Abstract
Routine exposure to artificial light at night (ALAN) in work, home, and community settings is linked with increased risk of breast and prostate cancer (BC, PC) in normally sighted women and men, the hypothesized biological rhythm mechanisms being frequent nocturnal melatonin synthesis suppression, circadian time structure (CTS) desynchronization, and sleep/wake cycle disruption with sleep deprivation. ALAN-induced perturbation of the CTS melatonin synchronizer signal is communicated maternally at the very onset of life and after birth via breast or artificial formula feedings. Nighttime use of personal computers, mobile phones, electronic tablets, televisions, and the like – now epidemic in adolescents and adults and highly prevalent in pre-school and school-aged children – is a new source of ALAN. However, ALAN exposure occurs concomitantly with almost complete absence of daytime sunlight, whose blue-violet (446–484 nm λ) spectrum synchronizes the CTS and whose UV-B (290–315 nm λ) spectrum stimulates vitamin D synthesis. Under natural conditions and clear skies, day/night and annual cycles of UV-B irradiation drive corresponding periodicities in vitamin D synthesis and numerous bioprocesses regulated by active metabolites augment and strengthen the biological time structure. Vitamin D insufficiency and deficiency are widespread in children and adults in developed and developing countries as a consequence of inadequate sunlight exposure. Past epidemiologic studies have focused either on exposure to too little daytime UV-B or too much ALAN, respectively, on vitamin D deficiency/insufficiency or melatonin suppression in relation to risk of cancer and other, e.g., psychiatric, hypertensive, cardiac, and vascular, so-called, diseases of civilization. The observed elevated incidence of medical conditions the two are alleged to influence through many complementary bioprocesses of cells, tissues, and organs led us to examine effects of the totality of the artificial light environment in which humans reside today. Never have chronobiologic or epidemiologic investigations comprehensively researched the potentially deleterious consequences of the combination of suppressed vitamin D plus melatonin synthesis due to life in today’s man-made artificial light environment, which in our opinion is long overdue.
Introduction
The physical and chemical environment of modern man differs radically from the one experienced during evolution that determined, through genetic adaptation, fitness for survival. One of the most rapid and radical changes that began at the end of the nineteenth century is substitution of the natural 24 h light(L)/dark (D) cycle for around-the-clock artificial light that differs markedly in spectrum, intensity, and temporal patterning. Canadian and US population-based surveys substantiate people nowadays spend very little time, on average ∼12%, outdoors, with half of this amount spent inside vehicles, and the rest, ∼88%, indoors (Klepeis et al., Citation2001; Leech et al., Citation1996; Matz et al., Citation2014). Studies utilizing personal light sensors, even though involving a relatively small number of subjects, provide additional perspective of the amount of time people spend in natural or artificial light of different intensities. One investigation (Espiritu et al., Citation1994) conducted in San Diego, California – known for its beautiful and comfortable environmental conditions throughout the year – reported the median duration of exposure of middle-aged adults to natural daylight of ≥1000 lux to be only ∼58 min/day. Another study (Cole et al., Citation1995) found the median exposure to light of ≥1000 lux in Rochester, Minnesota (USA) and San Diego, California (USA) to be, respectively, ∼2.4 h/day and ∼2.2 h/day in summer versus, respectively, ∼0.4 h/day and ∼1.3 h/day in winter. The situation is similar for residents of Quebec, Canada (Hébert et al., Citation1998), with the mean daily exposure to light of ≥1000 lux by young adults being 2.6 h in summer but only 0.4 h in winter and >50% of wake time being spent in ≤100 lux during both seasons, and also Great Britain, with the average amount of time spent outdoors during the summer being ∼1.4 h/day week days and 2.4 h/day weekends (Diffey, Citation2011).
Daytime sunlight is necessary for vitamin D synthesis, the active metabolites of which directly or indirectly regulate multiple biological processes beyond calcium and phosphorous homeostasis critical to bone health, and nighttime natural darkness is necessary for normal melatonin synthesis that is essential for biological timekeeping, sleep, and, directly or indirectly, many processes of cells, tissues, and organs. The 24 h L/D cycle of nature conveys crucial temporal cues to the body’s master biological clock – the suprachiasmatic nuclei (SCN) of the hypothalamus – and pineal gland to achieve internal synchronization of the period (τ) and phasing (φ) of the circadian time structure (CTS; see ) to ensure appropriate metabolic and other resources both to support activity during the day and restoration and repair during sleep at night, and all in proper external synchrony with expected periodic alterations and challenges of the ambient environment (Albrecht, Citation2012; Kanabrocki et al., Citation1990; Martinez-Nicolas et al., Citation2014; Reppert & Weaver, Citation2002; Schibler, Citation2006; Ticher et al., Citation1995). The impression gained from careful review of the literature is the health effects of melatonin suppression, on the one hand, and of vitamin D deficiency and insufficiency, on the other hand – consequences of life in artificial light – have been researched separately rather than in combination, the emphasis being either vitamin D or melatonin insufficiency/deficiency. The published literature, which is enormous, suggests melatonin and vitamin D regulate in a complementary manner many of the same processes of cells, tissues, organs, and systems, such that deficiencies of either one can result in similar effects on health and well-being. Thus, the intent of this editorial is to call attention to the potential challenges to health and well-being posed by the totality of the 24 h artificial light environment of today across the different stages of life due to both vitamin D and melatonin deficiency. Our emphasis is the combination of too little sunlight exposure during the day and too much artificial light exposure at night (ALAN), the situation so prevalent today almost everywhere throughout the world.
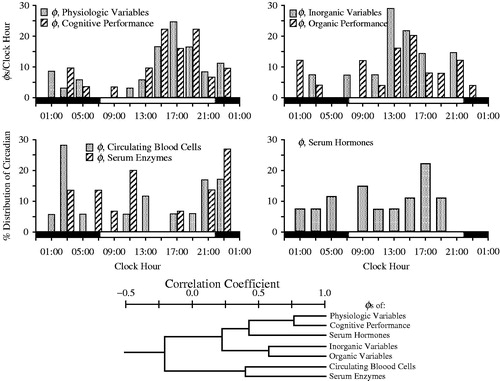
FIGURE 1. Top: Four histograms depict distribution according to clock hour of circadian acrophases (φs) of seven different categories of variables. Upper left histogram: 37 physiologic and 32 cognitive performance variables; Upper right histogram: 14 serum inorganic and 25 serum organic variables; Lower left histogram: 18 circulating blood cell and 15 serum enzyme variables; Lower right histogram: 27 serum hormone variables. Each vertical column indicates the proportion of circadian φs per variable category occurring according to clock-hour time. Bottom: Dendrogram depicts by line segment length correlation of the distribution of circadian φs between different pairings of the specified seven different categories of variables: the shorter the line segment length, the poorer the correlation and the greater the dissimilarity of the distribution of φs between designated categories of variables; the longer the line segment length, the stronger the correlation and the greater the similarity of the φs distribution between designated categories of variables. Circadian φs of the physiologic and cognitive categories are most strongly correlated (r = +0.8) (After Ticher et al., Citation1995; Reinberg et al., Citation2015).
Circadian timekeeping, melatonin, and 24 h L/D cycle
Circadian timekeeping is governed by a master brain oscillator, the paired nuclei of the SCN, through a cyclic feedback loop of ∼24 h duration entailing transcription of clock genes Per1, Per2, Per3, Cry1, Cry2, and RevErbα, following heteromerization of CLOCK and BMAL1, during nighttime darkness in diurnally active mammals, alternating with CLOCK/BMAL1 suppression by nuclear PER and CRY proteins in the early morning. The CTS is organized in a hierarchical manner, with the SCN regulating peripheral oscillators via humoral, endocrine, and neural signals, to sequentially and coherently organize virtually all biological processes in time throughout the 24 h (Albrecht, Citation2012; Reppert & Weaver, Citation2002; Schibler, Citation2006). The τ and φ of the CTS are primarily synchronized to the 24 h environment by perception of light onset (dawn) and offset (dusk) by specialized non-visual retinal ganglion cells most sensitive to the blue-violet (446–484 nm λ) spectrum that signal the SCN by the retinohypothalamic (RTH) projection (Arendt, Citation1992, Citation2011). The SCN regulates pineal gland melatonin synthesis by neural signals relayed through the paraventricular nucleus to the hindbrain, spinal cord, and finally postganglionic fibers originating from the superior cervical ganglion. Sympathetic input, primarily β1-receptors but also α1-receptors, governs melatonin synthesis (Arendt, Citation1992, Citation2011). Melatonin synthesis, which occurs exclusively during the darkness of night, except in the rare Smith–Magenis syndrome when produced during the daytime (De Leersnyder et al., Citation2001, Citation2006), takes place through a series of transformative enzymatic steps, commencing with the essential amino acid tryptophan: hydroxylation by tryptophan 5-hydroxylase to 5-hydroxytryptophan (5HTP), decarboxylation of 5HTP by 5HTP-decarboxylase to serotonin (5-hydoxytryptamine, 5HT), N-acetylation of serotonin by serotonin-N-acetyl transferase (NAT) to N-acetylserotonin (NAS), and culminating with O-methylation of NAS by hydroxyindole-O-methyl-transferase (HIOMT) to produce melatonin (Arendt, Citation1992, Citation2011). Once synthesized, melatonin is immediately released into systemic circulation, but due to its rapid liver metabolism to 6-hydroxymelatonin by cytochrome P450 enzyme CYP1A2, its half-life is rather short, with most studies reporting it being 20–40 min, although a few as long as 60 min (Arendt, Citation2011).
