for Cy2 (green) and 550 nm to 570 nm for Cy3 (red). Pictures from a Zeiss Axiocam camera (Carl Zeiss, Oberkochen, Germany) of pancreatic islets, using both filters, were merged together with Adobe Photoshop 7.0 software (Adobe, San Jose, CA, USA), in which a yellow colour indicated co-expression of SSTR subtypes with one of the four islet hormones tested in this study. Results were expressed as a percentage of SSTR-positive cells in relation to the total number of the respective islet cell type in a specific pancreatic islet.
Statistical analysis
Data are presented as means ± SEM, and groups of data were compared using Student’s t test, where p < 0.05 was considered as statistically significant. The computer program used was SigmaStat 2.0 (SPSS Science, Chicago, IL, USA).
Results
Immunocytochemical detection of SSTRs during rat development
To investigate the protein expression of SSTRs in different embryonic tissues, whole rat embryos from embryonic day 10 to birth were collected and immunostained for SSTR1–5. Tissues were identified using light microscopy, and SSTR expression was graded (for details see ). In representative sections from rat embryos immunostained for SSTRs at different stages of development are demonstrated.
Table II. Summarized protein expression of SSTR1–5 in major structures identified in rat embryos from day 10 to birth.
Figure 1. The immunohistochemical staining for SSTR subtypes. A: At embryonal day 11 (SSTR1, magnification 100×); B: day 15 (SSTR2, magnification 16×); and C: day 17 (SSTR5, magnification 16×) in rat embryos. Positive staining by SSTR antibodies is highlighted by red colour. D–G: Structures in rat embryos from day 15 to birth (magnification 200×). D: positive staining of SSTR3 in the lens at day 15. E: The outer part of the skin expresses SSTR4 at day 15; F: part of the eye is positive for SSTR1 at day 19; and G: the outer part of the skin is immunostained for SSTR5 in the newborn.
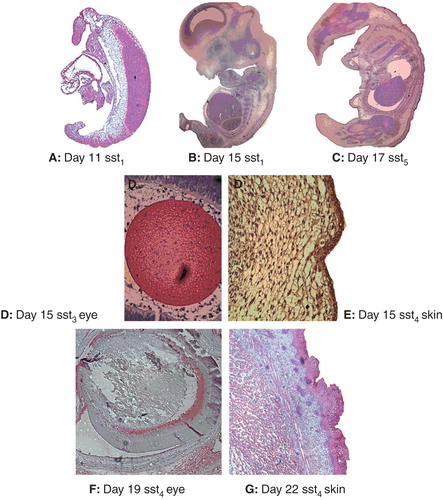
SSTR1 protein expression was identified in the neural tube, yolk sac, and aorta on day 10 and 11, and in aorta from day 11 (), while expression in other tissues was detected from day 14–15 and onwards. Retina was an exception, with SSTR1 expression present from day 19 (). The expression was usually weak, but in skin and cartilage it was more intense for 2 and 3 consecutive days.
Expression of SSTR2 was similar to SSTR1 with a few exceptions: expression in the heart was not detected until day 14, while SSTR2 was detected in the retina from day 15 ().
SSTR3 was expressed during early development in all organs except for the heart, where it was detected from day 11 and skin from day 14. The expression gradually decreased in some organs beginning in the cartilage and retina from day 17, later in the liver, and lens from day 19. Expression was usually weak, but the lens and muscle showed a more intense staining ().
SSTR4 expression was found early in the neural tube, yolk sac, skin, heart, liver, and brain, while cartilage, retina, and lens showed expression on day 15 or later (). SSTR4 was not expressed in muscle.
SSTR5 was the most universally expressed receptor, being present in all tissues investigated and with an intense staining in the skin, lens, muscle, and brain ().
SSTRs mRNA expression during development of the embryonic rat pancreas
SSTR1: The expression of SSTR1 was faint at day 14. At day 15 the levels were higher and peaked at day 17. Subsequently, at day 19 it disappeared, but returned with high expression in the newborn. In the adult pancreas, expression of SSTR1 was very weak ().
