Abstract
Ipilimumab 3 mg/kg was the first agent to improve survival of pretreated advanced melanoma patients. Nonconventional response patterns to ipilimumab have been reported widely, but most of these data were from studies with ipilimumab 10 mg/kg. Here, case reports from five patients treated within an expanded access program (EAP) with ipilimumab at its licensed dose of 3 mg/kg illustrate the efficacy of ipilimumab in an expanded access setting and the range of different tumor response patterns encountered. The durable clinical benefit seen in these patients despite the observed atypical response patterns highlights the necessity for comprehensive clinical decision making.
INTRODUCTION
Patients with advanced melanoma have had a dismal prognosis for decades, especially once distant metastases have developed beyond the limits for surgical resection. Melanoma has a particular propensity to metastasize to the brain, with up to half of patients with metastatic disease having brain metastases and an associated poor prognosis (Citation1). Median overall survival (OS) for these patients is only a few weeks if left untreated, and even after treatment, remains around 4–5 months. Many such patients are excluded from experimental studies, and therefore, data on the efficacy of novel therapies in patients with intracranial metastases, extensive visceral spread and a history of extensive prior treatment are lacking.
Ipilimumab (Yervoy™, BMS), a fully human monoclonal antibody against cytotoxic T-lymphocyte-associated antigen-4 (CTLA-4), was the first agent to show improved survival of patients with metastatic melanoma in a phase 3 trial (Citation2). In this study; in patients with pretreated, metastatic melanoma, ipilimumab monotherapy (3 mg/kg every 3 weeks for 4 doses) significantly improved median OS (Hazard ratio: 0.66, p = .003) from 6.4 months in patients treated with the gp100 vaccine control to 10.1 months. Rates of OS in the ipilimumab-alone group and the gp100 group, respectively, were 45.6% and 25.3% at 12 months and 23.5% and 13.7% at 24 months. Based on these results, ipilimumab 3 mg/kg received marketing authorization from both the Food and Drug Administration and European Commission, and is therefore available for clinical use in the USA (for all patients with metastatic melanoma) and Europe (for pretreated patients only). Notably, the majority of the pivotal phase 3 study population had characteristics associated with poor prognosis; patients with pretreated, asymptomatic brain metastases were not excluded, over 70% of patients had visceral metastases and over a third had elevated lactate dehydrogenase (LDH) levels (Citation2). Interestingly, responses to ipilimumab continued to improve beyond Week 24; in the ipilimumab-alone group, two patients with stable disease (SD) improved to a partial response (PR) and three with a PR improved to a complete response (CR) (Citation2). Moreover, among 31 eligible patients who received ipilimumab retreatment, 61% achieved durable disease control (CR, PR, or SD) lasting more than 2 years (Citation3). Due to the small number of patients, however, further studies will be required to confirm the impact of retreatment on outcome.
Table 1 Immune-Related Response Criteria in Relation to Conventional Criteria
Delayed responses and response patterns to ipilimumab that differ from those seen with conventional chemotherapy have been reported at both 3 and 10 mg/kg doses in phase 2 studies, including a slow, steady decrease in tumor burden after SD, a decrease in total tumor burden after progressive disease (PD), and responses developing or improving beyond 12 weeks (Citation4–7). Multiple reports of patients who developed late-onset, often durable responses to ipilimumab, sometimes after apparent disease progression, led to the proposal of alternative response criteria termed immune-related response criteria (irRC). These are based in essence on World Health Organization (WHO) measures, differing only in as much as the first assessment is deferred to Week 12, new lesions are considered as a part of the total tumor burden and are not an automatic indicator of PD (as long as the patient's performance status (PS) does not decrease and the overall increase in tumor burden remains below 25%) and PD is confirmed by a subsequent radiological assessment at least 4 weeks later () (Citation8–13). In a retrospective analysis of pooled data from prospective studies with ipilimumab, superior OS was seen in patients who had PD according to modified WHO (mWHO) criteria but could be considered nonprogressors according to irRC (Citation13).
Conventionally, antitumor treatments are stopped when progression occurs according to defined response criteria. The distinct, unusual tumor response characteristics associated with ipilimumab therapy therefore have implications for clinical practice, as they challenge the way treatment decisions are made. As melanoma patients enrolled in clinical studies are not necessarily representative of a wider, more challenging demographic, findings from an expanded access program (EAP) are of interest. Patients enrolled in such programs are typically more extensively pretreated, with multiple metastatic loci, often including the central nervous system (CNS). Although atypical response patterns similar to those described above have been reported from a compassionate use setting, these patients were treated with ipilimumab at 10 mg/kg, that is, before the 3 mg/kg licensed dose had been decided (Citation14, 15).
Between April 1, 2010 and May 1, 2011, 50 advanced pretreated melanoma patients were treated in the EAP at our institution (UZ Brussel, Brussels, Belgium). These patients represented an unbiased selection of the patients eligible for treatment with ipilimumab at our institution during the first year when ipilimumab became available in the EAP. The global clinical outcome of this group of patients closely resembles the outcome of patients treated in a randomized phase 3 trial using the 3 mg/kg dosing regimen in patients with pretreated advanced melanoma (Citation2, Citation16). In this manuscript, we report the detailed clinical outcome of five individual cases that showed a range of atypical response patterns to ipilimumab at its licensed 3 mg/kg dose. We believe these patients to be representative of some of the more demanding and typical cases oncologists are likely to encounter in their daily practice using ipilimumab according to its label, and discuss the implications of our findings on the routine assessment of clinical response and patient management decisions.
