Abstract
Individuals differ in their preferred timing of sleep and activity, which is referred to as a chronotype. The timing shows a wide distribution; extremely early chronotypes may wake up when the extremely late chronotypes fall asleep. The chronotype is supposed to be determined by the central circadian clock located in the suprachiasmatic nuclei (SCN) of the hypothalamus because the phasing of the pineal melatonin rhythm, which is driven by the SCN, correlates with the sleep timing preference. In addition to the SCN, circadian oscillators are also present in most if not all bodily cells. These peripheral clocks are synchronized by the central SCN clock and by other tissue-specific entraining cues. At the molecular level, the circadian oscillations are based on a complex, self-sustaining mechanism that drives the rhythmical expression of clock genes and their proteins. The aim of the present field study was to elucidate whether the changes in the internal timing of early and late chronotypes, as expressed by changes in the phases of their mid-sleep and melatonin secretion, can also be detected at the molecular clockwork level in subjects examined under real-life conditions. Ninety-five adult volunteers were chronotyped using an adapted Munich chronotype questionnaire to assess their mid-sleep phase, and 6 subjects with early chronotypes and 6 with late chronotypes were chosen for the study. For the assessment of the circadian phase, the subjects provided samples of saliva for the melatonin assay and samples of oral mucosa for the determination of clock gene Per1, Per2, and Rev-erbα mRNA levels every 4 h during a 24-h period. The significant correlation between the phase of the melatonin profile and timing of mid-sleep confirmed the classification of the subjects according to their chronotype. The circadian phases of the Per1, Per2, and Rev-erbα expression profiles in the oral mucosa were advanced in the early chronotypes compared with those in the late chronotypes (p < .001) and correlated significantly with the mid-sleep phase of the individual subjects. Moreover, the circadian phases of the Per1 expression profiles of individual subjects correlated significantly with the phases of their melatonin profiles (p < .05), whereas the correlation for the Per2 and Rev-erbα phases was nonsignificant, although the trend was the same. Our results demonstrate that the individual chronotype in humans living in real-life conditions affects not only the phasing of the daily melatonin rhythm in saliva but also the phasing of Per1, Per2, and Rev-erbα clock gene expression profiles in buccal mucosa cells. This report represents the first demonstration that the human peripheral circadian clock may sense the individual's chronotype under field study conditions. The data contribute to our understanding of the mechanisms underlying human chronotypes in real life. (Author correspondence: [email protected])
INTRODUCTION
Human physiology and behavior are under temporal control by an internal timekeeping system, which self-autonomously maintains rhythms of these functions running with an about a day, i.e., circadian, period. The persistence of these rhythms does not require changes in daylight and if subjects are maintained under constant conditions, their rhythms free run in line with the endogenous period, which may differ among individuals and typically ranges from 23.47 to 24.64 h (Carskadon et al., 1999; Czeisler et al., 1999; Dijk et al., 2000; Wyatt et al., 1999). In real-life conditions that allow individuals to perceive daily cycles of daylight, the rhythms are entrained to the solar day and run with a period of exactly 24 h. Due to the entrainment, various rhythmic functions are selectively phased relative to the daytime so that some of them peak at night and others during the day. The central oscillator that drives behavioral and humoral rhythms in mammals, including humans, is located in the suprachiasmatic nuclei (SCN) of the hypothalamus (Ralph et al., 1990). The SCN receives information concerning environmental light directly from the retinas and entrains its rhythmicity accordingly. Evening light, which intrudes into the early subjective night in individuals with shorter periods, phase delays the clock, whereas morning light, which intrudes into the late subjective night in individuals with longer periods, phase advances the clock (Pittendrigh, 1981). Therefore, subjects that are exposed to solar cycles should all be similarly entrained to the daytime. However, extensive research on human circadian behavior revealed that individuals differ significantly in their specific temporal relationship to the external light/dark cycle (for a review see Roenneberg et al., 2007). Although exposed to similar environmental lighting conditions, some individuals prefer going to bed earlier and some later than others, and they are thus referred to as various chronotypes. In fact, the differences are so significant that individuals of extreme chronotypes may not even meet one another, because when the very early chronotypes wake up, the very late ones fall asleep. The mechanism underlying this aspect of human entrainment has not yet been fully understood. It has been suggested that chronotype is likely associated with the endogenous period length because individuals whose clock is running with longer periods tend to be later chronotypes (Brown et al., 2005, 2008; Duffy et al., 2001; Emens et al., 2009; Pagani et al., 2010), but likely also with other properties of the clock driving the circadian rhythms, such as amplitude (Brown et al., 2008). The strength of the cue entraining the clock, i.e., the actual exposure to light, also plays an important role in maintaining the clock in a proper phase relationship with the external light/dark cycle (Roenneberg & Merrow, 2007). It appears that not only environmental but also social and genetic factors may influence the chronotype (Archer et al., 2003, 2008; Barclay et al., 2010; Carpen et al., 2005; Osland et al., 2011; Toh et al., 2001; Vanselow et al., 2006). Moreover, the chronotype is not uniform throughout life but changes remarkably, being later during the early adulthood and earlier during the childhood and in the elderly (Roenneberg et al., 2004, 2007). The chronotype also appears to be dependent on gender because in general, men tend to be later chronotypes than women (Randler, 2011a; Roenneberg & Merrow, 2007). Therefore, the factors determining an individual's chronotype might be complex.