Figure 2. Expression of SSTR mRNAs in the embryonic rat pancreas amplified using real-time PCR. A: SSTR1; B: SSTR2; C: SSTR3; D: SSTR4; E: SSTR5. Data are given as mean relative expression (2-Δ(cpssts-cpTBP)) of embryonal pancreases from the same day.
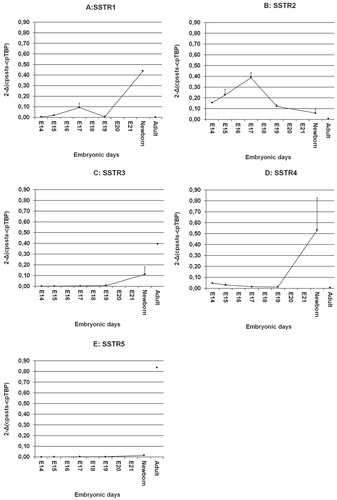
SSTR2: Expression of SSTR2 was present at day 14–15 with a peak at day 17, but then there was a decline in the mRNA levels. In the pancreas of adult rats SSTR2 expression was absent ().
SSTR3: Levels of SSTR3 were under the detection level at day 14–19. In the newborn the levels increased, and there was high expression of SSTR3 in the adult rat pancreas ().
SSTR4: Low SSTR4 levels were detected at days 14–19. In the newborn the levels were much higher and comparable to the high expression levels of SSTR1. In adult pancreatic tissues there was no SSTR4 expression ().
SSTR5: Expression of SSTR5 was low in all fetal pancreatic specimens. This was in contrast to the adult pancreas where mRNA levels were high ().
SSTRs mRNA expression in entire rat embryos
Total RNA of rat embryos (n = 11) was collected from embryonal day 10 up to birth. Embryos from one pregnant rat were considered as one observation.
SSTR1: There was no expression of SSTR1 in rat embryos 10–11 days old. It increased markedly from day 12 with a peak at day 14. At day 15–19 the SSTR1 expression decreased, but increased slightly again in the newborn ().
Figure 3. Expression of SSTR mRNAs of entire rat embryos amplified using real-time PCR. A: SSTR1; B: SSTR2; C: SSTR3; D: SSTR4; E): SSTR5. Embryos pooled from one pregnant rat (n = 11) were considered as one observation.
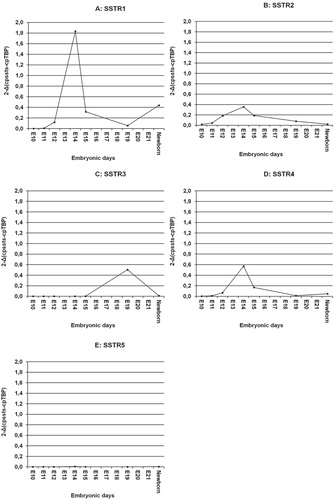
SSTR2: The expression of SSTR2 was barely detectable at day 10 in the embryos, but increased markedly up to day 14 followed by a decline during development ().
SSTR3: We were unable to detect SSTR3 mRNA before embryonic day 15. SSTR3 expression increased 5-fold at day 19 but disappeared in the newborn ().
SSTR4: There was some expression of SSTR4 at day 11, and it then increased with a peak at day 14 ().
SSTR5: At all time points investigated, there was no expression of SSTR5 mRNA ().
Presence of SSTRs in endocrine cells of the embryonal pancreas
Pancreatic specimens from embryonal days 19, 21, in the newborn, and from adult rats were used to investigate the distribution of SSTRs in pancreatic islet cells. In all investigated pancreatic sections at least 10 islets (n = 10–20) were identified and their islet cells counted. The results are summarized in . It should be noted that SSTR immunofluorescence was not analysed in the exocrine part of the pancreas.
Table III. Quantitative analysis of the expression of SSTRs in β-cells, α-cells, δ-cells, or PP-cells in the fetal rat pancreas from day 19, 21 and in the newborn. Percentages are proportion of cells in which immunofluorescence was detected.
SSTR1 was highly expressed in β- and PP-cells and had a moderate to low expression in α- and δ-cells. Expression was especially low in α-cells on day 21 and in the newborn.