CASE PRESENTATION
Case 1: Early Response to Therapy
Patient A was a 45-year-old man who had a successful excision of a primary melanoma on his back at which time he had no signs of locoregional or distant metastases, but who developed brain metastasis 5.5 years later, in February 2010. There were no sites of extracranial metastases. Adjuvant pancranial radiotherapy was administered. In March 2010, total body 18FDG-PET/CT scans revealed the appearance of 18FDG-avid lymph node, muscle, and lung metastases. First-line chemotherapy with dacarbazine was initiated. In July 2010, after three cycles of dacarbazine, repeat assessment showed PD of lymph node, muscle, lung, small intestine, and bone. There was no progression of disease within the CNS.
In August 2010 following referral to our hospital, Patient A provided signed informed consent for treatment with second-line ipilimumab 3 mg/kg every 3 weeks by intravenous infusion for a total of four doses in a European EAP. He had a baseline WHO PS of 1 and baseline laboratory tests showed a LDH level below the upper limit of normal (ULN) and slightly elevated C-reactive protein at 2 × ULN. Organ functions were normal. After his first dose of ipilimumab, he concomitantly received radiotherapy (30 Gy) for a symptomatic bone metastasis to the right knee. During ipilimumab treatment (every 3 weeks, for a total of 4 administrations), Patient A experienced grade 1 diarrhoea that resolved within 24 hr following therapy with loperamide, and grade 2 pruritus successfully palliated with a single 10 mg oral dose of cetirizine. At his first tumor response assessment at Week 12, Patient A had a documented PR by mWHO criteria. Only one muscular metastasis in the upper left leg, the irradiated skeletal metastasis in the right knee and a smaller bone metastasis in the right tibia remained 18FDG-avid; all other lesions had decreased significantly in size and had become 18FDG-negative (). An MRI of the brain confirmed that there was no progression in the CNS. There was also a marked increase in absolute lymphocyte count (ALC) compared to the baseline value (2371 vs. 1181/mm3, respectively).
Figure 1 Coronal maximal-intensity-projection PET whole body scans obtained at baseline and Weeks 12, 24, 38, and 50 during therapy with ipilimumab 3 mg/kg in Patient A. Melanoma metastases are indicated by red arrows. At baseline, scans showed metastatic muscular, lymph node, lung, intestinal, and bone lesions. A Week 12 assessment showed a rapid response with regression of most of the metastatic sites. Only a muscular metastasis in the upper left leg and the bone metastases in the right knee and tibia remained 18FDG-avid. At Week 24, there was a disappearance of the muscular upper left leg metastasis and the skeletal knee metastasis became 18FDG-negative. At Week 38, there was a reappearance of metastatic mediastinal lymph nodes (blue arrow) and the patient was given retreatment therapy with ipilimumab. Repeat scans after retreatment showed a stabilisation of the mediastinal lymph node and bone (right tibia) metastases.
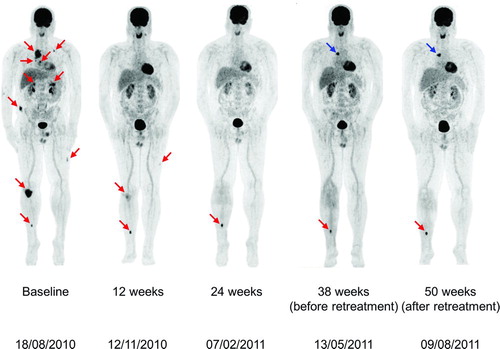
Tumor assessment at Week 24, in February 2011, confirmed the patient's PR, with complete disappearance of all muscular metastases. The bone metastasis in the right knee (which had been irradiated during treatment with ipilimumab) had also become 18FDG-negative (). A single metastasis to the right tibia had become more prominent on 18FDG-imaging; therefore a decision was taken to administer focal radiotherapy (30 Gy) to this residual solitary metastatic site. At Week 38, in May 2011, Patient A had reappearance of metastatic mediastinal lymph nodes but he remained asymptomatic with normal blood values and without progression in the CNS. He was given retreatment with ipilimumab and at repeat assessment in early August 2011, he was showing a stabilization of the mediastinal metastatic lymph nodes and the tibial bone metastasis. Mutation analysis of the patient's tumor material demonstrated the presence of a BRAFV600E mutation and the patient subsequently initiated treatment with a BRAF inhibitor in September 2011.