The pineal gland and SCN coordinate and order the CTS. The duration and onset and offset times of the melatonin signal chemically translate the duration and onset and offset times of the environmental scotophase (nighttime dark phase) from day to day over the course of the year, thereby communicating not only 24 h but annual time. This is accomplished by direct circulation of melatonin into cells, tissues, and organs and also through its binding to specialized G-protein MT1 and MT2, quinone reductase enzyme MT3, and nuclear receptors of cells (e.g., epithelial prostate and breast, immune, fat, ovary/granulosa, uterine, cardiac, skin, and neural), organs (e.g., liver, gallbladder, intestine, and kidney), and systems (e.g., gastrointestinal, nervous, and cardiovascular). Melatonin plays a major role in feedback regulation of the circadian patterning of several clock genes, e.g., Per1, Per2, BMAL1, RevErbα, CLOCK, and Cry1, both in central and peripheral tissues, along with certain non-photic synchronizers, e.g., temporal pattern of nutrient consumptions (Arendt, Citation2011; Ekmekcioglu, Citation2006; Hoogerwerf et al., Citation2007; Singh & Jadhav, Citation2014; Tosini et al., Citation2014). Accordingly, the τ and φ of peripheral cellular circadian clocks, and the many diverse circadian rhythms they drive, of tissues, organs, and systems are synchronized and ordered in time during the 24 h and year for optimal biological efficiency. Melatonin exerts many receptor-independent effects (for detailed review, see Pechanova et al., Citation2014). Overall, they involve reactive oxygen species scavenging and activation and over-expression of various antioxidant enzymes, i.e., protection from oxidative damage, plus elevated efficiency of the mitochondrial electron transport chain. In addition, melatonin serves as an endocrine regulator of nighttime blood pressure in healthy persons by contributing to its normal decline during sleep by 10–20% from its daytime mean level (Portaluppi et al., Citation1996). Thus, melatonin suppression by ALAN may play a role in the increasing incidence of nocturnal hypertension as substantiated by around-the-clock ambulatory blood pressure monitoring (Portaluppi et al., Citation1990). Furthermore, normal nighttime melatonin production reduces oxidative stress caused by arterial hypertension (Portaluppi et al., Citation2004). For these reasons melatonin is under investigation as a putative chronotherapy of hypertension (Hermida et al., Citation2011, Citation2013; Portaluppi & Smolensky, Citation2010). Also, melatonin is protective of the central nervous system; it reduces free-radical burden, improves endothelial dysfunction, reduces inflammation, modulates the response to endotoxemia (Alamili et al., Citation2013), and shifts the balance between sympathetic and parasympathetic drive in favor of the later. Finally, it may interact directly with calmodulin and inhibit Ca2+ channels or calcium pump stimulation, for example, in cardiomyocytes. Altogether, many of the pleiotropic effects of melatonin appear to be similar or complementary to the actions of vitamin D, as later discussed.
ALAN and melatonin
As aptly pointed out by Stevens et al. (Citation2014), ALAN is a very recent and significant perturbation of the natural environment, since 1879 with the invention by Thomas Edison of the carbon-filament incandescent electric light bulb. Early humans illuminated the nighttime by small brush/wood fires. Candles began to be used for light ∼5000 yrs ago, and in the fifteenth and sixteenth centuries gas lamps were a popular means of illuminating public spaces of many European cities, but it was not until the eighteenth century that they were widely used indoors. These relatively dim light sources are rich in yellow and red spectrum wavelengths (λs), but sparse in blue λ, as is the incandescent electric light bulb. The widely used electric lights of today, like the Cool White and Daylight White Fluorescent and energy-saving Compact Fluorescent ones, are richer in blue λ and able to suppress melatonin synthesis when used within 1 h of bedtime (Wahnschaffe et al., Citation2013). Carefully conduced research over the past two decades demonstrates retinal exposure to as little as ≤1 lux monochromatic blue 440–460 nm λ light, ∼100 lux broad spectrum λ fluorescent light, or other conventional sources of ALAN can markedly attenuate nocturnal melatonin synthesis (Brainard et al., Citation2001; Giménez et al., Citation2014; Glickman et al., Citation2002; Montagnese et al., Citation2015; Obayashi et al., Citation2014a), phase shift the CTS (Gooley et al., Citation2011; Lockley et al., Citation2003; Zeitzer et al., Citation2000), and elevate alertness and disrupt sleep (Brainard et al., Citation2001; Cajochen et al., Citation2000; Lockley et al., Citation2006). Blocking the blue λ spectrum of light sources prevents such effects (Rahman et al., Citation2013; van de Werken et al., Citation2013).
Numerous studies identify a large number of potentially detrimental effects of short-term and life-long ALAN exposure – from traditional industrial, community, and home sources but nowadays increasingly from televisions, personal computers, electric tablets (e-tablets), readers (e-readers), and the like – on sleep, metabolism, reproductive capability, aging, and various medical conditions, including hormone-responsive tumors of adults (Bouchlariotou et al., Citation2014; Chau et al., Citation2014; Flynn-Evans et al., Citation2013; Haim & Portnov, Citation2013; Kloog et al., Citation2009, Citation2010, Citation2011; Obayashi et al., Citation2014b,Citationc, Citation2015; Pukkala et al., Citation2006; Reiter et al., Citation2012, Citation2014a; Rybnikova et al., Citation2015; Stevens, Citation2006; Yu & Weaver, Citation2011; Zhu et al., Citation2006), and on altered sleep-associated degradation of cognitive performance, neurodevelopmental, certain cancers, and other health conditions of children and adolescents (Arora et al., Citation2014; Cajochen et al., Citation2011; Chahal et al., Citation2013; Chang et al., Citation2015; Chen et al., Citation2012; Erren, Citation2005; Falbe et al., Citation2015; Gamble et al., Citation2014; Gitto et al., Citation2013; Gradisar et al., Citation2013; Rossignol & Frye, Citation2011; Stevens, Citation2012; Tordjman et al., Citation2013, Citation2015). Many epidemiologic investigations report association between ALAN exposure and BC in shift-working women and prostate specific antigen level and PC in shift-working men, and numerous laboratory animal studies report findings consistent with these observations (Haus & Smolensky, Citation2013; Stevens, Citation2006; Stevens et al., Citation2014), with some showing ALAN-induced resistance to first-line tamoxifen and doxorubicin cancer chemotherapies (Dauchy et al., Citation2014; Xiang et al., Citation2015), of obvious relevance to the clinical management of BC. This large body of findings relating to shift work, ALAN, and cancer led the expert working group of the World Health Organization’s International Agency for Cancer Research (I.A.R.C., Citation2010) to conclude the evidence: (i) from laboratory animal experiments is sufficient for the carcinogenicity of ALAN, (ii) in humans is limited for the carcinogenicity of shift schedules that involve night work, and (iii) is probable in humans for shift work that involves circadian disruption as being carcinogenic (Group 2 A Carcinogen).
A great amount of research has been conducted to understand the cellular and molecular mechanisms of the observed increased risk of ALAN-associated cancers (Ackermann et al., Citation2013; deHaro et al., Citation2014; Haim & Zubidat, Citation2015; Haus & Smolensky, Citation2013; Hill et al., Citation2015; Schwimmer et al., Citation2014). The hypothesized circadian rhythm mechanisms are: repeated ALAN-induced nocturnal melatonin synthesis suppression, frequent CTS disruption, i.e., phase delays or advances according to the light phase-response curve, and/or altered sleep/wake 24 h cycle with sleep deprivation (deHaro et al., Citation2014; Haus & Smolensky, Citation2013; Hill et al., Citation2015). At this time there are no established threshold criteria that define melatonin insufficiency or deficiency based on risks to health and well-being, like there are for vitamin D (as later discussed) and conventional clinical laboratory variables, particularly with respect to circadian time. Furthermore, although progress is being realized toward achieving consensus regarding proper description of ALAN exposure, such as spectrum, intensity, frequency, and duration – hours/night, nights/week, and years – in home, community, and work settings, including in relation to work arrangements (Stevens et al., Citation2011), there is as yet no broad agreement on the definition of CTS disruption, or certain other key factors. Population-based research associates antecedent 10-yr environmental ALAN exposure assessed by satellite with BC incidence of women (Rybnikova et al., Citation2015) and PC of men (Kloog et al., Citation2009), especially in Western countries. Yet, so far conducted epidemiology studies on the relationship between night work, BC and PC incidence, and ALAN, which are likely to entail melatonin suppression and/or CTS disruption, have neglected likely confounding by ever-prevalent ALAN experienced by both cases and controls that commences at the very beginning of life, i.e., in utero, and continues throughout all life’s stages – infancy, childhood, adolescence, and adulthood.