The contribution of the circadian clock to chronotype was demonstrated in studies in which the subject's preference for sleep timing was mostly correlated with the profiles of the pineal hormone melatonin secreted into bodily fluids, such as blood or saliva (Burgess & Fogg, 2008; Duffy et al., 1999, 2001; Roemer et al., 2003). Melatonin has been a marker of choice for the phase of the circadian clock, because its production and secretion from the pineal is under direct control of the SCN (Illnerová, 1991). However, melatonin synthesis is also sensitive to light because light exposure of humans may suppress elevated nocturnal melatonin levels in a dose-dependent manner (Lewy et al., 1980; Zeitzer et al., 2000). Advances in the understanding of the circadian system, especially the finding that apart from the SCN cells, other bodily cells are also genetically equipped to generate circadian rhythms (for review see Hastings et al., 2003), have allowed an exploration of the molecular mechanisms of the circadian clock in human biopsy samples. The mechanism of rhythm generation is based on interactions of the protein products of clock genes, i.e., the genes essential for clockwork, with promoters of their own or other clock genes, thereby activating or inhibiting their expression. Together with posttranslational modifications of these proteins, the mechanism results in circadian rhythm in expression of clock genes and levels of their proteins (for review see Takahashi et al., 2008). The mechanism appears to be conserved, with little deviation in the SCN and peripheral bodily clocks. The peripheral clocks receive entraining signals from the SCN and, therefore, the periods and phases of the peripheral clocks are determined by the central clock. In humans, the period of the central SCN clock, as determined by the period of the melatonin rhythm in saliva, has been shown to be directly proportional to the period of the peripheral circadian clock in fibroblasts (Pagani et al., 2010). However, in another study, the in vivo period assessment by melatonin rhythm did not correlate with the in vitro period of the individual's fibroblast clock (Hasan et al., 2012). It is possible that in some circumstances, in vitro conditions may affect the basic properties of the cellular clock, including its period and particularly its phase. Therefore, for the determination of the phase, in vivo experiments appear to be more appropriate. In a detailed and well-controlled study, a correlation was demonstrated between the phase of leukocyte Per3 clock gene rhythmic expression and the timing of sleep/wake cycle and plasma melatonin levels, whereas the correlation of these variables with Per2 and Bmal1 expression profiles was not significant (Archer et al., 2008). Other studies have also demonstrated a correlation between the phases of Per1, Per2, and Dec1 gene expression in peripheral blood mononuclear cells and melatonin profile (Boivin et al., 2003; Kusanagi et al., 2004). All these studies were performed under a constant routine protocol, which unmasks any direct effects of the external environment on the endogenous properties of the clocks and did not study these correlations in chronotypes. In real-life conditions, melatonin profiles were also proven to be a valuable marker of the choronotype because even in these conditions, the hormone synthesis was advanced in the early chronotypes compared with the late chronotypes (Burgess & Fogg, 2008; Duffy et al., 1999, 2001; Roemer et al., 2003). However, data on the modulation of the molecular clock in early and late chronotypes in real-life conditions are lacking. Therefore, in this study we assessed subjects living in their natural environment in a semicontrolled field study to elucidate whether their chronotype was encoded in the phasing of their peripheral clock and whether and how the phasing of the peripheral clock correlated with their melatonin profile.
MATERIALS AND METHODS
Subjects
Ninty-five adult volunteers of both genders (58% of males and 42% of females), 33.7 ± 11.4 (mean ± SD) yrs old, were chronotyped using a modified Munich ChronoType Questionaire (MCTQ) (Roenneberg et al., 2003). The midpoint of sleep on the free days, i.e., mid-sleep phase (MSF) was used as a marker of individual chronotype (Roenneberg et al., 2004). In the 95 subjects, the mean MSF was 04:52 ± 0:08 h (mean ± SEM); 9% of the subjects had MSF between 02:00 h and 03:00 h, 22% between 03:05 h and 04:00 h, 29% between 04:05 h and 05:00 h, 17% between 05:05 h and 06:00 h, 12% between 06:05 h and 07:00 h, and 11% between 07:05 h and 08:00 h. For the study, 6 subjects with the latest MSF (i.e., at 07:05–08:00 h) and 6 subjects with the earliest MSF (i.e., at 02:00–03:00 h) were selected and their MSF was corrected for the sleep dept accumulated during the workdays (MSFSC) (see ). The subjects selected as early or late chronotypes were free of medication throughout the study, self-reported good health, and had no sleep problems. The subjects were not obese. None of the subjects had worked night shift, flown across time zones, or lived on a nonregular daily schedule during the previous month. The subjects were informed in detail of the purpose and procedure of the study and signed the informed consent form. The protocol and consent form were approved by the Ethical Committee of the Institute of Physiology, Academy of Sciences of the Czech Republic, and were in agreement with the Declaration of Helsinki. All experiments were conducted under the standards of the Journal (Portaluppi et al., 2010).
TABLE 1. Characterization of subjects involved in the study
Protocol of the Study and Sample Collection
Sampling was performed in the subject's home environment. Before the study, the subjects were trained how to collect saliva samples and buccal scrubs. One week prior to sampling, the subjects were asked to maintain a regular daily regime with a sleep duration and timing to match each subject's habitual sleep times. On the day of the sampling (typically on free day), they were asked to avoid exposure to bright light before going to bed and after waking and to keep the curtains closed at the scheduled bedtime and until the last sampling. To collect samples during the night hours, the subjects could only use dim light from a lamp with a red bulb of intensity below 20 lux. The subjects were instructed to protect themselves from the exposure to light in the fridge/freezer during the night hours. Drinking alcoholic and caffeinated beverages, using chewing gum, and brushing their teeth were prohibited during the entire day of sampling, and 1 h before each sampling, no eating or drinking was allowed. Sampling began at 11:00 h and continued every 4 h throughout the 24 h until 11:00 h on the next day. The subjects provided saliva samples directly into a test tube, and these samples were stored at −20°C until assay. Immediately after the saliva sampling, buccal mucosa samples were taken by gently scratching off the inner cheek on both sides using a cytological brush. The oral mucosa samples were collected in RNAlater reagent (Sigma-Aldrich, St. Louis, USA) at room temperature and were then maintained at −20°C until assay. Additional time points, when only saliva but not buccal scrubs were collected, were at 21:00 and 09:00 h. This arrangement allowed us to more precisely estimate the time of melatonin rise and decline and prevented damaging the oral mucosa because of too frequent brushing. The sampling procedure was tolerated by all participants without any complaints.