Case 2: Response After Progressive Disease
A 44-year-old man (Patient B), who in September 2007 had undergone a successful resection of a primary melanoma lesion on his back with no evidence of metastatic spread and no adjuvant treatment, subsequently developed liver metastasis in June 2008. He achieved CR after two cycles of dacarbazine and radiofrequency ablation of the liver lesion. A further 9 months later, however, he was diagnosed with two subcortical brain metastases and multiple metastases localized in the para-aortic lymph nodes and the lungs. He received radiosurgery for the brain metastases (1 × 20 Gy per lesion), but systemic chemotherapy for the other lesions with two cycles of dacarbazine was unsuccessful, with PD comprising significant growth of baseline lesions and appearance of new gluteal and mesenteric lymph node metastases. Patient B underwent second-line therapy in a phase 2 trial of oral sunitinib malate (Sutent®, Pfizer) 50 mg four times daily during which time he had stabilization of his extracranial metastases (Citation17). Four months after starting sunitinib, however, he developed two new brain lesions; 2 months later these had grown and were treated with radiosurgery (1 × 20 Gy per lesion). Although there was no further CNS progression, 8 months after starting sunitinib Patient B had progression of his known visceral metastases and developed two new metastases—a lesion in the quadriceps muscle and a paravertebral lesion.
Figure 2 (Top) Axial sections of total body 18FDG-PET/CT scans in Patient B at baseline and evaluation in Weeks 12, 24, and 33 following initiation of ipilimumab 3 mg/kg. (A) Baseline: presence of metastases in the quadriceps and hamstring muscles, mesenteric, duodenal, lung, and several mediastinal locations. Melanoma metastases are indicated by red circles. (B) Images at 12 weeks showing disappearance of metastases in the quadriceps and hamstring muscles, metabolic normalization of mesenteric metastases and disappearance of lung metastases; metabolic activity persisted in the duodenal lesion and two new mediastinal lymph node metastases and a new peri-hepatic lymph node metastasis appeared (blue circles). (C) Images at 24 weeks after initiation of ipilimumab showing disappearance of mediastinal and peri-hepatic lymph node metastases and persistent activity of the duodenal lesion. (D) At 33 weeks after initiation of ipilimumab, there was a continuing response of all previously active lesions with the exception of persistence of 18FDG-uptake in the duodenal lesion. (Bottom) Corresponding coronal maximal-intensity-projection PET whole body scans of Patient B at baseline and subsequent assessments.
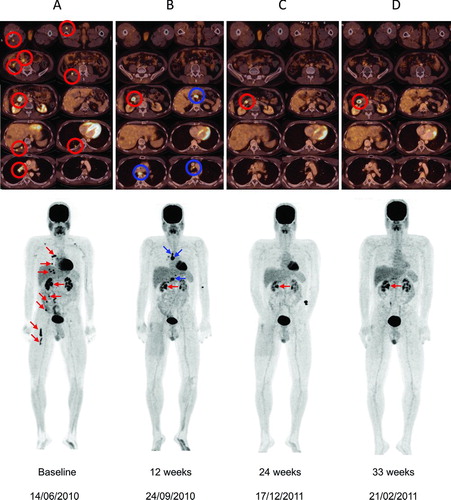
After giving written informed consent, Patient B was entered into a European EAP and received third-line systemic therapy with intravenous infusion of ipilimumab 3 mg/kg every 3 weeks for a total of four doses. After the first ipilimumab administration, the patient received radiotherapy for his symptomatic paravertebral and muscular (quadriceps) lesion. At first evaluation 12 weeks after starting ipilimumab, a new 18FDG-PET/CT scan revealed normalization of 18FDG-uptake of most metastases and complete regression of muscle metastases (). A single duodenal metastasis was persistently 18FDG-avid without radiological evidence for growth but new metastatic localizations affecting the mediastinal lymph nodes and a peri-hepatic lymph node were found. According to conventional mWHO tumor response criteria or Response Evaluation Criteria in Solid Tumors (RECIST), Patient B had PD but qualified for SD according to irRC as his overall burden of disease had decreased. Given this patient's preserved general well-being, absence of biological evidence of endangered organ function and decrease in total tumor burden, we believed that an atypical response to ipilimumab was still possible and therefore, no alternative therapy was initiated. During ipilimumab treatment, patient B showed an initial decrease of his ALC (from 1,232 to 695/mm3). This was possibly influenced by the administered radiotherapy for the symptomatic paravertebral and quadriceps lesion between the first and second iplimumab dose. At Week 9, there was a partial recovery of the lymphodepletion and the ALC was 1044/mm3. On repeat assessment 24 weeks after he started ipilimumab, Patient B had complete regression of his 18FDG-avid mediastinal lymph node metastases and confirmed complete regression in all other baseline lesions except for the persistently 18FDG-avid duodenal lesion which remained stable ().
Figure 3 (Top) Axial sections of total body 18FDG-PET/CT scans in Patient C. Views A1 and B1 show 18FDG-avid right retroclavicular and retroperitoneal lymph node metastases. (Bottom) Corresponding coronal maximal-intensity-projection PET images at baseline and subsequent assessments. Baseline scan shows 18FDG-avid right retroclavicular and retroperitoneal lymph node metastases. At 12 weeks after starting treatment with ipilimumab 3 mg/kg, scan showed normalization of both the retroclavicular lesion and two out of four retroperitoneal lymph node metastases (red arrows) with simultaneous growth of the other two retroperitoneal lymph node metastases (blue arrows). At Week 24, there was a regression of all lymph node metastases, resulting in a CR at Week 36.