Most human melatonin studies have been conducted on individuals subjected to the man-made light environment of today – little exposure to daytime sunlight and extensive exposure to evening and nighttime relatively bright artificial light, conditions that differ dramatically from ones under which our ancestors evolved and that existed essentially until the electrification of the modern world. Thus, the findings of at least some, or perhaps many, studies pertaining to the features of the human melatonin circadian rhythm and even CTS, may not be entirely representative. Nonetheless, it is becoming increasingly evident that the modern light environment can negatively impact human beings during every stage of life, thereby further raising concerns about their potential risks to health and well-being. Indeed, some propose artificial lighting and extensive industrialization have significantly altered the natural human 24 h wake/sleep pattern and sleep duration. Historical accounts plus thus far limited laboratory studies (Ekirch, Citation2001, Citation2005; Fagioli et al., Citation2001; Wehr, Citation1992; Wehr et al., Citation2001) suggest the natural ancestral sleep of humans before the industrial revolution, particularly under short photoperiod, was biphasic – a first sleep commencing shortly after sunset of ∼3–4 h duration, followed by a 1–3 h wake span, followed by a second sleep phase lasting until sunrise of ∼3–4 h duration – with differences, compared to the sleep mode of today, in both the amount and circadian patterning of melatonin synthesis (Wehr, Citation1992).
During pregnancy, the maternal melatonin circadian rhythm serves as time-of-day and time-of-year synchronizing cues to the immature SCN and biology of the fetus (Reppert, Citation1985). Maternal-derived melatonin also protects the fetus against oxidative stressors and exerts positive effects on growth and development (Reiter et al., Citation2014b; Seron-Ferre et al., Citation2007; Tamura et al., Citation2008; Tauman et al., Citation2002; Thomas et al., Citation1998; Torres-Farfan et al., Citation2006; Voiculescu et al., Citation2014). Until the middle of the past century, labor and parturition typically took place during the darkness of night (Reiter et al., Citation2014b; Smolensky et al., Citation1972), and neonates were raised under the natural 24 h L/D cycle. All infants were nourished by breast milk that varies extensively in melatonin concentration in a predictable-in-time nyctohemeral pattern that served to synchronize the τ and φ of the neonatal and early childhood CTS in accord with maternal and natural environmental L/D time cues. Over the past six or so generations, particularly in Western countries, pregnant women have been habitually exposed to ALAN inside and outside the home, with a growing proportion of them in many countries also experiencing such through night work sometimes until delivery, potentially disrupting on a daily basis the biological time structure of the developing fetus. Nowadays, in developed countries most births take place in hospitals with term neonates usually housed around-the-clock under relatively bright artificial light until mother and neonate are discharged home. At-risk pre-term newborns are housed in neonatal intensive care unit (NICU) facilities having this same light environment for a much greater duration of time – weeks to months. Several studies suggest these conditions delay organization, or even promote free-running (circadian τ≠24.0 h), of the immature CTS. Neonates whose life after birth is spent in a 24 h cycled L/D hospital nursery setting designed to mimic the natural environment synthesize significantly more melatonin nocturnally and more quickly develop the normal 24 h sleep/wake cycle than those kept under constant bright light (Jaldo-Alba et al., Citation1993; Martin-du-Pan, Citation1970; McMillen et al., Citation1991). This is particularly true for pre-term neonates; when the NICU environment is 24 h L/D cycled, rather than continuously lighted, weight gain is significantly faster and greater, requirement for mechanical respiration shorter, and length of NICU residence reduced (Mann et al., Citation1986; Morag & Ohlsson, Citation2013; Rivkees et al., Citation2004; Vásquez-Ruiz et al., Citation2014).
Today, breast feeding is encouraged, although not always pursued, or done only for a relatively brief time. Instead, the infant is likely to be fed by bottle with a formulated nutrient product devoid of constituents needed to convey synchronizing signals to the still immature CTS. Studies reveal neonates nourished naturally with breast milk around-the-clock or special day/night differentially constituted, with respect to circadian requirements, formula feedings more rapidly develop the mature 24 h sleep/wake cycle than babies bottle-fed with conventionally formulated products (Arslanoglu et al., Citation2011; Cubero et al., Citation2005, Citation2006; Illnerová et al., Citation1993). However, breast feeding practices vary between mothers. Some elect to naturally nourish their infants on demand at all times during the day and night for many months. Others prefer to collect breast milk by manual ‘pumping’ during the daytime as convenient – but when melatonin and other constituents of nighttime breast milk that convey crucial time cues to help synchronize the τ and φ of the neonate’s developing CTS are absent – for overnight feedings delivered by bottle by another caretaker. Still others rely entirely on artificial formulas for around-the-clock feedings that are devoid of melatonin and other constituents that would ordinarily provide time cues to the neonate. Moreover, the immediate postpartum home light environment of mother and infant pairs may be atypical. One study found 7-week postpartum mothers and infants on average spent, respectively, ∼71% and ∼80% of the time between 06:00 and 21:59 h in <50 lux dim light and, respectively, only∼6% and ∼2% in >1000 lux bright light (Tsai et al., Citation2009). In this regard, disruption of both the sleep/wake and melatonin circadian rhythms for at least 8 wks after hospital discharge was found in 20–30% of mothers of term and 30–40% of pre-term neonates (McMillen et al., Citation1993). Thus, disruption of the CTS plus absence or abnormality of the maternal melatonin time cue, both during fetal development and early neonatal life, seem to be common phenomena in today’s artificial light society.
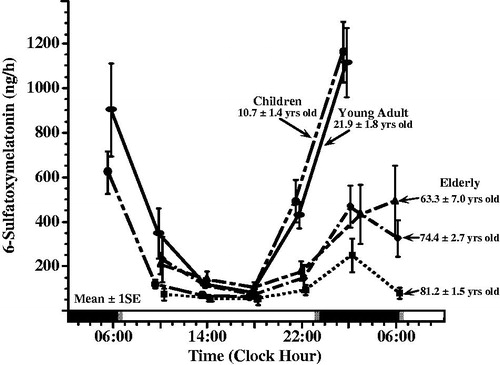
Melatonin synthesis and features of its circadian rhythm change with age (Kennaway et al., Citation1999; Mahlberg et al., Citation2006; Zhao et al., Citation2002), as illustrated in . In healthy term neonates, melatonin synthesis increases rapidly during the first 2 months of life, with circadian patterning typically developed 8–12 wks after parturition (Ardura et al., Citation2003; Kennaway et al., Citation1992). In pre-term neonates, in comparison, melatonin circadian rhythmicity is usually delayed by several weeks (Kennaway et al., Citation1992). The nocturnal and morning concentrations of melatonin and its metabolite, 6-sulfatoxymelatonin (aMT6s), in blood, urine, and saliva increase after birth, being greatest between 1.5 and ∼6 yrs of age. Thereafter, such levels decrease by ∼35–40% on average in those 6–8 yrs of age and another ∼20–25% on average prior to or around puberty – ages 8–13, but with slight increase ∼13–15 yrs of age. Nighttime melatonin and aMT6s decline much more slowly during adulthood, some investigators reporting decreases only to ∼25–30 yrs of age and others reporting continuous attenuation throughout life, but with appreciable between-individual variation (Kennaway et al., Citation1999; Mahlberg et al., Citation2006; Molina Carballo et al., Citation1996; Zhao et al., Citation2002).
FIGURE 2. Age-related changes in melatonin circadian rhythm investigated by around-the-clock assessment of the urinary excretion of its metabolite – 6-sulfatoxymelatonin (aMT6s). Urines were collected from subjects adhering to a routine of nocturnal sleep in darkness (dark shading along bottom time axis) alternating with diurnal activity (non-shaded portion of bottom time axis) at ∼4 h intervals during a single 24 h span. aMT6s values ( ± S.E.) are plotted as midpoint time of the respective 4 h urine collections. Across all age groups, aMT6s concentration is greatest in the middle of the night and lowest midday. Mean nocturnal aMT6s is somewhat greater in young adults ∼22 yrs of age (n = 43) than children ∼11 yrs of age (n = 193), and lowest in the elderly (n = 271 in total), particularly the oldest (∼81 yrs of age) age group (Previously unpublished data of E. Haus & L. Sackett-Lundeen).