In a separate experiment, samples of saliva and buccal scrubs were collected throughout a 24-h period in one subject with an average chonotype (MSFSC: 04:30 h). To evaluate the reliability of the methods used for determination of the chronotypes in the field study, the subject was exposed to three separate 24-h sampling sessions that were at least 2 wks apart. Especially, the reliability of the newly introduced method for determining clock gene expression profiles was compared with that of the method for determining melatonin profiles. The subject followed the identical procedure as described above.
Melatonin Assay
A direct double-antibody radioimmunoassay was used for the melatonin assay (Bühlmann Laboratories, Allschwil, Switzerland) as previously described (Nováková et al., 2011). The kit was used according to the manufacturer's instructions. The analytical sensitivity was .2 pg/mL. The intra-assay coefficient of variation was 3% for samples of 18.5 ± 1.0 pg/mL and 4% for samples of 2.4 ± .2 pg/mL. The interassay coefficient of variation was 12% for samples of 18.5 ± 1.0 pg/mL and 14% for samples of 2.4 ± .2 pg/mL.
The daily melatonin profiles of each subject were expressed as the ratio of their highest nighttime melatonin concentration.
Quantification of Clock Gene Expression by Quantitative Real-Time Polymerase Chain Reaction (qRT PCR)
The clock gene expression was determined as previously described (Nováková et al., 2012). Briefly, mRNA was isolated using the Dynabeads mRNA Direct Micro Kit (Invitrogen, Carlsbad, California, USA). cDNA was generated using the SuperScript VILO cDNA Synthesis Kit (Invitrogen) in 10-µL reactions incubated at 42°C for 1 h. The cDNA was then diluted 1:2 with RNase-free water and 2 µL was used for each LightCycler (Roche, Basel, Switzerland) PCR reaction in glass capillary tubes. The capillary tubes also contained 1× SYBR green PCR Mix (Sigma, Taufkirchen, Germany) and primers (1 µM) for a clock gene or a housekeeping gene. The following primer sequences were used: β-2-microglobulin (B2M, NM_004048): forward 5'-GTACTACACTGAATTCACCCCCACTG-3', reverse 5'-TGCGGCATCTTCAAACCTCCAT-3'; Glyceraldehyde-3-phosphate dehydrogenase (GAPDH, NM_002046): forward 5'-AAGGTGAAGGTCGGAGTCAA-3', reverse 5'-AATGAAGGGGTCATTGATGG-3'; Rev-erbα (NR1D1, NM 021724): forward 5'-TCCCCCAGCAAGAGCACCAGC-AACAT-3', reverse 5'-CCCGAGGCAACGTCCCCACAC-3'; Period1 (Per1, NM 002616): forward 5'-ATTCCGCCTAACCCCGTATGTGACC-3', reverse 5'-GTGTGCCGCGTAGTGAAAATCCTCTTGT-3'; and Period2 (Per2, NM_022817): forward 5'-CCCTTCCGCATGACGCCCTACCTG-3', reverse 5'-GACCGCCCTTTCATCCACATCCTG-3'. Primers for B2M, Per1, Per2, and Rev-erbα were designed in our laboratory using Lasergene Primer Select (DNAStar, Madison, Wisconsin, USA). The GAPDH primer sequence was taken from a public online database (http://primerdepot.nci.nih.gov).
The PCR reactions were amplified in a LightCycler 2.0 (Roche) during 50 cycles of 15 s of denaturation at 94°C, 20 s of annealing at 60°C, and 10 s of elongation at 72°C. At the end of each run, a melting curve analysis was performed to ascertain the presence of a single amplicon. Standard curves were generated for each PCR run from the serially diluted cDNA of a human fibroblast cell line. The threshold cycles were quantified using LightCycler Analysis software version 3.5 (Roche) via the second derivative maximum method. The levels of expression of Per1, Per2, and Rev-erbα were normalized to the expression of each of the housekeeping gene, i.e., GAPDH and B2M, separately and the arithmetic mean of the relative expression was calculated as in our previously published data (Nováková et al., 2012). The identity of the PCR products was verified by sequencing.
Statistical Analysis
The data for the 24-h profiles of melatonin and clock gene expression levels were depicted individually for each subject. The data were also expressed as the mean ± SEM for each time point of the group of subjects with early or late chronotypes. The profiles were fitted with single cosine curves (Nelson et al., 1979) defined by the equation y = mesor + {amplitude × cos[2π·(x − acrophase)/wavelength]}, with a constant wavelength of 24 h. The least squares regression method was applied using Prism 5 software (GraphPad, La Jolla, California, USA). The acrophase and coefficient of determination R2 (i.e., the goodness of fit) were calculated. The acrophases of the profiles were compared by Student's t test and p < .05 was required for significance. Moreover, the acrophases of the individual melatonin and clock gene expression profiles were correlated with the corresponding MSFSC and with each other. Correlation analysis was performed to confirm the same phase of the profiles of clock gene expression from repeated sampling in one subject (see Materials and Methods).