SSTR2 displayed a very weak expression in embryonal β-cells, while the expression was moderate in adult rats. In the other pancreatic islet cell types SSTR2 showed a high to moderate expression.
SSTR3 showed a low to moderate expression in β- and -cells, while the expression was high in the other two cell types, although declining with time in PP-cells.
SSTR4 showed a high expression in embryonal β-cells at day 21 and in the newborn, while the expression in the other islet cells was low or absent. In the adult pancreatic islets expression of SSTR4 was low to moderate except for the δ-cells.
SSTR5 was not expressed at all in embryonal β-cells, while adult rat pancreas had a high expression. The highest expression was found in -cells, while δ- and PP-cells had a moderate expression.
Discussion
mRNA expression in whole rat embryos
In whole rat embryos the SSTR mRNA expression was very weak at day 10 but showed a peak already at day 14, followed by a decrease at day 15 in the fetus. A similar expression profile was demonstrated in the optic pathways and cerebellum during rat development (Citation22). The most highly expressed mRNA was SSTR1, followed by SSTR4 and SSTR2 mRNAs (). Interestingly, according to a phylogenetic analysis, the SSTR1 and SSTR4 subtypes are more closely related to each other, forming a subfamily, than to the other three SSTRs which form a separate subfamily (Citation25). Thus, our finding of similar gene regulation for SSTR1 and SSTR4 is consistent with their closer evolutionary relationship. The expression of SSTR5 was weak, while SSTR3 expression was absent during day 10–14 in the fetus. Recent studies demonstrated that the SSTRs genes might be age-dependent. This may suggest that different SSTRs are expressed not only in a particular tissue, but also in a critical time frame during development of rodents (Citation25,32,33). This is in line with our data in that the SSTRs mRNA expression varied over time during development of the pancreas as well as the whole fetus.
Organ-specific protein expression
To investigate the organ-specific SSTR protein expression, subtype-specific SSTR antibodies were used on paraffin-embedded embryos collected at embryonal days 10 to birth. Because of their size, newly born fetuses could not be cut, therefore only the neck and head were cut and stained. Due to this, the comparison of protein expression data obtained from these tissues with SSTR mRNA expressions from pooled whole embryos must be interpreted with caution. This circumstance may explain the discrepancy with, for example, the rapid drop for SSTR1 at E19 to E21 (80% to 5%). This pattern was observed in several of the animals included in this study, but further studies are needed to explore this issue. In the skin, SSTR4 and SSTR5 were the subtypes first detected, and then the other SSTRs followed at day 14 (). In a previous study proliferating epithelial cells were demonstrated to express mainly SSTR2 and SSTR5, which may suggest a developmental role for SSTRs in angiogenesis (Citation34). Another study reported that all five SSTRs mRNAs were expressed in adult retina, with SSTR2 and SSTR4 as the most abundant, suggesting an important role for the SSTRs in the eye (Citation35). In our study the retina expressed only SSTR3 and SSTR5 at embryonic day 14, and SSTR2 appeared later. SSTR4 expression was first observed at day 17 in the retina, which was at the same time point as the SSTR3 expression disappeared. The immunostaining of SSTR1 was not detectable until day 19 in the retina. We showed that all five SSTRs were present in the brain at day 14 and that SSTR4–5 were strongly expressed. In diencephalon the intensity of SSTR4 seemed to decline with time. The function and role of SSTR4 is as yet mainly unknown. In our study we demonstrate that during development the SSTR4 expression is detected almost in all tissues studied, with time suggesting an important developmental role for SSTR4, but further studies will be needed to confirm this. Furthermore, a counter-regulatory pattern of the SSTRs expression was demonstrated in various tissues in the present study. For example, in diencephalon the expression of SSTR1 increased with time, while SSTR4–5 decreased. Interestingly, the protein expression of SSTR3–4 seemed to vary reciprocally in the neural tube as well as in cartilage, lens, and retina. Moreover, SSTR2–3 had a counter-regulatory pattern in lens, retina, and cartilage tissue. Both SSTR2–3 are known to be involved in the induction of apoptosis (Citation14,15). This may suggest that the protein expression of SSTRs during rat development is influenced differently by developmental signals, and may thereby have opposing effects during differentiation of specific tissues. Alternatively, it cannot be excluded that such changes suggest that different SSTRs may substitute for each other in order to maintain biological actions of somatostatin in developing tissues.