Several investigations suggest a causal or predictive link between low melatonin level during the initial weeks of life and abnormal neurodevelopment, e.g., delayed psychomotor achievements at 3 and 6 months of age, and some others (Rossignol & Frye, Citation2011; Tordjman et al., Citation2015) have posed the question whether ALAN-induced melatonin suppression plays a role in other primarily childhood neurological medical conditions, such as autism spectrum disorder, including Asperger’s syndrome, among other pervasive disorders. Precocious puberty in children as young as 4–6 yrs of age is associated with premature attenuation of nocturnal melatonin synthesis (Waldhauser et al., Citation1991). Melatonin receptors are found in the hypothalamus, pituitary, and reproductive tissues, and although precocious puberty typically results from pineal tumor and abnormal hypothalamic gonadotrophin mechanisms (Arendt, Citation2011; Commentz & Helmke, Citation1995), it is unknown if ALAN-caused suppression of nocturnal melatonin synthesis – temporal patterning and/or total amount – plays a permissive or contributory role in idiopathic precocious puberty (Aleandri et al., Citation1997; Alikasifoglu et al., Citation2015; Cavallo, Citation1993). It is unclear if advancement in age of puberty onset in boys and girls over the past several decades in most countries, often ascribed to nutritional factors or endocrine-disrupting environmental pollutants (Anderson & Must, Citation2005; Euling et al., Citation2008; Jaruratanasirikul & Sriplung, Citation2014; Morris et al., Citation2011; Song et al., Citation2014; Talma et al., Citation2013), to some extent might involve ALAN exposure that commences at the very onset of life.
ALAN now originates in an increasing proportion of countries in more ways than from traditional work, community, and home lighting. Computers, televisions, electronic tablets (e-tablets and e-readers), and mobile telephones, which beginning in early childhood are routinely utilized in close proximity to the eyes before bedtime, constitute prevalent new blue λ-rich sources. According to the Mobile technology Fact Sheet (http://www.pewinternet.org/fact-sheets/mobile-technology-fact-sheet/), as of September 2013, 91% of American adults owned a cell phone and 55% a smart phone – with 44% of owners sleeping close by them to receive calls, text messages, or other updates during the night, 42% a tablet computer, and 32% an e-book reader. Moreover, in 2013, 83.8% of U.S. homes had a computer, with 78.5% a desktop or laptop and 63.6% a handheld one (http://www.census.gov/content/dam/Census/library/publications/2014/acs/acs-28.pdf). A small sample survey of 53 persons 23–80 yrs of age reported e-tablet use ≤1 h before bedtime by at least 60% (http://appleinsider.com/articles/11/12/22/apples_ipad_has_84_satisfaction_rating_17_of_users_nstall_80_apps), and a large US 2009 telephone survey of Americans of different ages reported operation by 90% of a technological light-emitting device ≤1 h of retiring to bed. Before bedtime, 60% of the respondents, regardless of age, reported viewing TV, and 72% of adolescents and 67% of young adults admitted to using cell phones versus 36% of middle-aged and 16% of older adults. Sleep onset of younger respondents was significantly later than older ones, both on weekday and weekend nights, with utilization of the more interactive technological devices, such as desktop and laptop computers, cell phones, and video game consoles, causing greatest delay in falling asleep and most numerous complaints of not attaining refreshing sleep, results in line with other American studies dealing with small screen smart phone and other electronic devices (Falbe et al., Citation2015; Gradisar et al., Citation2013). A 2014 published Australian internet survey of >1000 adolescents 11–17 yrs of age found >70% possessed ≥2 devices in their bedroom at night, with in-bed operation at least a few nights/wk of cell phone by 46.8%, computer by 38.5%, and TV by 23.2%, that resulted in delayed onset, offset, and shortened duration of sleep – the severity of which varied according to exposure, i.e., nights/wk of use (Gamble et al., Citation2014). Furthermore, a Canadian survey found 64% of parents of 5th grade school children acknowledged their child had access to ≥1 electronic entertainment and communication devices in their bedroom that was associated with his/her shortened sleep duration, excess body weight, poor diet quality, and low physical activity (Chahal et al., Citation2013). Similar findings were derived from survey of British 11–13 yr olds (Arora et al., Citation2014), Norwegian 16–19 yr olds (Hysing et al., Citation2015), and Belgian secondary school students (Van den Bulck, Citation2004). Before-bedtime use of light-emitting electronic devices, based on findings of both experimental laboratory and in-home studies on children and adults, results not only in reduced sleep length and delayed sleep phasing but suppressed melatonin synthesis (Burgess & Molina, Citation2014; Chang et al., Citation2015; Higuchi et al., Citation2014; Peixoto et al., Citation2009). Finally, recent research findings suggest blue λ-rich ALAN delivered in immediate proximity to the eyelids while closed during nighttime sleep, but not when delivered extraocularly, transcranially, or transauditory canal (Bromundt et al., Citation2014; Jurvelin et al., Citation2014), is able to suppress melatonin synthesis, disorganize or phase-shift the CTS, and/or alter the 24 h sleep/wake pattern (Figueiro & Rea, Citation2012; Figueiro et al., Citation2014).
Children are highly sensitive to ALAN, more so than adults, and perhaps especially prone to its disruptive effects; well-controlled nighttime illumination studies show melatonin suppression response is ∼2-fold greater in youngsters than middle-age adults (Higuchi et al., Citation2014; Turner & Mainster, Citation2008). Frequent disruption of the 24h sleep/wake cycle as a consequence of ALAN exposure, often with shortened sleep duration, melatonin suppression, and CTS desynchronization, is quite common in evening chronotype children, adolescents, and young adults (Adan et al., Citation2012; Wittmann et al., Citation2006), but also in neither evening nor morning chronotypes when staying up late to study, complete school/college assignments, and partake in late-night, typically weekend, social events (Collado Mateo et al., Citation2012; Touitou, Citation2013). Additionally, short-term, and presumably long-term, late day or evening treatment of infants and children with β1-adrenoreceptor antagonist medications, e.g., to manage hemangioma (Ng et al., Citation2015) and migraine (Bidabadi & Mashouf, Citation2010), and of adults with β1-adrenoreceptor (e.g., atenolol, bisoprolol, metoprol, and propranolol) and α1-adrenoreceptor (e.g., alfuzosin, doxazosin, and terazosin) antagonists, e.g., to manage benign prostatic hyperplasia, cardiac arrhythmias, glaucoma, hypertension, ischemic heart disease, and situational anxiety (Akbar & Alorainy, Citation2014; Chon et al., Citation1999; DiNicolantonio et al., Citation2015; Zhang et al., Citation2015), mimics the melatonin suppression of ALAN exposure (for review, see Haus & Smolensky, Citation2013; Smolensky et al., Citation2012), with possible risk of CTS disruption. Melatonin is considered to be a valuable treatment for dyssomnias of youngsters, especially those diagnosed with delayed sleep phase syndrome, attention-deficit hyperactivity disorder, autism spectrum disorders, mood and anxiety syndromes, and certain neurologic conditions (Appleton et al., Citation2012; Bruni et al., Citation2015; Sánchez-Barceló et al., Citation2011). However, it is also used more often than recognized in countries where it can be obtained without doctor’s prescription to achieve compliance of healthy infants, children, and adolescents to the bedtime schedule mandated by parents. Most likely, parents have little or no knowledge of its potential disruptive effects – undesired advance or delay of the 24 h sleep/wake cycle or CTS – that can arise by inconsistent or ill-timed administrations. In this regard, it is of interest that a Norwegian study reports the recent doubling of melatonin use as a hypnotic to induce sleep of children, particularly boys 6–12 yrs of age (Hartz et al., Citation2012).