RESULTS
Phases of Daily Melatonin Profiles in Saliva Correlate With Human Early and Late Chronotypes Estimated by the MCTQ
The melatonin profiles of all subjects exhibited significant circadian variations, because cosinor analysis revealed significant sinusoidal fits (). The profiles were grouped according to the subject's chronotype as determined by the MCTQ (for details see Materials and Methods). The melatonin profiles of both groups did not differ in their amplitudes or mesors (t10 = .275, p = .789 and t10 = −1.719, p = .116, respectively). The average acrophase ± SD was 2.02 ± .31 h (n = 6) for the profiles of subjects selected as early chronotypes and 5.84 ± .34 h (n = 6) for those selected as late chronotypes (A). The acrophase of the late chronotypes was significantly delayed compared with that of the early chronotypes (t10 = −2.524, p < .001). Moreover, the acrophases determined for each subject's melatonin profiles () positively correlated with the corresponding individual MSFSC (B).
FIGURE 1. Melatonin profiles and their correlation with chronotype. (A) Grouped 24-h melatonin profiles for subjects with early (full circle) and late (full square) chronotypes. The data were expressed as the mean ± SEM (each chronotype n = 6) and fitted with a cosine curve to determine the acrophase of the rhythms. The acrophase of the early chronotypes (full line) occurred earlier than those of the late chronotypes (dashed line). (B) Relationship between acrophases of the 12 individual melatonin profiles (means ± SD) and their corresponding individual mid-sleep phases corrected for the sleep debt accumulated during workdays (MSFSC). The solid line shows the best-fit linear regression, the dashed lines represent 95% confidence intervals to the regression line, and R2 represents the goodness of fit. For details see Materials and Methods.
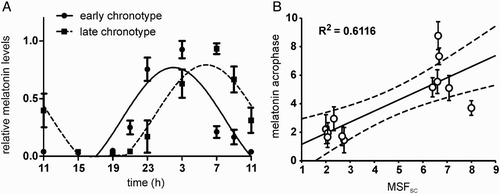
TABLE 2. Results of cosinor analysis of individual daily profiles of melatonin and clock gene expression levels
FIGURE 3. Clock gene expression profiles and their correlation with chronotype. Left panel: Grouped 24-h Per1 (upper), Per2 (middle), and Rev-erbα (lower) expression profiles for subjects with early (full circle) and late (full square) chronotypes. The data were expressed as the mean ± SEM (each chronotype n = 6) and fitted with a cosine curve to determine the acrophase of the rhythms. The acrophase of the early chronotypes (full line) occurred earlier than those of the late chronotypes (dashed line). Right panel: Relationship between the acrophases of the individual Per1 (upper), Per2 (middle), and Rev-erbα (lower) expression profiles (means ± SD) and the individual's mid-sleep phase corrected for the sleep debt accumulated during workdays (MSFSC). Only profiles with significant cosine fits (see Table 2) were considered for correlation (Per1: n = 11; Per2: n = 10; Rev-erbα: n = 9). The solid line shows the best-fit linear regression, the dashed lines represent 95% confidence intervals to the regression line, and R2 represents the goodness of fit. For details see Materials and Methods.
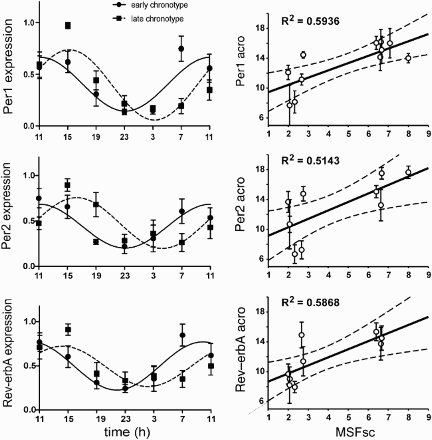
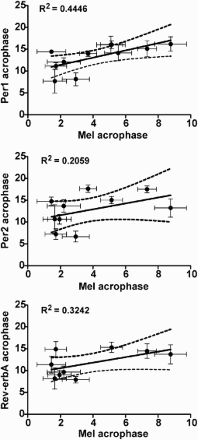
FIGURE 4. The relationship between the acrophases of the individual melatonin profiles and individual profiles of Per1 (upper), Per2 (middle), and Rev-erbα (lower) expression (means ± SD). Only profiles with significant cosine fits (see Table 2) were considered for correlation (Per1: n = 11; Per2: n = 10; Rev-erbα: n = 9). The solid line shows the best-fit linear regression, the dashed lines represent 95% confidence intervals to the regression line, and R2 represents the goodness of fit. For details see Materials and Methods.
Phases of the Daily Clock Gene Expression Profiles in Buccal Mucosa Differ in Subjects With Early and Late Chronotypes Estimated by the MCTQ
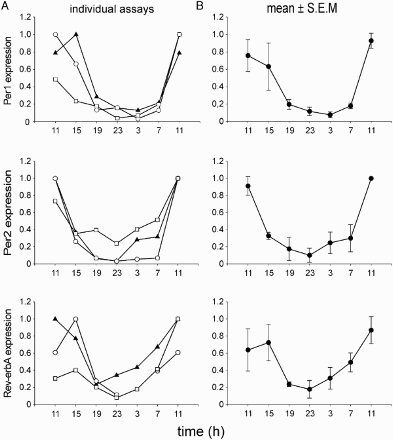
First, the Per1, Per2, and Rev-erbα expression profiles in the buccal mucosa samples were assessed for their reliability as a marker of the circadian phase in humans in the field study conditions. The profiles were determined in samples collected in three separate sessions from a single subject with an average chronotype (for details see Materials and Methods). The results demonstrated a high reproducibility of the assay () for determination of the circadian phase of the profiles because the correlation analysis revealed that for the interval of the clock gene expression rise (i.e., 19:00–11:00 h), all correlations between the pairs of the three independent measurements were significant with R values from .90 to .99 (p = .05).