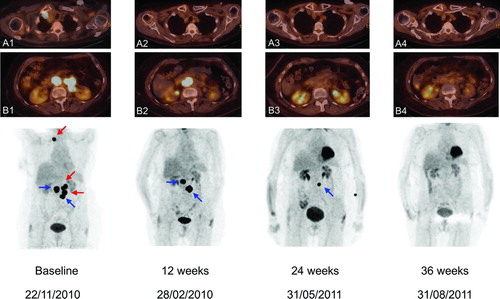
A control 18FDG-PET/CT scan obtained 33 weeks after the start of ipilimumab treatment showed no residual 18FDG uptake at any metastases with the exception of a single lesion located in the duodenum (). The patient remained clinically stable except for an increase of frontal headaches. An MRI of the brain revealed the reappearance of a lesion anterior in the right frontal lobe (at the exact same anatomical localization where the irradiated frontal metastasis had been) with important surrounding edema. The additional lesions within the brain had remained stable and there was no evidence of new metastases within the CNS. A neurosurgical resection of that frontal lesion was performed and the histopathological examination revealed the presence of radiation necrosis (Citation18). Following this neurosurgical resection, Patient B's clinical course remained uneventful (WHO PS 0) and he required no further antimelanoma therapy up to latest follow-up 56 weeks after the initiation of ipilimumab. Following documented persistent nonprogressive 18FDG-uptake of the single duodenal lesion in August 2011, the patient requested not to undergo further endoscopic investigation of this asymptomatic lesion.
Case 3: Gradual/Delayed Response
Patient C was an elderly woman (aged 74 years) who had undergone resection of a mucosal melanoma of the left maxillary sinus in November 2005 followed by a course of adjuvant radiotherapy (30 Gy) 6 weeks postoperatively. In September 2006, a MRI scan of her head and neck showed a pathologic lymph node on the left side. She underwent a radical left lymph node dissection which revealed 9/44 metastatic nodes. Four years later, she presented with PD with appearance of 18FDG-avid right retroclavicular and retroperitoneal (aorto-caval and para-aortic) lymph node metastases (). A c-Kit mutation analysis was negative and the patient was started on first-line therapy with dacarbazine (1,000 mg/m2) and received two cycles over the next 2 months. After completion of chemotherapy in November 2010, she had an increase in diameter of the metastatic retroclavicular and retroperitoneal lymph nodes and was assessed as having PD (by RECIST criteria).
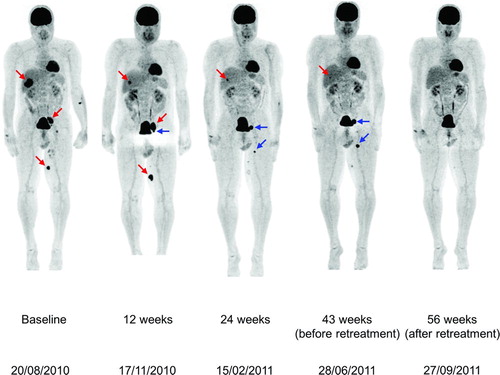
Figure 4 Coronal maximal-intensity-projection PET whole body scans obtained at baseline and Weeks 12, 24, 43, and 56 during therapy with ipilimumab 3 mg/kg in patient D. At baseline, before he started ipilimumab 3 mg/kg, there were 18FDG-avid metastases in the liver, para-iliac lymph node and distally in the inner left upper leg (red arrows). At the first evaluation in Week 12, there was regression of the liver metastasis but increases in the dimensions of (and 18FDG uptake in) the para-iliac lymph node and distal upper leg metastases and the appearance of a new iliaco-femoral lymph node metastatic lesion (blue arrows). Evaluation in Week 24 showed further regression of the liver metastasis and regression of the para-iliac metastasis and the distal metastasis in the left leg with complete loss of 18FDG uptake in these two lesions; there was an increase in size and 18FDG uptake in the iliaco-femoral metastasis and appearance of a new proximal lesion in the left leg. Immediately before retreatment, there was near-complete regression of the liver and para-iliac metastases but further progression of the other three lesions. After retreatment, all lesions further regressed in size with complete loss of 18FDG uptake.
In December 2010, Patient C was started on treatment with ipilimumab 3 mg/kg. An imaging series is shown in . At 12 weeks following treatment, scans showed normalization of the retroclavicular lymph node metastasis and two out of four retroperitoneal lymph node metastases; however, there was an increase in diameter of the other two retroperitoneal lymph node metastases. Repeat scans at 24 weeks showed regression of all retroperitoneal lymph node metastases and loss of 18FDG-uptake, except for one retroperitoneal lesion. At 36 weeks after she started on ipilimumab 3 mg/kg, scans showed disappearance of all 18FDG-avid lymph node metastases and the patient had achieved a CR (by both RECIST and irRC). Her LDH levels decreased from 696 U/L at the start of ipilimumab therapy to 484 U/L at 36 weeks while her ALC rose during therapy, from 1,218/mm3 at the start peaking at 2,488/mm3 approximately 2 weeks after the 4th ipilimumab dose, that is, at Week 11. During therapy with ipilimumab the patient's only adverse event was grade 1 pruritus, treated with oral antihistamines.