Considerable current debate focuses on the extent to which the increased risk of BC of shift-working women and PC of shift-working men, who frequently experience occupational ALAN, entails circadian rhythm mechanisms (): (i) melatonin synthesis suppression (Borges et al., Citation2007; Dumont & Paquet, Citation2014; Grundy et al., Citation2009; Kennaway, Citation2014; Schernhammer et al., Citation2004), particularly for postmenopausal BC (Brown et al., Citation2015; Schernhammer & Hankinson, Citation2009; Schernhammer et al., Citation2008), (ii) CTS disruption (Carter et al., Citation2014; Kubo et al., Citation2006; Schernhammer et al., Citation2001), (iii) activity/sleep 24 h cycle perturbation with sleep deprivation (Grundy et al., Citation2009; Wu et al., Citation2008), or/and (iv) some or all of these. Limited studies of blind women with no light perception (NLP), i.e., absence of SCN and pineal gland RHT projection signaling thereby precluding ALAN-induction of melatonin suppression, reveal BC incidence is much lower than it is in less visually impaired and normally sighted women (Feychting et al., Citation1998; Flynn-Evans et al., Citation2009; Hahn, Citation1991; Kliukiene et al., Citation2001). Many of these cited studies further indicate decreasing incidence of BC in women in close association with the extent of visual impairment, with one study revealing a comparable, although less consistent, trend for PC in men (Pukkala et al., Citation2006). Such findings are consistent with the view that BC and PC risk results primarily from ALAN-caused melatonin suppression; however, they do not exclude the possibility that CTS disruption also plays a role, because investigations conducted on visually impaired women and men, especially those with NLP, reveal alteration or disruption of the circadian τ and/or φ of the sleep/wake, melatonin, and probably other rhythms of the CTS is quite prevalent, especially in the absence of strong non-photic social and other time cues (Emens et al., Citation2013; Flynn-Evans et al., Citation2014; Lockley et al., Citation1997a, Citation1997b, Citation1999; Skene et al., Citation1999). Presently, it is unknown whether the incidence of BC and PC of NLP persons differs between those who are more versus less prone to circadian disruption. Thus, results of the broad array of investigations entailing women and men with NLP, either since birth or early in life, plus women and men of varying degrees of visual impairment are consistent with the position that melatonin suppression, alone, need not be the sole cause and mechanism of ALAN-associated elevated incidence of hormone-responsive tumors in normally sighted individuals. It is important to recall many studies report disruptive effects of ALAN are already prominent early in life – during fetal development, formative years of childhood and adolescence, and thereafter during adulthood, including during the schooling and training of those who later assume day and nightshift employment. Dayshift female and male employees with such antecedent light history are likely to continue to habitually experience ALAN from home and community sources, although presumably not to the same extent as those who regularly experience it during night work. This raises the question whether dayshift workers constitute appropriate controls for case-control investigations designed to explore the differential incidence of BC in female and of PC in male rotating and nightshift employees.
FIGURE 3. Mean (±S.E.) 24 h urinary melatonin metabolite 6-sulfatoxymelatonin (aMT6s) concentration expressed relative to creatinine concentration of dayshift nurses (n = 7), who maintained their diurnal activity/nocturnal sleep routine, studied on work and off days (n = 268 urine samples in total) and nightshift nurses (n = 12), who adopted a daytime sleep/nighttime activity routine when working nightshifts but with napping at work allowed when feasible, studied during duty nights (n = 224 urine samples in total) and also off nights (n = 275 urine samples). Void-by-void urines were self-collected around the clock by day nurses who worked 6 h shifts 6 day/wk and also by night nurses who worked 12 h nightshifts every other night and who had 36 h off-work periods every other "day". Night nurses exhibit a significantly lower mean aMT6s (i.e., melatonin suppression) on both work and off days compared to dayshift nurses on work and also off days (ANOVA; p < 0.0001); mean 24 h urinary 6-sulfatoxymelatonin/ng creatinine concentration of nightshift nurses, both on and off work nights, is ∼40% less than that of dayshift only nurses, both on work days and off days. [Figure constructed using previously unpublished data collected by Borges et al. (Citation2007, Citation2008)].
![FIGURE 3. Mean (±S.E.) 24 h urinary melatonin metabolite 6-sulfatoxymelatonin (aMT6s) concentration expressed relative to creatinine concentration of dayshift nurses (n = 7), who maintained their diurnal activity/nocturnal sleep routine, studied on work and off days (n = 268 urine samples in total) and nightshift nurses (n = 12), who adopted a daytime sleep/nighttime activity routine when working nightshifts but with napping at work allowed when feasible, studied during duty nights (n = 224 urine samples in total) and also off nights (n = 275 urine samples). Void-by-void urines were self-collected around the clock by day nurses who worked 6 h shifts 6 day/wk and also by night nurses who worked 12 h nightshifts every other night and who had 36 h off-work periods every other "day". Night nurses exhibit a significantly lower mean aMT6s (i.e., melatonin suppression) on both work and off days compared to dayshift nurses on work and also off days (ANOVA; p < 0.0001); mean 24 h urinary 6-sulfatoxymelatonin/ng creatinine concentration of nightshift nurses, both on and off work nights, is ∼40% less than that of dayshift only nurses, both on work days and off days. [Figure constructed using previously unpublished data collected by Borges et al. (Citation2007, Citation2008)].](/cms/asset/040c25a5-7539-4ea2-bf38-7bf15693bed0/icbi_a_1072002_f0003_b.jpg)
FIGURE 4. Circadian timing (acrophase, φ) of each of six urinary variables (volume = urine volume; potassium = urinary potassium concentration; sodium = urinary sodium concentration; HMMA = 4-hydroxy-3-methoxymandelic acid: measure of urinary catecholamines concentration; 17-OHCS = 17-hydroxycorticosteroids: urinary metabolite of glucocorticosteroids, and 5-HIAA = 5-hydroxyindoleacetic acid: urinary metabolite of serotonin) determined from voidings collected from (n = 5) male shift workers upon awakening, at regular intervals during daytime waking, and before retiring to sleep on work and off days throughout a continuous ≥50-day span while adhering to an employee-selected (and industry approved) backward-in-direction rapid 3 to 4-day rotation shift schedule (dayshift: 07:00–16:00 h; nightshift: 21:00–06:00 h; morning shift: 06:00–13:00 h; evening shift: 13:00–21:00 h). Each graph shows the group circadian acrophase (φ: peak time and also 95% confidence interval when group circadian rhythmicity is substantiated by Cosinor analysis) per study variable for the 1st, 2nd, and 3rd + 4th day of the respective shifts in relation to both the associated sleep/wake pattern of the workers (blackened portion of lower time axis plus gray shaded background of each of graph) and midwork time (bold black vertical line). The φ of each of the variables for the daytime activity/nocturnal sleep routine of off days (OD) and normal day (ND) shifts occurs before or around the middle of the activity span, within the 6 h following waking. The φs of the study variables are most misaligned in timing relative to the sleep/wake schedule and midwork time when the nighshift is worked, which constitutes greatest alteration (∼8 h change) in the sleep/wake circadian rhythm relative to the dayshift or off days conditions; this is particularly apparent the 1st nightshift when some φs are timed during daytime sleep rather than during nighttime work. By the 3rd and 4th day, no matter the work shift, nearly complete or complete adjustment of the φ relative to worker sleep/wake cycle of most if not all the studied circadian variables is achieved (Redrawn from Vieux et al., Citation1979).
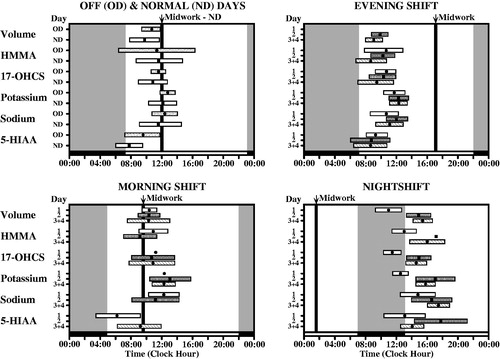
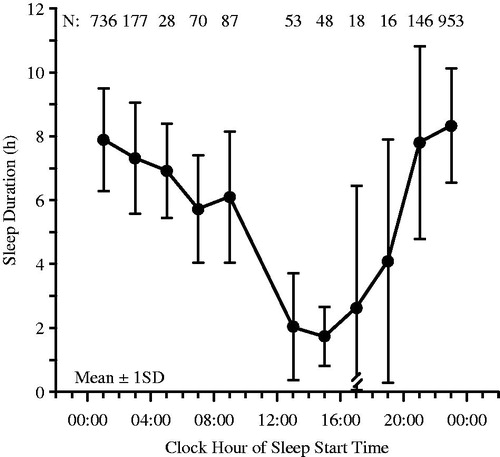
FIGURE 5. Sleep duration (x ± S.D.) of (n = 304) radio and television and airport transport and freight shift workers according to the clock time when sleep was initiated (starting time of sleep). When sleep must be scheduled during the day due to night work, it is never >6 h in duration on average, and when initiated, in particular, between 13:00 and 19:00 h, it is not >4 h in duration on average (Redrawn from Knauth & Rutenfranz, Citation1981).