FIGURE 2. Evaluation of the method used for determining the phase of the clock gene expression profiles. Samples of buccal scrubs were collected throughout the 24 h in the same subject in three independent sessions. (A) Individual profiles of Per1 (upper), Per2 (middle), and Rev-erbα (lower) resulting from three independent assays. (B) Average profiles (means ± SEM.) of Per1 (upper), Per2 (middle), and Rev-erbα (lower) of the three measurements. For details see Materials and Methods.
Then, the clock gene expression profiles were determined in subjects assigned as early and late chronotypes according to their MSFSC (for details see Materials and Methods). Individual Per1, Per2, and Rev-erbα expression profiles of most subjects exhibited significant circadian variations, because cosinor analysis revealed significant sinusoidal fits (with the exception of Per1 for subject E5, Per2 for subjects L4 and L6, and Rev-erbα for subjects L4–L6) (). The profiles were grouped according to the chronotype, and the data were expressed as the mean ± SD for early (n = 6) and late (n = 6) chronotypes (; left column). The mean acrophase of the Per1 expression profile was 11.12 ± .91 h for early chronotypes and 15.24 ± .57 h for late chronotypes. For Per2 expression, the mean acrophase was 11.05 ± 1.01 h for early chronotypes and 16.30 ± .78 h for late chronotypes. For Rev-erbα expression profile, the mean acrophase was 9.84 ± .89 h for early chronotypes and 14.20 ± 1.02 h for late chronotypes. From a comparison between the early and late chronotypes, it appeared that the mean acrophases of the late chronotypes for all studied clock genes were significantly delayed compared with those of the early chronotypes (Per1: t10 = −9.446, p < .001; Per2: t10 = −1.104, p < .001; Rev-erbα: t10 = −7.883, p < .001). The dependence of the profile phases on chronotype was also obvious from the values for individual subjects because the acrophases of the individual clock gene expression profiles (see ) of the subjects with the earlier MSFSC occurred earlier than those of the subjects with the later MSFSC (; right column). The profiles with nonsignificant fits (see above) were excluded from these comparisons.
In contrast to acrophase, chronotype does not appear to affect other characteristics of the clock gene expression rhythms () because no significant differences in amplitudes (Per1: t10 = −1.719, p = .116; Per2: t10 = −1.084, p = .304; Rev-erbα: t10 = 1.278, p = .230) or mesors (Per1: t10 = .309, p = .763; Per2: t10 = −1.359, p = .204; Rev-erbα: t10 = .0736, p = .943) were observed between the early and late chronotypes.
Individual Phases of the Melatonin Profiles Correlate With the Clock Gene Expression Profiles in Subjects With Early and Late Chronotypes
The above-described results demonstrated that the phases of melatonin and clock gene expression profiles positively correlated with the individual's MSFSC. These profiles represent markers of the central and the peripheral clocks, respectively. To determine whether the phases of the central and peripheral clocks were similarly affected by the chronotype, mutual correlations between the phases of the melatonin profiles with the phases of the Per1, Per2, and Rev-erbα expression profiles were evaluated in the individual subjects with early and late chronotypes. The data revealed a positive correlation between the phase of melatonin and Per1 expression profiles (p < .05). Correlation with the phase of the melatonin profile was only suggested for Per2 and Rev-erbα expression profiles ().
DISCUSSION
The results of this study demonstrated that in humans living in real-life conditions, not only the phasing of the daily melatonin rhythm in saliva but also the phasing of Per1, Per2, and Rev-erbα clock gene expression profiles in buccal mucosa cells reflected individual chronotypes assessed by the MSFSC. This study is the first demonstration that the human peripheral circadian clock may reflect the individual's chronotype under field conditions. Moreover, the results of this study provided evidence that clock gene expression profiles in buccal mucosa may serve not only as a marker to assess the functional state of the peripheral clock, as shown previously (Nováková et al., 2012), but also as a marker to assess the actual phase of the human circadian system.
Previous studies repeatedly demonstrated (Duffy et al., 1999, 2001; Gibertini et al., 1999; Roemer et al., 2003) that the phases of melatonin profiles correlated well with the chronotype. In our field study, the determination of chronotype was highly dependent on a self-assessment via responding to questions regarding the preferences in sleep timing. Therefore, it was important to confirm that the self-assessment was accurate and the subjects belonged to the reported early or late chronotypes. The MSFSC values of the subjects assigned in our study as the early or late chronotypes, with MSFSC at 02:18 ± 0:19 and 06:54 ± 00:35 h, respectively, corresponded well with the extreme chronotypes in the population of subjects living mostly in Germany, Switzerland, The Netherlands, and Austria (Roenneberg et al., 2007). In this population, MSFSC exhibited almost normal distribution and most subjects had MSFSC at 04:14 h. In our study, the most prevalent MSF in the population of 95 subjects (29% of subjects) living in the Czech Republic occurred also between 04:05 and 05:00 h. The correlation between mid-sleep phase and melatonin acrophase was proven for the groups of early and late chronotypes as well as for the individual subjects of each group. The subjects differed significantly only in phases but not in the amplitudes or mesors of their melatonin profiles. Based on a correlation analysis, the self-assessment of the early chronotype appeared more accurate because in the group of the late chronotypes, the correlation of MSFSC and melatonin profiles was slightly less pronounced. We cannot exclude the possibility that in two subjects of this group, which fall outside of the confidence interval of the correlation curve, the chronotype was under- or overestimated. Nevertheless, the correlation we observed was roughly as high as in a study performed under constant routine conditions (Archer et al., 2008). Therefore, the less-controlled conditions of our field study did not likely contribute to this variability. These data are thus further evidence for the presence of differences in the phasing of melatonin rhythms in a subset of individuals exposed to environmental conditions.