The occasionally observed discrepancies between levels of mRNA transcripts detected by PCR and protein expressions as detected by immunocytochemical methods may reflect methodological limitations concerning antibody specificity and cross-reactivity and very low abundant expressions of mRNA transcripts. On the other hand, it could also reflect dynamic and rapid changes during embryonic development. For individual findings in our investigation it is thus difficult to be certain to what extent such a result represented a true biological discrepancy.
SSTR mRNA expression in endocrine cells of the pancreas
Pancreatic specimens were collected from rat fetuses at embryonic days 19, 21, and after birth. Sections were double-stained with SSTR subtype-specific antibodies and antibodies directed towards the four major islet hormones (). In a previous study in adult rat pancreas we suggested that there exists a possible cross-talk between SSTR2 and SSTR5 on β-cells (Citation18,19), as also supported by others (Citation10,12,25,36). In the present study it was found that the protein expression of SSTR5 in embryonic pancreas was not detectable by the experimental settings chosen for this study. This was also the case for the SSTR5 mRNA levels in embryonic rat pancreas () as well as for the whole rat embryo (). However, both the protein expression of SSTR5 and the mRNA levels were very high in adult pancreatic tissue (, ). The receptor has been shown to play a role during insulin secretion; and the low prenatal pancreatic expression indicates that at this time point the physiological need for glucose sensitization is not yet prioritized in the rat fetus, while this is required in the rat after birth.
Glucagon secretion is thought to be inhibited by SSTR2 (Citation12), and the high co-expression of SSTR2 observed in rat pancreatic α-cells all through rat pancreatic development and adult pancreatic tissue may suggest that glucagon secretion has an important function already in the rat embryo. Moreover, a peak at embryonic day 14 was observed for SSTR2 during pancreas development (also seen for SSTR1) (), suggesting that this may be an important time point in glucagon secretion development. In line with this the majority of SSTR1 was co-expressed on α-cells early in development. This finding is in line with previous observations showing that the cross-talk between SSTR1 and SSTR2 is important for a maximal inhibitory effect on glucagon secretion (Citation18,19,30). In addition, the majority of α-cells also express SSTR5, making it possible that the cross-talk also includes this receptor for maximum regulatory effects, but further studies are needed to support this.
There is little information about δ-cells in the pancreas, but our findings indicate that the co-expression of SSTR2–3 is similar in the newborn and in the adult pancreatic tissue, suggesting a possible basic role in somatostatin secretion. Moreover, the high co-expression of SSTR3 at embryonic day 21, in the newborn, and in adult tissue is in line with the increasing mRNA levels of the same receptor (). PP-cells are also poorly investigated, and not much is known about their function and role in the developing rat pancreas. Therefore we can only put forward the idea that the high co-expression of SSTR1–3 in these cells during development compared with that in the adult pancreas may indicate a possible important role for these SSTRs in developmental PP regulation. The co-localization of SSTR4–5 remained more or less the same during the development as well as in adult pancreas (about 20%). The reason for this is not clear, and more studies are needed to explore this issue further.
In summary, we have described the expression profiles of SSTRs both as total mRNA and as protein in the developing rat embryo and especially so in the pancreas. Expression profiles revealed mostly increased expression of the SSTRs up to embryonic day 14 and thereafter a decline. These results suggest an important role for SSTRs during the developmental process, and subsequent functional studies may elucidate the role of specific SSTRs for the growth, differentiation, and metabolism of different organs.
Acknowledgements
The technical expertise by Lisbeth Sagulin and handling of the animals by Dr Mattias Gäreskog and Dr Parri Wentzel are gratefully acknowledged. Funding: This work was supported by grants from the Swedish Research Council [72X-8273], the Swedish Diabetes Association, the Swedish Cancer Foundation, the Novo Nordisk Foundation, The Swedish Childhood Diabetes Foundation, the Family Ernfors Fund, the European Foundation for the Study of Diabetes, Magnus Bergvalls Foundation, and the Lions Foundation for Cancer Research at the University Hospital, Uppsala, Sweden.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Brazeau P, Vale W, Burgus R, Ling N, Butcher M, Rivier J, et al. Hypothalamic polypeptide that inhibits the secretion of immunoreactive pituitary growth hormone. Science. 1973;179:77–9.