Daytime sunlight, vitamin D, health, and well-being
The topic of vitamin D synthesis, deficiency, and sufficiency has been the subject of a vast number of publications, including several extensive reviews in part relied upon in this section (Hilger et al., Citation2014; Hossein-nezhad & Holick, Citation2013; Obaidi et al., Citation2014; Wacker & Holick, Citation2013a,Citationb). The term “vitamin D” is somewhat nonspecific, referring in common usage to either ergocalciferol (vitamin D2), which is normally derived from diet, cholecalciferol (vitamin D3), which is generated by endogenous natural synthesis, and/or 25-hydroxyvitamin D (25(OH)D3), the form clinically assessed to determine adequacy of vitamin D status. The behavior and actions of the biologically active forms – alphacalcidol (1-hydroxyvitamin D3), doxercalciferol (1-hydroxyvitamin D2), and calcitriol (1,25-dihydroxyvitamin D3 [1,25(OH)2D3]) – are essentially those of hormones (Mandarino et al., Citation2015; Wacker & Holick, Citation2013a). Ordinarily, only ∼10% of the total daily vitamin D requirement, as both vitamin D2 and vitamin D3, is derived from the non-supplemented diet through consumption of foods, such as certain vegetables, mushrooms, egg yolk, cheese, fatty fish, fish oils, and beef liver; the major natural source is endogenous synthesis initiated through absorption of sunlight irradiation (UV-B: 290–315 nm λ spectrum) by 7-dehydrocholesterol in living cells of the epidermis. This produces unstable previtamin D3 that is rapidly transformed via thermal isomerization into vitamin D3 and exported into systemic circulation as a protein-bound complex. Vitamin D3 is hydroxylated at carbon 25 to produce 25-hydroxyvitamin D3 [25(OH)D] in the liver and then hydroxylated at carbon 1 by 1α-hydroxylase (1α-OHase) to yield 1,25-dihydroxyvitamin D3 [1,25(OH)2D3] primarily in mitochondria of the proximal convoluted tubular cells of the kidney but also certain other peripheral tissues. 1,25(OH)2D3 is highly regulated by parathyroid hormone, whose circulation is induced by low levels of serum calcium to enhance renal calcium reabsorption, skeletal calcium liberation, and renal 1,25(OH)2D3 synthesis (Mandarino et al., Citation2015; Wacker & Holick, Citation2013a). This active form of vitamin D crosses the cell membrane and binds to cytoplasmic vitamin D receptor (VDR). Thereafter, the 1,25(OH)2D3-VDR complex locates to the nucleus where it heterodimerizes with retinoic acid receptor (RXR). The 1,25(OH)2D3-VDR-RXR complex subsequently binds to response elements of vitamin D (VDRE) in the promoter region of target genes to stimulate RNA transcription and protein expression that mediates many diverse actions (Mandarino et al., Citation2015; Min, Citation2013; Wacker & Holick, Citation2013a). Such vitamin D-regulated gene expression occurs in a broad array of cells, including β-pancreatic, epidermal small intestine, hematopoietic, parathyroid gland, placental, renal tubular, vascular endothelial, and smooth muscle ones, plus cardiomyocytes, lymphocytes, myocytes, osteoblasts, and osteoclasts (Mandarino et al., Citation2015; Wacker & Holick, Citation2013a). In this manner, vitamin D directly or indirectly controls and regulates a wide array of processes of cells plus endocrine, immune, nervous, and cardiovascular systems (Bouillon et al., Citation2008; Mandarino et al., Citation2015; Wacker & Holick, Citation2013a). This is not only the basis for the diverse beneficial actions of vitamin D, but also the deleterious effects of vitamin D insufficiency and deficiency especially in the presence VDR gene polymorphisms, e.g., autoimmune and chronic inflammatory diseases, endocrine abnormalities, hypertension, vascular pathologies, and various cancers (Chen et al., Citation2005; Gandini et al., Citation2014; Hossein-nezhad & Holick, Citation2013; Juniku-Shkololli et al., Citation2015; Koroshi & Idrizi, Citation2011; Li et al., Citation2004; Mandarino et al., Citation2015; Min, Citation2013; Mozos & Marginean, Citation2015; Ordóñez Mena & Brenner, Citation2014; Santoro et al., Citation2015; Shui & Giovannucci, Citation2014; Wacker & Holick, Citation2013a,Citationb).
Although 1,25(OH)2D3 is the most biologically active form of vitamin D, it is the 25(OH)D blood level that is assessed clinically. Guidelines issued by the World Health Organization (WHO), US Institute of Medicine (IOM), and US Endocrine Society (ES) for interpretation of such values vary somewhat. ES guidelines are based on the evaluation and treatment of at-risk vitamin D-deficient patients with specific diseases, while those of the IOM are based on dietary reference intake of vitamin D in the healthy North American population (Gallagher & Sai, Citation2010; Pramyothin & Holick, Citation2012; Wacker & Holick, Citation2013b). The IOM regards a 25(OH)D level of 16 ng/ml (40 nmol/l) sufficient to meet the requirement of ∼50% the population and 20 ng/ml (50 nmol/l) sufficient to meet the requirement of ≥97.5% of the population. Thus, IOM guidelines view a 25(OH)D level <12 ng/mL (<30 nmol/L) as deficient and 12–20 ng/mL (30–50 nmol/L) as insufficient. The ES, on the other hand, lists the corresponding thresholds as <20 ng/mL (50 nmol/L) and 21–29 ng/mL (∼50–75 nmol/L). The WHO Scientific Group on the Prevention and Management of Osteoporosis (Citation2003) defines vitamin D insufficiency as a serum level of 25(OH)D <20 ng/ml (50 nmol/L).
Vitamin D insufficiency and deficiency are quite common worldwide (Hilger et al., Citation2014; Holick, Citation2007; Hossein-nezhad & Holick, Citation2012; Looker et al., Citation2011). Findings of the 2001–2006 cycle of the US National Health and Nutrition Survey, based on US IOM reference thresholds, revealed ∼73% of African Americans, ∼42% of Mexican Americans, and 34% of Caucasian Americans to be at risk to vitamin D deficiency and inadequacy (Looker et al., Citation2011). Another report utilizing ES guidelines estimates ∼75% of the adult US population has a vitamin D level that is too low (Binkley et al., Citation2010). Hypovitaminosis is comparable or even worse in many other countries. A 2012 Guiyang, China survey conducted on healthy adult males reported vitamin D deficiency in ∼50% and vitamin D insufficiency in another ∼32% (Zhang et al., Citation2012), findings consistent with a more recent and larger multicenter Chinese survey involving both men and women (Yu et al., Citation2015). A 2011 northern India survey conducted on men >50 yrs of age determined hypovitaminosis D in that region to be almost universal, with vitamin D deficiency found in ∼91% and vitamin D insufficiency in another ∼7% (Marwaha et al., Citation2011a). Finally, a Niigata, Japan survey discovered only ∼9% of adults 40–74 yrs of age to be vitamin D sufficient (Nakamura et al., Citation2015). Even in Australia – often referred to as the skin cancer capital of the world – some 40% of the population is vitamin D deficient, either due to prevalent reliance on UV-B sunscreen for protection against melanoma and other skin cancers and conditions or lack of sunlight exposure (Daly et al., Citation2012; Working Group of the Australian and New Zealand Bone and Mineral Society et al., Citation2005).
As earlier discussed, increased incidence of BC is reported in women employed in night and rotating shift work as well as those who reside in community and home environments rich in ALAN. However, risk of BC (Goodwin et al., Citation2009; Imtiaz & Siddiqui, Citation2014; John et al., Citation1999; Obaidi et al., Citation2014; Ordóñez Mena & Brenner, Citation2014; Rose et al., Citation2013) and other cancers (Wacker & Holick, Citation2013a) is also known to be vitamin D status-dependent. Meta-analysis of reported findings from >100 countries reveals an inverse relationship between incident solar UV-B irradiation and incidence of several cancers of both men and women – bladder, colorectal, esophageal, gastric, lung, pancreatic, rectal, renal, and Hodgkin’s and non-Hodgkin’s lymphoma – and specifically in women – breast, cervical, endometrial, ovarian, and vulvar cancers (Grant, Citation2012). Such an association is also suggested for PC incidence of men (Hanchette & Schwartz, Citation1992; Luscombe et al., Citation2001; Schwartz & Hulka, Citation1990). In this regard, hypovitaminosis D is especially prevalent in night workers (Munter et al., Citation2015), perhaps, more so in females than males (Ward et al., Citation2011), and it is also prevalent in pregnant and postpartum breast feeding women and their newborns (Dawodu & Tsang, Citation2012; Jan Mohamed et al., Citation2014; Marwaha et al., Citation2011b; Xiang et al., Citation2013). Temporal variation of vitamin D concentration in breast milk is likely due to time-of-day patterns in the ingestion of foods and supplements containing vitamin D and also sunlight exposure. The health effects of Vitamin D insufficiency and deficiency are many and seem to play an important role in various prevalent so-called diseases of civilization beyond bone health and cancer risk, e.g., hypertension – through compromised suppression of renin resulting in over-expression of the renin–angiotensin system – and cardiovascular disease – through disturbed endothelial, vascular smooth muscle, and cardiomyocyte regulation (Mandarino et al., Citation2015; Min, Citation2013; Wacker & Holick, Citation2013a).