Case 4: Initial New Lesions with Superior Response on Retreatment
A 61-year-old man (Patient D) had presented with an acral ulcerated melanoma on the third toe of his left foot in January 2009. In February, the affected toe was surgically removed and a sentinel node biopsy showed evidence of metastasis. The following month, a left iliaco-inguinal lymphadenectomy did not show any additional metastatic nodes and the final pathologic staging was pT4bN1Mx. One year and 2 months later, in April 2010, follow-up PET-CT scans revealed stage IV-M1c disease with liver, lymph node and subcutaneous metastases. Patient D started first-line dacarbazine (1 g/m2 every 3 weeks) in July of 2010 but on August 20, after two cycles of chemotherapy, he was assessed as having PD (by RECIST criteria).
Between August 27 and October 29, 2010 Patient D received ipilimumab 3 mg/kg in a European EAP. At baseline he had 18FDG-avid metastases in the liver, para-iliac lymph node and distally in the inner left upper leg (). At Patient D's first evaluation in Week 12, he had regression of the liver metastasis but increases in both the dimensions and 18FDG uptake in the para-iliac and inner left upper leg distal lesions. There was also the appearance of a new iliaco-femoral lymph node metastasis. This corresponded to an assessment of PD by mWHO criteria or RECIST, but qualified for SD by irRC. Evaluation in Week 24 showed further regression of the liver metastasis and regression of the para-iliac metastasis and distal metastasis in the left leg accompanied by complete loss of 18FDG uptake. At the same time, there was an increase in size and 18FDG uptake in the iliaco-femoral lymph node metastasis and the appearance of a new proximal lesion in the inner left upper leg. Although the patient still had PD by conventional response criteria, he now qualified for a PR by irRC. Before the start of retreatment, this patient had near-complete regression of the liver and para-iliac metastases but further progression of the iliaco-femoral, inner left upper leg distal and inner left upper leg proximal lesions (). His ALC increased after treatment peaking at approximately 3,000/mm3 at Week 20.
Figure 5 (A) In-transit melanoma metastases on the right leg prior to therapy with ipilimumab 3 mg/kg. (B) During the first weeks of treatment, there was an increase in size and number of the right leg in-transit lesions. C. Two months after starting ipilimumab therapy, the in-transit metastases slowly regressed and all disappeared (as illustrated 1 year after ipilimumab treatment).
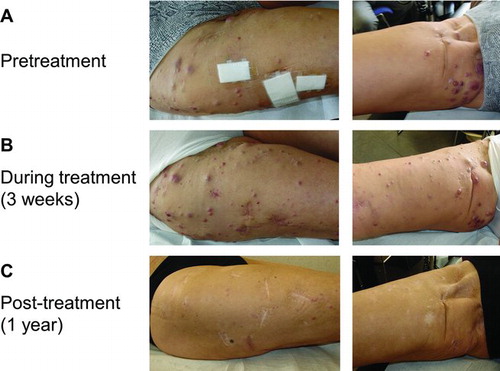
Patient D had normal blood values prior to and during ipilimumab treatment but did experience grade 1 pruritus, treated with oral antihistamines. After the end of ipilimumab therapy, he developed vitiligo of the face. Retreatment therapy with ipilimumab 3 mg/kg was started on July 8, 2011. At his first evaluation after retreatment (September 27, 2011), all lesions further regressed in size, with a complete loss of 18FDG uptake. The patient's clinical status (Karnofsky PS score = 100%) and laboratory values remained normal throughout treatment with ipilimumab.
Case 5: Mixed Response with Response on Retreatment
Patient E, a 67-year-old woman, underwent resection of an acrolentiginous melanoma from the sole of her right foot in March 2008, at which time she had a positive popliteal and inguinal sentinel-node biopsy. In May 2008, a popliteal-inguinal-crural and ilio-obturator right lymphadenectomy was performed and melanoma metastases were found in two additional lymph nodes. In August 2008, she was recruited into a phase 1 pilot clinical trial of intradermal autologous mRNA electroporated dendritic cells (TriMix-DC) and additional interferon-alpha-2b (5 MU subcutaneously 3 times weekly) but on February 20, 2009; she had further unresectable in-transit metastases in her right leg. On May 6, 2009; patient E underwent limb perfusion with melphalan, tumor necrosis factor-alpha and interferon-gamma and on May 28, 2009; she had a PR of the in-transit metastases in her right leg by both mWHO response criteria and RECIST. In November 2009, however, this patient had PD with in-transit metastases in her right leg () and inguinal and peri-iliacal lymph node metastases. She was started on chemotherapy with dacarbazine on March 15, 2010 but in May 2010, after three cycles of chemotherapy, she had PD.