- Moller LN, Stidsen CE, Hartmann B, Holst JJ. Somatostatin receptors. Biochim Biophys Acta. 2003;1616:1–84.
- Patel YC. Somatostatin and its receptor family. Front Neuroendocrinol. 1999;20:157–98.
- Raynor K, O’Carroll AM, Kong H, Yasuda K, Mahan LC, Bell GI, et al. Characterization of cloned somatostatin receptors SSTR4 and SSTR5. Mol Pharmacol. 1993;44:385–92.
- Yamada Y, Kagimoto S, Kubota A, Yasuda K, Masuda K, Someya Y, et al. Cloning, functional expression and pharmacological characterization of a fourth (hSSTR4) and a fifth (hSSTR5) human somatostatin receptor subtype. Biochem Biophys Res Commun. 1993;195:844–52.
- Yamada Y, Post SR, Wang K, Tager HS, Bell GI, Seino S. Cloning and functional characterization of a family of human and mouse somatostatin receptors expressed in brain, gastrointestinal tract, and kidney. Proc Natl Acad Sci USA. 1992;89:251–5.
- Yamada Y, Reisine T, Law SF, Ihara Y, Kubota A, Kagimoto S, et al. Somatostatin receptors, an expanding gene family: cloning and functional characterization of human SSTR3, a protein coupled to adenylyl cyclase. Mol Endocrinol. 1992;6:2136–42.
- Florio T, Schettini G. Multiple intracellular effectors modulate physiological functions of the cloned somatostatin receptors. J Mol Endocrinol. 1996;17:89–100.
- Bousquet C, Puente E, Buscail L, Vaysse N, Susini C. Antiproliferative effect of somatostatin and analogs. Chemotherapy. 2001;47:30–9.
- Strowski MZ, Kohler M, Chen HY, Trumbauer ME, Li Z, Szalkowski D, et al. Somatostatin receptor subtype 5 regulates insulin secretion and glucose homeostasis. Mol Endocrinol. 2003;17:93–106.
- Rossowski WJ, Coy DH. Specific inhibition of rat pancreatic insulin or glucagon release by receptor-selective somatostatin analogs. Biochem Biophys Res Commun. 1994;205:341–6.
- Strowski MZ, Parmar RM, Blake AD, Schaeffer JM. Somatostatin inhibits insulin and glucagon secretion via two receptors subtypes: an in vitro study of pancreatic islets from somatostatin receptor 2 knockout mice. Endocrinology. 2000;141:111–17.
- Shimon I, Yan X, Taylor JE, Weiss MH, Culler MD, Melmed S. Somatostatin receptor (SSTR) subtype-selective analogues differentially suppress in vitro growth hormone and prolactin in human pituitary adenomas. Novel potential therapy for functional pituitary tumors. J Clin Invest. 1997;100:2386–92.
- Sharma K, Patel YC, Srikant CB. Subtype-selective induction of wild-type p53 and apoptosis, but not cell cycle arrest, by human somatostatin receptor 3. Mol Endocrinol. 1996;10:1688–96.
- Teijeiro R, Rios R, Costoya JA, Castro R, Bello JL, Devesa J, et al. Activation of human somatostatin receptor 2 promotes apoptosis through a mechanism that is independent from induction of p53. Cell Physiol Biochem. 2002;12:31–8.
- Bates CM, Kegg H, Petrevski C, Grady S. Expression of somatostatin receptors 3, 4, and 5 in mouse kidney proximal tubules. Kidney Int. 2003;63:53–63.
- Raulf F, Perez J, Hoyer D, Bruns C. Differential expression of five somatostatin receptor subtypes, SSTR1-5, in the CNS and peripheral tissue. Digestion. 1994;55:46–53.
- Ludvigsen E, Stridsberg M, Taylor JE, Culler MD, Öberg K, Janson ET, et al. Regulation of insulin and glucagon secretion from rat pancreatic islets in vitro by somatostatin analogues. Regul Pept. 2007;138:1–9.