Sunlight-induced vitamin D synthesis is affected by several factors, the more important ones being age, latitude of residence, pigmentation of skin, and chemical, physical, and other barriers of UV-B irradiation, e.g., sunscreen creams and sprays, clothing, air pollutants, and glass and plastic material blockade (Wacker & Holick, Citation2013a). In actuality, only ∼1% of solar UV-B irradiation reaches the earth’s surface, even in summer at noontime, because 100% of the λs up to ∼290 nm and ∼99% of those between 291 and 320 nm are absorbed by the ozone layer of the stratosphere. The amount of incident UV-B irradiation that arrives to the earth’s atmosphere exhibits predictable time-of-day variation due to predictable time-of-day change in sun zenith angle, i.e., angle of sunlight with respect to its vertical position when directly overhead. As the angle progressively changes during the day, the distance through which UV-B irradiation traverses the ozone layer differs. This results in temporal patterning in the amount of UV-B irradiation absorbed by the ozone layer and, thus, the amount reaching the skin, wherein a large proportion is absorbed by melanin, proteins, and other constituents of the stratum corneum or back scattered and reflected. Zenith angle is greater after morning sunrise and before evening sunset; accordingly, natural vitamin D synthesis tends to be least in the hours around dawn and dusk and greatest midday and early afternoon (Halloran et al., Citation1985; Masood et al., Citation2015; Rejnmark et al., Citation2002). In our opinion, orderly biological timekeeping during the 24 h and year, particularly synchronization of the human CTS, entails the daytime sunlight-derived blue-violet (446–484 nm) λ spectrum, in particular, that signals the SCN plus the UV-B (290–315 nm) λ spectrum that initiates vitamin D synthesis with subsequent pervasive actions of the active forms on many diverse processes of cells, tissues, and organs. Incident UV-B irradiation and vitamin D also exhibit predictable variation over the year due to change in sun position north and south of the equator (Berry et al., Citation2013; Cinar et al., Citation2014; Hoteit et al., Citation2014; Millen et al., Citation2010; Porojnicu et al., Citation2007), thereby modulating aspects of the annual biological time structure. This seems to be especially true for people residing beyond ∼33° latitude who are able to synthesize little, if any, vitamin D3 during winter; residents of Boston, MA (USA) at 42° North latitude, even if fully exposed to sunlight, are essentially unable to synthesize vitamin D3 between November and February, and residents of Edmonton, Canada at 52° North latitude, Bergen, Norway at 60° North latitude, and Ushuaia, Argentina at 55° South latitude are unable to generate significant D3 synthesis for ∼6 months of the year (Wacker & Holick, Citation2013a).
Discussion
Living organisms, including humanoids, are genetically adapted to the natural environment experienced by their ancestors during evolution. This environment is defined by sunlight during the day alternating with darkness, except for the dim illumination of stars, planets, and moon, during the nighttime. It is no surprise that living organisms developed biological clocks to optimally regulate and coordinate biological processes in the form of the CTS as a means of efficiently predicting and responding to the temporally varying metabolic and other demands of the cyclic ambient environment. The CTS is viewed as being orchestrated by a master brain clock – the paired nuclei of SCN located within the hypothalamus – that coordinates subservient peripheral clocks – with τ set to 24 h and phasing appropriately staged to environmental time. The diurnal cycle of blue λ spectrum sunlight, defined by its onset and offset times and therefore daily duration, modulates the clock genes in the SCN plus melatonin synthesis in the pineal gland to internally synchronize the τ and φ of human circadian and circannual rhythms in external synchrony with expected, based on evolutionary history, corresponding periodic variations and challenges of the natural environment. The diurnal pattern of incident (sensible) UV-B irradiation, also defined by its onset and offset times, and therefore daily duration – all of which also undergo annual modulation – induces corresponding patterning of vitamin D synthesis. It is our opinion the diurnal cycle of sunlight-derived UV-B (290–315 nm λ) onset (dawn) and offset (dusk) plus the dependent nyctohemeral pattern of vitamin D synthesis convey temporal information of high relevance to orderly circadian timekeeping, although the latter has received relatively little study. The thus far limited laboratory research shows vitamin D is able to synchronize BMAL1 and Per2 circadian clock gene expression in adipose-derived stem cells (Gutierrez-Monreal et al., Citation2014), and several human subject studies (Chang et al., Citation2011; Hashimoto et al., Citation1997; Hébert et al., Citation2002; Mishima et al., Citation2001; Park & Tokura, Citation1999; Smith et al., Citation2004; Takasu et al., Citation2006) document the quality of the daytime light environment, i.e., recent routine wake-time light-intensity history, affects nocturnal melatonin synthesis plus its vulnerability to suppression and circadian phase alteration by ALAN: usual wake-time exposure of subjects to relatively dim compared to bright illumination is associated with lower nighttime melatonin synthesis plus greater melatonin suppression and circadian rhythm phase shift responses to test ALAN illumination.
The environment of today is far different than that experienced during evolution; in most parts of the world artificial light has replaced natural sunlight during the day and ALAN has replaced darkness during the night. Moreover, polluted skies and dense residential housing of cities reduce the amount of daytime UV-B irradiation reaching the earth’s surface thereby further contributing to the risk for vitamin D deficiency (Wacker & Holick, Citation2013a). A growing number of reports, several cited in this editorial, substantiate linkage between ALAN exposure and excess incidence of hormone-dependent cancers, both in community dwelling and shift-working women and men. The literature further suggests ALAN-induced melatonin suppression, likely in conjunction with CTS disruption, begins very early in life and continues throughout childhood, adolescence, and adulthood, thereby raising the possibility of risk for a broad array of medical conditions and pathology in children and adults, as new diseases of modern civilization. Proposed, but as yet incompletely verified, contributing circadian rhythm mechanisms underlying increased risk of human cancers and other pathologies are: melatonin synthesis suppression, CTS disruption, 24 h activity/sleep cycle perturbation with sleep deprivation, or a combination of some or all of these. Typically, melatonin suppression is defined in terms of changes in blood, saliva, or urinary melatonin or aMT6s levels before and after ALAN exposure, because currently there are no established threshold criteria, e.g., as established for vitamin D status, particularly with respect to circadian time. Vitamin supplementation is recommended for hypovitaminosis. If threshold values existed for defining and diagnosing hypomelatoninosis, would they be the basis for recommending melatonin supplementation?
There is as yet no consensus among researchers as to exactly which single circadian rhythm or set of circadian rhythms is appropriately indicative of the extent of CTS synchronization or desynchronization. Some chronobiologists rely on the dim light melatonin onset (DLMO) time relative to the mid-sleep point (Lewy, Citation2007; Lewy et al., Citation1999; Pandi-Perumal et al., Citation2007), while others rely on the peak or trough time primarily of the core body temperature circadian rhythm (Giebel et al., Citation2008; Goel, Citation2005; Klein & Wegman, Citation1979) or several circadian rhythms in combination (Adan et al., Citation2012; Chaumont et al., Citation1979; Gronfier et al., Citation2004; Reinberg et al., Citation2013; Vieux et al., Citation1979). Perhaps, assessment of specific clock genes in blood or white cells using convenient around-the-clock self-sampling methods, e.g., new dried blood technology (http://www.spotonsciences.com), may prove to be the best means of determining CTS status. Presently, the decision of which circadian marker rhythm(s) to select is difficult. A recent investigation entailing firefighters found co-existence in the same individual of both weak and strong oscillators, the former being more prone to circadian τ disruption than the latter (Reinberg et al., Citation2013). Finally, a limited number of studies indicate susceptibility of the τ of circadian rhythms to desynchronize from 24.0 h has a genetic basis, thereby suggesting an explanation for observed differences between individuals in risk to and extent of CTS disruption when subjected to rotating shift work and other perturbing challenges (Ashkenazi et al., Citation1993; Motohashi et al., Citation1995; Reinberg et al., Citation2015). Interestingly, individual differences in biological tolerance to CTS disruption of rotating shift workers were earlier substantiated based on sleep, mood, and other complaints surveyed by questionnaire or interview plus disease and pathology determined by routine annual clinical assessment (Reinberg et al., Citation2007). CTS disruption in some rotating shift workers was associated with severe intolerance with manifestation of symptoms and complaints (dyschronism) but not in some others who displayed good tolerance and without symptoms or complaints, the latter phenomenon termed allochronism (another way to react but without overt complaints, symptoms, or disability). However, both types of workers were not followed long enough to assess the risk for detrimental long-term medical outcomes of particular concern today, especially cancers.