On June 11, 2010; Patient E started ipilimumab 3 mg/kg in the EAP. During the first weeks of ipilimumab treatment, there was an increase in size and number of the in-transit metastases in her right leg (). From Week 8, there was a slow regression of the in-transit metastases that resulted in complete disappearance at 1 year (). Patient E had a modest increase of her absolute lymphocyte count during ipilimumab treatment (from 1,648/mm3 at baseline to 2,052/mm3 at the 4th ipilimumab dose). Meanwhile, PET-CT imaging in February 2011, 34 weeks after starting ipilimumab, showed PD of the inguinal and peri-iliacal lymph nodes and the patient started retreatment therapy with ipilimumab 3 mg/kg on February 21. A repeat assessment 10 weeks after retreatment showed a regression of the metastatic lymph nodes which qualified for SD by all tumor response criteria, but with a marked regression (33% decrease by mWHO criteria). A second assessment in Week 26 after retreatment showed a further regression of the inguinal and peri-iliacal lymph nodes, which was classified as a PR according to both mWHO criteria and RECIST. She had no adverse-events during ipilimumab treatment. At further follow-up in November 2011 (Week 38 after retreatment), progressive disease of the iliacal lymph nodes and new in-transit metastases in the right leg were documented and the patient was subsequently treated with a selective BRAF-inhibitor (vemurafenib).
DISCUSSION
These five cases illustrate the activity of ipilimumab in patients with pretreated metastatic melanoma. All five cases had failed on previous chemotherapy and two (B and E) had also failed a second line systemic therapy; in these patients, therefore, ipilimumab was administered as a third-line therapy for metastatic disease. In addition, the five cases were characterized according to variable poor prognostic features, including male gender (Patients A, B, and D), older age (Patients C, D, and E), CNS metastases (Patients A and B), multiple visceral metastases (Patients A–D), and elevated baseline LDH (Patient C) (Citation19–24). Despite having these poor prognostic baseline factors and some having been heavily pretreated, all five patients showed an objective tumor response and derived clinical benefit from treatment with ipilimumab at a dose of 3 mg/kg. The best overall responses achieved were three PRs (Patients A, B, and E) and two CRs (Patients C and D); therefore, our cases are consistent with the findings from the pivotal phase 3 trial whereby the performance of ipilimumab within relevant prognostic subgroups was consistent with the survival advantage demonstrated for the overall study population (Citation2).
Of interest is the observation that Patients D and E had improved responses following retreatment. Patient D, who had had prolonged disease control (for over 10 months) after treatment with ipilimumab achieved a CR upon retreatment and Patient E, who had a mixed response (regression in lower limb in-transit metastases with progression in nodal metastases), achieved a PR following retreatment. This is consistent with a subanalysis of patients from the phase 3 study which showed that retreatment in eligible patients increased the likelihood of obtaining a better overall response (Citation3).
As with the pivotal study, the observed responses were also found to be durable, and a significant percentage of patients continued to improve beyond their second clinical assessment. The CR achieved by Patient C, for example, was attained 36 weeks after treatment with ipilimumab was initiated, and was associated with a concomitant decline in elevated LDH.
In terms of tumor response patterns, these were also consistent with the range of different and atypical response patterns reported for ipilimumab at 3 and 10 mg/kg doses from both phase 3 and phase 2 studies (Citation2, Citation4–7). Our five patients showed the following variations: an early response akin to traditional chemotherapy responses (Patient A); a response after apparent disease progression (Patient B), a slowly building/delayed response (Patient C); initial new lesions with a CR on retreatment (Patient D), and a mixed response followed by gradual development of a PR after retreatment (Patient E).
It is possible that progressive disease during initial ipilimumab therapy in Patients B and E reflected tumor enlargement and subsequent inflammation as a result of T-cell infiltration and potentiation, respectively, rather than true disease progression resulting solely from tumor cell proliferation (Citation25, 26). Likewise, previously undetectable lesions may have become visible and manifested as newly arising lesions (Patient B) while existing lesions may have appeared to grow (Patient E). This possibility is supported by Patient B having a reduction in his total tumor burden by irRC while being classified as having PD, and Patient E showing a mixed response with her in-transit metastases reducing while her nodal metastases increased, suggesting that some antitumor activity of ipilimumab had started quite early in both patients. In patient E, some covert antitumor activity of ipilimumab post-treatment is also supported by her subsequent response after retreatment; previous analysis has shown that response after retreatment is rare in patients who have not responded initially to treatment with ipilimumab; indeed, a response to ipilimumab treatment is a qualifying criterion for retreatment eligibility (Citation3).
Interestingly, three of the patients reported here had a marked increase in ALC during treatment. In patient A, this coincided with his response at 12 weeks, in Patient C, it predated a gradual evolution of her response and in Patient D ALC increased gradually after both treatment and retreatment. Lymphopenia has been shown to be a negative prognostic factor in various advanced solid cancers (Citation27) and higher ALC has been correlated previously with clinical benefit and survival in patients with metastatic melanoma receiving ipilimumab 10 mg/kg in a compassionate use setting (Citation14) It is likely that patients must have a responsive immune system before they can benefit from immunotherapy, including ipilimumab retreatment (Citation28). In some patients, such as Patients D and E, retreatment may be required to give the immune system an extra “boost” before disease stabilisation can be converted to disease reduction or elimination or a clinical response can be detected.
In phase 3, retreatment with ipilimumab was often more effective than the initial treatment in the same patient, irrespective of poor prognostic indicators (Citation3). Both slowly building response (as in Patient C) and mixed responses (as in Patient E) could be a reflection of the intricate dynamics between the patient's immune response and their tumor (Citation28). Tumors can be antigenically heterogeneous both within and between individual lesions in the same patient, and as lesions grow or new ones arise time may be needed for clonal expansion of antigen-specific T cells to occur and for these cells to become potentiated by ipilimumab. Sometimes, a retreatment cycle may be needed for this to happen. In patient D, for example, the development of vitiligo by the end of the treatment cycle may have signaled a robust immune response which was subsequently consolidated during retreatment. Vitiligo is beginning to be seen as reflection of T-cell responsiveness against melanocytic differentiation antigens in melanoma (Citation29, 30).