In this study, we attempted to correlate the phases of melatonin profiles of subjects with early and late chronotypes with the phases of clock gene expressions in oral mucosa cells. Unlike in an earlier study in which oral mucosa biopsy samples were obtained under local anesthesia (Bjarnason et al., 2001), the cells in this study were self-sampled noninvasively by the subjects in their own homes. Therefore, the gene expression profiles may provide information concerning the actual phase of their circadian clock. This method has been used previously to assess the functional state of the human molecular clock affected by acute light exposure (Cajochen et al., 2006) or neurological disease (Nováková et al., 2012). In this study, Per1, Per2, and Rev-erbα expression profiles were mutually phased so that the Rev-erbα expression profile slightly advanced the Per1 and Per2 expression profiles, which were in approximately identical phases. Similar phasing was also observed in healthy subjects in our previous study (Nováková et al., 2012). The mutual phasing likely resulted from the entraining signals of the SCN because in patients with Smith-Magenis syndrome, whose melatonin secretion lacks proper temporal timing, the mutual phasing of the clock gene expression was significantly distorted (Nováková et al., 2012). Because the assessment of the clock gene expression profiles in oral mucosa samples is highly sensitive to the quality of the samples in terms of the RNA integrity, first we had to prove a high reproducibility of circadian phase assessment of the studied clock gene expression profiles in a subject. The reliability of the method for the assessment of circadian phase was also obvious from the comparison of our data with the results of a study by Cajochen and colleagues (Cajochen et al., 2006), in which the phasing of the Per2 expression profile in the oral mucosa of subjects without an extreme chronotype fall into the range of the phases we observed for the early and late chronotypes. In our study, the phases of all three studied clock genes appeared to be dependent to the same extent on MSFSC and were all significantly advanced in the group of early chronotypes compared with those in the late chronotypes. Moreover, the phases of the clock gene expression profiles occurred earlier in individual subjects with the early chronotypes than in those with the late chronotypes, which was demonstrated for all studied genes. The amount of the phase difference of the melatonin profiles between the early and late chronotypes approximately corresponded with the differences observed for all of the clock gene expression profiles. However, when the phases of clock gene expression were correlated with the phase of the melatonin profile in each individual, a significant correlation was observed only for Per1 expression. Therefore, whereas Per1 expression correlated with MSFSC and melatonin profile, Per2 and Rev-erbα expression only correlated with MSFSC but not with the timing of melatonin maximum. Theoretically, this might be caused by the fact that due to methodological constraints and discomfort of the subjects, we were only able to collect 24-h data, which may affect reliability of the acrophase estimation by cosinor analysis. Another possibility is that this result might be due to less frequent sampling, which represents the main limitation of this study. This sampling schedule was employed to prevent subjects' discomfort from oral mucosa damage due to the repeated brushing to sample the mucosa cells. In profiles resulting from the 4-h sampling, the acrophase of the melatonin rhythm is only a rough estimate of the actual peak time. Nevertheless, in the study of Archer and colleagues, which was performed under constant routine using subjects with average chronotypes that were hourly sampled during the 24-h period, the Per3 expression profile in human leukocytes was correlated with the time of melatonin onset rather than the melatonin maximum, and the phases of the Per2 and Bmal1 expression profiles even did not correlate with the mid-sleep phase (Archer et al., 2008). Moreover, in their study, only 79% of Per3 and 33% of Per2 and Bmal1 expression profiles were significantly rhythmic. Although their study was much more extensive than the present one and the absolute levels of the percentage thus cannot be compared, they appeared to be similar to our study in which 83% of Per1, 66% of Per2, and 50% of Rev-erbα expression profiles were significantly rhythmic. From the comparison between the results of both studies, it appears that the method of determination of the phase of clock gene expression profiles in the oral mucosa is also valid for field studies because it likely is not directly masked by environmental conditions.
Our data demonstrate that chronotype is not only reflected at the level of the phase of the central clock in the SCN, but also at the level of the phase of the peripheral clocks. The result indicates that individuals with early or late chronotypes have their circadian system phased differently compared with other major populations. Nevertheless, these individuals must often adapt their behavior to the social schedules dictated by the majority. Proper internal phasing of the circadian system that is in sync with the external environment appears crucial for our health (for a review see Takahashi et al., 2008). The situation, when the internal clock is discordant with the outer world, which occurs when extreme chronotypes are forced to adapt to social time, is called social jet lag (Wittmann et al., 2006). Social jet lag more likely occurs in late chronotypes, whose sleep onset is determined by their clock but wake-up time is forced by social cues. Consequently, these individuals suffer from, for example, cumulated sleep debt and higher daytime sleepiness, attention problems, higher risk of depression, and they often exhibit higher consumption of alcohol and cigarettes (Giannotti et al., 2002; Hidalgo et al., 2009; Meliska et al.; Taillard et al., 1999; Urban et al.; Wittmann et al., 2006, 2010). Recently, accumulated data have suggested that the late chronotype may be a nonspecific risk factor for mental health (Gaspar-Barba et al., 2009; Randler, 2011b) because it may increase susceptibility to mood disorders (Kitamura et al., 2010). Therefore, understanding the mechanisms underlying chronotype in real life appears important for human well-being and for revealing interactions of temporal timing with various aspects of human behavior, brain functions, and physiological processes in our body.
Declaration of Interest: The study was supported by the Internal Grant Agency of the Ministry of Health of the Czech Republic, grant no. NT11474-4/2010; the Grant Agency of the Charles University in Prague, no. 22810; and research project nos. AV0Z50110509 and RVO:7985823.
The authors report no conflict of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Archer SN, Robilliard DL, Skene DJ, Smits M, Williams A, Arendt J, von Schantz M. (2003). A length polymorphism in the circadian clock gene Per3 is linked to delayed sleep phase syndrome and extreme diurnal preference. Sleep 26:413–415.