- Ludvigsen E. Somatostatin receptor expression and biological functions in endocrine pancreatic cells. Ups J Med Sci. 2007;112:1–20.
- Bodenant C, Leroux P, Vaudry H. Localization of somatostatin receptors in subcortical visual centres of the rat during development. Effect of neonatal enucleation on the expression of somatostatin receptors. Neuroscience. 1993;53:1097–102.
- Gonzalez BJ, Leroux P, Bodenant C, Laquerriere A, Coy DH, Vaudry H. Ontogeny of somatostatin receptors in the rat brain: biochemical and autoradiographic study. Neuroscience. 1989;29:629–44.
- Maubert E, Slama A, Ciofi P, Viollet C, Tramu G, Dupouy JP, et al. Developmental patterns of somatostatin-receptors and somatostatin-immunoreactivity during early neurogenesis in the rat. Neuroscience. 1994;62:317–25.
- Thoss VS, Perez J, Duc D, Hoyer D. Embryonic and postnatal mRNA distribution of five somatostatin receptor subtypes in the rat brain. Neuropharmacology. 1995;34:1673–88.
- Haiyan D, Wensheng L, Haoran L. Comparative analyses of sequence structure, evolution, and expression of four somatostatin receptors in orange-spotted grouper (Epinephelus coioides). Mol Cell Endocrinol. 2010;323:125–36.
- Ocampo Daza D, Sundström G, Bergqvist CA, Larhammar D. The evolution of vertebrate somatostatin receptors and their gene regions involves extensive chromosomal rearrangements. BMC Evol Biol. 2012;12:231.
- Ballian N, Brunicardi FC, Wang XP. Somatostatin and its receptors in the development of the endocrine pancreas. Pancreas. 2006;33:1–12.
- Jensen J. Gene regulatory factors in pancreatic development. Dev Dyn. 2004;229:176–200.
- Teitelman G, Alpert S, Polak JM, Martinez A, Hanahan D. Precursor cells of mouse endocrine pancreas coexpress insulin, glucagon and the neuronal proteins tyrosine hydroxylase and neuropeptide Y, but not pancreatic polypeptide. Development. 1993;118:1031–9.
- Eriksson U, Dahlström E, Larsson KS, Hellerström C. Increased incidence of congenital malformations in the offspring of diabetic rats and their prevention by maternal insulin therapy. Diabetes. 1982;31:1–6.
- Ludvigsen E, Olsson R, Stridsberg M, Janson ET, Sandler S. Expression and distribution of somatostatin receptor subtypes in the pancreatic islets of mice and rats. J Histochem Cytochem. 2004;52:391–400.
- Portela-Gomes GM, Stridsberg M, Grimelius L, Öberg K, Janson ET. Expression of the five different somatostatin receptor subtypes in endocrine cells of the pancreas. Appl Immunohistochem Mol Morphol. 2000;8:126–32.
- Reed DK, Korytko AI, Hipkin RW, Wehrenberg WB, Schonbrunn A, Cuttler L. Pituitary somatostatin receptor (sst)1-5 expression during rat development: age-dependent expression of sst2. Endocrinology. 1999;140:4739–44.
- Wang XP, Norman M, Yang J, Liu SH, Magnusson J, DeMayo FJ, et al. The effect of global SSTR5 gene ablation on the endocrine pancreas and glucose regulation in aging mice. J Surg Res. 2005;129:64–72.
- Adams RL, Adams IP, Lindow SW, Zhong W, Atkin SL. Somatostatin receptors 2 and 5 are preferentially expressed in proliferating endothelium. Br J Cancer. 2005;92:1493–8.
- Cristiani R, Petrucci C, Dal Monte M, Bagnoli P. Somatostatin (SRIF) and SRIF receptors in the mouse retina. Brain Res. 2002;936:1–14.
- Sprecher U, Mohr P, Martin RE, Maerki HP, Sanchez RA, Binggeli A, et al. Novel, non-peptidic somatostatin receptor subtype 5 antagonists improve glucose tolerance in rodents. Regul Pept. 2010;159:19–27.