CONCLUSIONS
The five patients discussed herein would not have qualified for enrolment in many conventional clinical studies and their options were few, if any, hence their inclusion in an EAP on compassionate grounds. These five cases show that in the compassionate use setting in heavily pretreated patients with more than one negative prognostic indicator, ipilimumab 3 mg/kg can provide clinical benefit and was generally well tolerated; these patients had adverse events that were mild-to-moderate and could be managed with conventional therapies. Moreover, their differing atypical tumor response patterns illustrate the importance of a comprehensive assessment of the clinical benefit before abandoning or switching therapy in patients on ipilimumab prematurely, and certainly allowing sufficient time for a response to occur before doing so as long as the patient's general condition and well-being permit this. Certainly, confirmation of PD is important but equally, clinical judgment must play a pivotal role when treating these poorly served patients.
DECLARATION OF INTEREST
Bart Neyns has received financial compensation from Bristol-Myers Squibb for providing expert opinion. The other authors declare that they have no competing interests.
ACKNOWLEDGMENTS
We thank the patients and their families; Katrien Van den Bossche and Cindy Aerts for data management. We confirm that the content of this publication accurately reflects our viewpoint and medical expertise. We wish to acknowledge that StemScientific, funded by Bristol-Myers Squibb, provided writing and editorial support. Neither Bristol-Myers Squibb nor StemScientific influenced the content of the manuscript, nor did the authors receive financial compensation for authoring the manuscript. Sofie Wilgenhof is a PhD fellow of the FWO-Vlaanderen and Stephanie Du Four is a PhD fellow of the Vlaamse Liga Tegen Kanker.
REFERENCES
- Margolin KA, Di Giacomo AM, Maio M. Brain metastasis in melanoma: clinical activity of CTLA-4 antibody therapy. Semin Oncol October 2010;37(5):468–472.
- Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJM, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbé C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med August 2010;363(8):711–723.
- Robert C, Hodi FS, O'Day SJ, Peschel C, Ottensmeier CH, Trefzer U, Lorigan P, Messina M, Ibrahim R, Schadendorf D. Re-induction with ipilimumab, gp100 peptide vaccine, or a combination of both in a phase III study of previously-treated patients with advanced melanoma: update of clinical characteristics of patients. Ann Oncol 2010;21(viii403):1330.
- O'Day SJ, Maio M, Chiarion-Sileni V, Gajewski TF, Pehamberger H, Bondarenko IN, Queirolo P, Lundgren L, Mikhailov S, Roman L, Verschraegen C, Humphrey R, Ibrahim R, de Pril V, Hoos A, Wolchok JD. Efficacy and safety of ipilimumab monotherapy in patients with pretreated advanced melanoma: a multicenter single-arm phase II study. Ann Oncol August 2010;21(8):1712–1717.
- Weber J, Thompson JA, Hamid O, Minor D, Amin A, Ron I, Ridolfi R, Assi H, Maraveyas A, Berman D, Siegel J, O'Day SJ. A randomized, double-blind, placebo-controlled, phase II study comparing the tolerability and efficacy of ipilimumab administered with or without prophylactic budesonide in patients with unresectable stage III or IV melanoma. Clin Cancer Res September 1, 2009;15(17):5591–5598.
- Wolchok JD, Neyns B, Linette G, Negrier S, Lutzky J, Thomas L, Waterfield W, Schadendorf D, Smylie M, Guthrie T, Jr., Grob JJ, Chesney J, Chin K, Chen K, Hoos A, O'Day SJ, Lebbe C. Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol February 2010;11(2): 155–164.
- Hersh EM, O'Day SJ, Powderly J, Khan KD, Pavlick AC, Cranmer LD, Samlowski WE, Nichol GM, Yellin MJ, Weber JS. A phase II multicenter study of ipilimumab with or without dacarbazine in chemotherapy-naive patients with advanced melanoma. Invest New Drugs Junuary 2011;29(3):489–498.
- Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. January 2009;45(2):228–247.
- James K, Eisenhauer E, Christian M, Terenziani M, Vena D, Muldal A, Therasse P. Measuring response in solid tumors: unidimensional versus bidimensional measurement. J Natl Cancer Inst. March 17, 1999;91(6):523–528.
- Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer. January 1, 1981;47(1):207–214.
- Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG. New guidelines to evaluate the response to treatment in solid tumors. European organization for research and treatment of cancer, national cancer institute of the United States, national cancer institute of Canada. J Natl Cancer Inst February 2, 2000;92(3):205–216.
- Weber J. Ipilimumab: Controversies in its development, utility and autoimmune adverse events. Cancer Immunol Immunother May 2009;58(5):823–830.
- Wolchok JD, Hoos A, O'Day S, Weber JS, Hamid O, Lebbé C, Maio M, Binder M, Bohnsack O, Nichol G, Humphrey R, Hodi FS. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res December 2009;15(23):7412–7420.