- Archer SN, Viola AU, Kyriakopoulou V, von Schantz M, Dijk DJ. (2008). Inter-individual differences in habitual sleep timing and entrained phase of endogenous circadian rhythms of BMAL1, PER2 and PER3 mRNA in human leukocytes. Sleep 31:608–617.
- Barclay NL, Eley TC, Buysse DJ, Archer SN, Gregory AM. (2010). Diurnal preference and sleep quality: same genes? A study of young adult twins. Chronobiol. Int. 27:278–296.
- Bjarnason GA, Jordan RC, Wood PA, Li Q, Lincoln DW, Sothern RB, Hrushesky WJ, Ben-David Y. (2001). Circadian expression of clock genes in human oral mucosa and skin: association with specific cell-cycle phases. Am. J. Pathol. 158:1793–1801.
- Boivin DB, James FO, Wu A, Cho-Park PF, Xiong H, Sun ZS. (2003). Circadian clock genes oscillate in human peripheral blood mononuclear cells. Blood 102:4143–4145.
- Brown SA, Fleury-Olela F, Nagoshi E, Hauser C, Juge C, Meier CA, Chicheportiche R, Dayer JM, Albrecht U, Schibler U. (2005). The period length of fibroblast circadian gene expression varies widely among human individuals. PLoS Biol. 3:e338.
- Brown SA, Kunz D, Dumas A, Westermark PO, Vanselow K, Tilmann-Wahnschaffe A, Herzel H, Kramer A. (2008). Molecular insights into human daily behavior. Proc. Natl. Acad. Sci. U. S. A. 105:1602–1607.
- Burgess HJ and Fogg LF. (2008). Individual differences in the amount and timing of salivary melatonin secretion. PLoS ONE 3:e3055.
- Cajochen C, Jud C, Munch M, Kobialka S, Wirz-Justice A, Albrecht U. (2006). Evening exposure to blue light stimulates the expression of the clock gene PER2 in humans. Eur. J. Neurosci. 23:1082–1086.
- Carpen JD, Archer SN, Skene DJ, Smits M, von Schantz M. (2005). A single-nucleotide polymorphism in the 5'-untranslated region of the hPER2 gene is associated with diurnal preference. J. Sleep Res. 14:293–297.
- Carskadon MA, Labyak SE, Acebo C, Seifer R. (1999). Intrinsic circadian period of adolescent humans measured in conditions of forced desynchrony. Neurosci. Lett. 260:129–132.
- Czeisler CA, Duffy JF, Shanahan TL, Brown EN, Mitchell JF, Rimmer DW, Ronda JM, Silva EJ, Allan JS, Emens JS, Dijk DJ, Kronauer RE. (1999). Stability, precision, and near-24-hour period of the human circadian pacemaker. Science 284:2177–2181.
- Dijk DJ, Duffy JF, Czeisler CA. (2000). Contribution of circadian physiology and sleep homeostasis to age-related changes in human sleep. Chronobiol. Int. 17:285–311.
- Duffy JF, Dijk DJ, Hall EF, Czeisler CA. (1999). Relationship of endogenous circadian melatonin and temperature rhythms to self-reported preference for morning or evening activity in young and older individulas. J. Invest. Med. 47:141–150.
- Duffy JF, Rimmer DW, Czeisler CA. (2001). Association of intrinsic circadian period with morningness-eveningness, usual wake time, and circadian phase. Behav. Neurosci. 115:895–899.
- Emens JS, Yuhas K, Rough J, Kochar N, Peters D, Lewy AJ. (2009). Phase angle of entrainment in morning- and evening-types under naturalistic conditions. Chronobiol. Int. 26:474–493.
- Gaspar-Barba E, Calati R, Cruz-Fuentes CS, Ontiveros-Uribe MP, Natale V, De Ronchi D, Serretti A. (2009). Depressive symptomatology is influenced by chronotypes. J. Affect. Disord. 119:100–106.
- Giannotti F, Cortesi F, Sebastiani T, Ottaviano S. (2002). Circadian preference, sleep and daytime behaviour in adolescence. J. Sleep Res. 11:191–199.
- Gibertini M, Graham C, Cook MR. (1999). Self-report of circadian type reflects the phase of the melatonin rhythm. Biol. Psychol. 50:19–33.
- Hasan S, Santhi N, Lazar AS, Slak A, Lo J, von Schantz M, Archer SN, Johnston JD, Dijk DJ. (2012). Assessment of circadian rhythms in humans: comparison of real-time fibroblast reporter imaging with plasma melatonin. FASEB J. 26:2414–2423.
- Hastings MH, Reddy AB, Maywood ES. (2003). A clockwork web: circadian timing in brain and periphery, in health and disease. Nat. Rev. Neurosci. 4:649–661.
- Hidalgo MP, Caumo W, Posser M, Coccaro SB, Camozzato AL, Chaves ML. (2009). Relationship between depressive mood and chronotype in healthy subjects. Psychiatry Clin. Neurosci. 63:283–290.
- Illnerová H. (1991). The suprachiasmatic nucleus and rhythmic pineal melatonin production. Klein DC, Moore RJ, Reppert SM. Suprachiasmatic nucleus: the mind's clock. New York: Oxford University Press, 197–216.
- Kitamura S, Hida A, Watanabe M, Enomoto M, Aritake-Okada S, Moriguchi Y, Kamei Y, Mishima K. (2010). Evening preference is related to the incidence of depressive states independent of sleep-wake conditions. Chronobiol. Int. 27:1797–1812.