- Ku GY, Yuan J, Page DB, Schroeder SE, Panageas KS, Carvajal RD, Chapman PB, Schwartz GK, Allison JP, Wolchok JD. Single-institution experience with ipilimumab in advanced melanoma patients in the compassionate use setting: lymphocyte count after 2 doses correlates with survival. Cancer April 1, 2010;116(7):1767–1775.
- Di Giacomo AM, Danielli R, Calabro L, Bertocci E, Nannicini C, Giannarelli D, Balestrazzi A, Vigni F, Riversi V, Miracco C, Biagioli M, Altomonte M, Maio M. Ipilimumab experience in heavily pretreated patients with melanoma in an expanded access program at the University Hospital of Siena (Italy). Cancer Immunol Immunother April 2011;60(4):467–477.
- Wilgenhof S, Seghers AC, Du Four S, Lienard D, Salmon I, Del Marmol V, Neyns B. Single-center experience with ipilimumab in patients with pretreated advanced melanoma. ASCO Meeting Abstracts May 30, 2012;30(15_suppl):e19026.
- Decoster L, Neyns B, Vande Broek I, Anckaert E, De Clerck D, De Mey J, Majois F, Baurain J, Denys H, De Greve J. Activity of sunitinib in advanced malignant melanoma and its correlation with potential predictive biomarkers. ASCO Meeting Abstracts June 14, 2010;28(15_suppl):8518.
- Du Four S, Wilgenhof S, Duerinck J, Michotte A, Binst AV, Ridder MD, Neyns B. Radiation necrosis of the brain in melanoma patients successfully treated with ipilimumab, three case studies. Eur J Cancer June 22, 2012.
- Manola J, Atkins M, Ibrahim J, Kirkwood J. Prognostic factors in metastatic melanoma: a pooled analysis of Eastern Cooperative Oncology Group trials. J Clin Oncol November 15, 2000;18(22):3782–3793.
- Balch CM, Gershenwald JE, Soong SJ, Thompson JF, Atkins MB, Byrd DR, Buzaid AC, Cochran AJ, Coit DG, Ding S, Eggermont AM, Flaherty KT, Gimotty PA, Kirkwood JM, McMasters KM, Mihm MC, Jr., Morton DL, Ross MI, Sober AJ, Sondak VK. Final Version of 2009 AJCC Melanoma Staging and Classification. J Clin Oncol November 2009.;27:6199–6206.
- Staudt M, Lasithiotakis K, Leiter U, Meier F, Eigentler T, Bamberg M, Tatagiba M, Brossart P, Garbe C. Determinants of survival in patients with brain metastases from cutaneous melanoma. Br J Cancer April 13, 2010;102(8):1213–1218.
- Gupta G, Robertson AG, MacKie RM. Cerebral metastases of cutaneous melanoma. Br J Cancer 1997;76(2):256–259.
- Lasithiotakis K, Leiter U, Meier F, Eigentler T, Metzler G, Moehrle M, Breuninger H, Garbe C. Age and gender are significant independent predictors of survival in primary cutaneous melanoma. Cancer April 15, 2008;112(8):1795–1804.
- Minor DR, Moore D, Kim C, Kashani-Sabet M, Venna SS, Wang W, Boasberg P, O'Day S. Prognostic factors in metastatic melanoma patients treated with biochemotherapy and maintenance immunotherapy. Oncologist October 2009;14(10):995–1002.
- Hodi FS, Butler M, Oble DA, Seiden MV, Haluska FG, Kruse A, MacRae S, Nelson M, Canning C, Lowy I, Korman A, Lautz D, Russell S, Jaklitsch MT, Ramaiya N, Chen TC, Neuberg D, Allison JP, Mihm MC, Dranoff G. Immunologic and clinical effects of antibody blockade of cytotoxic T lymphocyte-associated antigen 4 in previously vaccinated cancer patients. Proc Natl Acad Sci USA February 2008;105(8):3005–3010.
- Ribas A, Chmielowski B, Glaspy JA. Do we need a different set of response assessment criteria for tumor immunotherapy? Clin Cancer Res December 1, 2009;15(23):7116–7118.
- Ray-Coquard I, Cropet C, Van Glabbeke M, Sebban C, Le Cesne A, Judson I, Tredan O, Verweij J, Biron P, Labidi I, Guastalla JP, Bachelot T, Perol D, Chabaud S, Hogendoorn PC, Cassier P, Dufresne A, Blay JY. Lymphopenia as a prognostic factor for overall survival in advanced carcinomas, sarcomas, and lymphomas. Cancer Res July 1, 2009;69(13):5383–5391.
- Wang E, Panelli MC, Marincola FM. Understanding the response to immunotherapy in humans. Springer Semin Immunopathol June 2005;27(1):105–117.
- Byrne KT, Turk MJ. New perspectives on the role of vitiligo in immune responses to melanoma. Oncotarget September 2011;2(9):684–694.
- Chang GY, Kohrt HE, Stuge TB, Schwartz EJ, Weber JS, Lee PP. Cytotoxic T lymphocyte responses against melanocytes and melanoma. J Transl Med 2011;9(1):122–131.