- Kusanagi H, Mishima K, Satoh K, Echizenya M, Katoh T, Shimizu T. (2004). Similar profiles in human period1 gene expression in peripheral mononuclear and polymorphonuclear cells. Neurosci. Lett. 365:124–127.
- Lewy AJ, Wehr TA, Goodwin FK, Newsome DA, Markey SP. (1980). Light suppresses melatonin secretion in humans. Science 210:1267–1269.
- Meliska CJ, Martinez LF, Lopez AM, Sorenson DL, Nowakowski S, Parry BL. (2011). Relationship of morningness-eveningness questionnaire score to melatonin and sleep timing, body mass index and atypical depressive symptoms in peri- and post-menopausal women. Psychiatry Res. 188:88–95.
- Nelson W, Tong YL, Lee JK, Halberg F. (1979). Methods for cosinor-rhythmometry. Chronobiologia 6:305–323.
- Nováková M, Paclt I, Ptáček R, Kuželová H, Hájek I, Sumová A. (2011). Salivary melatonin rhythm as a marker of the circadian system in healthy children and those with attention-deficit/hyperactivity disorder. Chronobiol. Int. 28:630–637.
- Nováková M, Nevšímalová S, Příhodová I, Sládek M, Sumová A. (2012). Alteration of the circadian clock in children with Smith-Magenis syndrome. J. Clin. Endocrinol. Metab. 97:E312–E318.
- Osland TM, Bjorvatn BR, Steen VM, Pallesen S. (2011). Association study of a variable-number tandem repeat polymorphism in the clock gene PERIOD3 and chronotype in Norwegian university students. Chronobiol. Int. 28:764–770.
- Pagani L, Semenova EA, Moriggi E, Revell VL, Hack LM, Lockley SW, Arendt J, Skene DJ, Meier F, Izakovic J, Wirz-Justice A, Cajochen C, Sergeeva OJ, Cheresiz SV, Danilenko KV, Eckert A, Brown SA. (2010). The physiological period length of the human circadian clock in vivo is directly proportional to period in human fibroblasts. PLoS ONE 5:e13376.
- Pittendrigh CL. (1981). Circadian systems: entrainment. Aschoff J. Biological rhythms. Handbook of behavioral neurology. New York: Plenum, 95–124.
- Portaluppi F, Smolensky MH, Touitou Y. (2010). Ethics and methods for biological rhythm research on animals and human beings. Chronobiol. Int. 27:1911–1929.
- Ralph MR, Foster RG, Davis FC, Menaker M. (1990). Transplanted suprachiasmatic nucleus determines circadian period. Science 247:975–978.
- Randler C. (2011a). Age and gender differences in morningness-eveningness during adolescence. J. Genet. Psychol. 172:302–308.
- Randler C. (2011b). Association between morningness-eveningness and mental and physical health in adolescents. Psychol. Health Med. 16:29–38.
- Roemer HC, Griefahn B, Kuenemund C, Blaszkewicz M, Gerngross H. (2003). The reliability of melatonin synthesis as an indicator of the individual circadian phase position. Mil. Med. 168:674–678.
- Roenneberg T, Merrow M. (2007). Entrainment of the human circadian clock. Cold Spring Harb. Symp. Quant. Biol. 72:293–299.
- Roenneberg T, Wirz-Justice A, Merrow M. (2003). Life between clocks: daily temporal patterns of human chronotypes. J. Biol. Rhythms 18:80–90.
- Roenneberg T, Kuehnle T, Pramstaller PP, Ricken J, Havel M, Guth A, Merrow M. (2004). A marker for the end of adolescence. Curr. Biol. 14:R1038–R1039.
- Roenneberg T, Kuehnle T, Juda M, Kantermann T, Allebrandt K, Gordijn M, Merrow M. (2007). Epidemiology of the human circadian clock. Sleep Med. Rev. 11:429–438.
- Taillard J, Philip P, Bioulac B. (1999). Morningness/eveningness and the need for sleep. J. Sleep Res. 8:291–295.
- Takahashi JS, Hong HK, Ko CH, McDearmon EL. (2008). The genetics of mammalian circadian order and disorder: implications for physiology and disease. Nat. Rev. Genet. 9:764–775.
- Toh KL, Jones CR, He Y, Eide EJ, Hinz WA, Virshup DM, Ptacek LJ, Fu YH. (2001). An hPer2 phosphorylation site mutation in familial advanced sleep phase syndrome. Science 291:1040–1043.
- Urban R, Magyarodi T, Rigo A. (2011). Morningness-eveningness, chronotypes and health-impairing behaviors in adolescents. Chronobiol. Int. 28:238–247.
- Vanselow K, Vanselow JT, Westermark PO, Reischl S, Maier B, Korte T, Herrmann A, Herzel H, Schlosser A, Kramer A. (2006). Differential effects of PER2 phosphorylation: molecular basis for the human familial advanced sleep phase syndrome (FASPS). Genes Dev. 20:2660–2672.
- Wittmann M, Dinich J, Merrow M, Roenneberg T. (2006). Social jetlag: misalignment of biological and social time. Chronobiol. Int. 23:497–509.
- Wittmann M, Paulus M, Roenneberg T. (2010). Decreased psychological well-being in late 'chronotypes' is mediated by smoking and alcohol consumption. Subst. Use Misuse 45:15–30.
- Wyatt JK, Ritz-De Cecco A, Czeisler CA, Dijk DJ. (1999). Circadian temperature and melatonin rhythms, sleep, and neurobehavioral function in humans living on a 20-h day. Am. J. Physiol. 277:R1152–R1163.
- Zeitzer JM, Dijk DJ, Kronauer R, Brown E, Czeisler C. (2000). Sensitivity of the human circadian pacemaker to nocturnal light: melatonin phase resetting and suppression. J. Physiol. 526:695–702.