If CTS disruption, however it be defined, plays a role in the etiology of ALAN-induced cancers and other pathology, exposure thresholds predictive of such detrimental outcomes, e.g., according to frequency (times per week, month, or year), duration (years vs. decades), and extent (ΔΨ ≥ 3 h but <12 h vs. ΔΨ = 1–3 h) of change in the 24 h socioecologic or sleep/wake synchronizer schedule, are at this time unknown. Endogenous thresholds predictive of detrimental outcomes also are unknown. In the case of rotating shift workers, these include extent of CTS disruption, which may differ according to the specific aspects, e.g., direction (delay vs. advance) and rate (slow vs. rapid) of rotation plus start and end times (relative to the diurnal activity in L/nocturnal sleep in D cycle typical for each individual employee according to chronotype) of shift schedule, but also clock gene polymorphisms (Gamble et al., Citation2011) that over the long-term perhaps confer differential risk, e.g., for BC of female employees (Dai et al., Citation2011; Rabstein et al., Citation2014; Reszka et al., Citation2013; Truong et al., Citation2014; Zienolddiny et al., Citation2013). Rapid 2–4-day shift rotations, specifically designed to respect as much as possible the usual human nyctohemeral sleep/wake and social rhythms, tend to be less disruptive of the CTS than slower 5–7-day ones (Chaumont et al., Citation1979; Vieux et al., Citation1979). Additionally, susceptibility to CTS disruption shows between-individual differences. A limited number of thus far small-subject field studies suggest workers who possess a strong CTS, expressed as large amplitude temperature, urinary glucocorticoid, and other key circadian rhythms, are more resistant to phase alteration, i.e., exhibit slower rate of φ shift of circadian rhythms, when rotating between day and night shifts, than those who possess a weak CTS, expressed as small amplitude circadian rhythms (Reinberg et al., Citation1979; Vidacek et al., Citation1993). Thus, if inheritance of a "weak" versus a "strong" CTS favors better tolerance to rotating shift work, does it also pose over the long-term higher risk to its harmful effects, especially when combined with bright ALAN? A hint, perhaps, of the answer to this question is suggested by findings of a small pilot study of day and nightshift nurses (Borges et al., Citation2007, Citation2008); nightshift nurses exposed to ALAN who reported less biological tolerance, i.e., disturbed sleep, excessive fatigue, bad mood or other psychological changes, etc., compared to those who reported more biological tolerance, i.e., no or only 1 of the symptoms, as a group evidenced on both work and off nights statistically significantly lower 24 h mean urinary aMT6s concentration (). However, it is unknown whether low melatonin level is a predictor or consequence of nightshift intolerance. Overall, even though the impact of frequent CTS disruption on health and well-being is a topic of current concern, there is as yet no consensus based on medical outcomes investigations or other acceptable criteria of those circadian processes that are most indicative of CTS status – circadian desynchronization versus synchronization. Neither is it known if animal models can be used to reliably derive such criteria and thresholds. Frequent CTS disruption in its own right has been demonstrated to decrease longevity in some, but not all, animal studies. The median life span of cardiomyopathic Syrian hamsters when subjected to weekly shift in the environmental L/D cycle, which induces alteration and accommodation of the activity/rest and other circadian rhythms, is shortened by 11% compared to controls maintained on the typical non-varying L/D schedule (Penev et al., Citation1998). Other laboratory rat studies found shifting every 3 days between 14 L/10D and 10D/14 L (Li & Xu, Citation1997) or exposure to constant light (Valdés-Tovar et al., Citation2015) impaired immune responsivity. On the other hand, other laboratory animal research entailing insects – codling moth and face fly – and rodents maintained on diverse combined L/D and feeding regimens found no effects on longevity (Hayes, Citation1989; Nelson & Halberg, Citation1986).
FIGURE 6. Data (x ± SE) from the same aMT6 nursing study as for , however, with focus only on nightshift nurses (12 h night shift [19:00–07:00 h] followed by 36 h off time) categorized according to more (n = 8) versus less (n = 4) tolerance to night work (Borges et al., Citation2008). Tolerance to night work was determined by self-assessment of fatigue, sleep disturbances, insomnia, sleepiness, minor psychological symptoms, and satisfaction with free (off-work) time, with those presenting ≥2 of these complaints considered less tolerant and those presenting with none or only 1 of them considered more tolerant. Nightshift nurses having more compared to less tolerance to their working arrangement exhibit higher mean aMT6s concentration when working during the night and sleeping during the day as well as during off days when adhering to a normal diurnal activity/nocturnal sleep routine. The difference between the less and more tolerant nightshift groups in mean aMT6s concentration for the combination of the work and off days is statistically significant (ANOVA; p < 0.05). [Figure constructed using previously unpublished data collected by Borges et al. (Citation2007, Citation2008)].
![FIGURE 6. Data (x ± SE) from the same aMT6 nursing study as for Figure 3, however, with focus only on nightshift nurses (12 h night shift [19:00–07:00 h] followed by 36 h off time) categorized according to more (n = 8) versus less (n = 4) tolerance to night work (Borges et al., Citation2008). Tolerance to night work was determined by self-assessment of fatigue, sleep disturbances, insomnia, sleepiness, minor psychological symptoms, and satisfaction with free (off-work) time, with those presenting ≥2 of these complaints considered less tolerant and those presenting with none or only 1 of them considered more tolerant. Nightshift nurses having more compared to less tolerance to their working arrangement exhibit higher mean aMT6s concentration when working during the night and sleeping during the day as well as during off days when adhering to a normal diurnal activity/nocturnal sleep routine. The difference between the less and more tolerant nightshift groups in mean aMT6s concentration for the combination of the work and off days is statistically significant (ANOVA; p < 0.05). [Figure constructed using previously unpublished data collected by Borges et al. (Citation2007, Citation2008)].](/cms/asset/a0c3011a-9be9-46f3-910c-3023055a8cef/icbi_a_1072002_f0006_b.jpg)
In this editorial, we introduce the novel idea that the diurnal pattern of incident sunlight UV-B irradiation – especially its onset and offset times during the day – may constitute an additional important, but as yet unappreciated, temporal cue that serves to bolster synchronization of the CTS through stimulation of vitamin D synthesis and subsequent actions of active metabolites at all hierarchical levels. Under the natural environmental conditions experienced during evolution by our ancestors, orderly biological timekeeping, particularly synchronization of the CTS, in our opinion, involved complementary alternating-in-time dual ambient signaling phenomena and biological response mechanisms. During the daytime activity span, we propose they entailed primarily the sunlight blue-violet (446–484 nm λ) spectrum that modulates the SCN plus the UV-B (290–315 nm λ) spectrum that initiates vitamin D synthesis and generates its biologically active forms. During the nighttime rest span, we propose this entailed darkness (absence of biologically active light intensity) that under the control of the SCN and pineal gland results in melatonin synthesis and thereafter actions of this hormone on many of the same cells, tissues, and organs as vitamin D. This position is consistent with the concept of a dual, morning (dawn) and evening (dusk), oscillator timekeeping system (Benstaali et al., Citation2001; Bywalez et al., Citation2012; Oda et al., Citation2000; Wehr et al., Citation1995; Yoshii et al., Citation2012). We view the health concerns currently ascribed primarily to ALAN-induced melatonin suppression and/or CTS disruption as potentially being the consequence of pervasive perturbation of the entire natural L/D environment of our evolutionary past. We propose the deleterious effects currently attributed to ALAN, alone, may also include insufficient, abnormally timed, or near absence of natural daytime sunlight exposure and associated time cues, including those conveyed by the active metabolites of vitamin D synthesis that show diurnal and annual patterning. This hypothesis, of course, requires rigorous exploration and validation. Exposure to today’s abnormal artificial light environment devoid of daytime UV-B and other biologically active λs and that is rich in ALAN, causing vitamin D insufficiency plus melatonin suppression often accompanied with CTS disruption, begins at the very commencement of life. Thus, antecedent and continuing day/night artificial light exposure plus routine behaviors, such as ingestion of certain classes of medications like α1- and β1-adrenoreceptor antagonist medications that mimic the effects of ALAN on melatonin suppression and possibly induce CTS perturbation, must be taken into consideration to completely understand the mechanism(s) responsible for the increased incidence of hormone-dependent tumor incidence, and perhaps other not yet recognized resultant medical conditions, as well as their prevention. We call on human ecologists, epidemiologists, chronobiologists, and scientists of other disciplines to expend their efforts to assess in a comprehensive manner the effects and potential negative consequences of life today in an environment defined by too little sunlight during the daytime in combination with too much artificial light during the nighttime.
Declaration of Interest
Linda Sackett-Lundeen and Francesco Portaluppi have no conflict of interests to state. Michael Smolensky is a consultant to Spot on Sciences (Austin, Texas USA), a company that specializes in dried blood self-sampling technology.
References
- Ackermann K, Plomp R, Lao O, et al. (2013). Effect of sleep deprivation on rhythms of clock gene expression and melatonin in humans. Chronobiol Int. 30:901–9
- Adan A, Archer SN, Hidalgo MP, et al. (2012). Circadian typology: A comprehensive review. Chronobiol Int. 29:1153–75
- Akbar S, Alorainy MS. (2014). The current status of beta blockers' use in the management of hypertension. Saudi Med J. 35:1307–17
- Alamili M, Klein M, Lykkesfeldt J, et al. (2013). Circadian variation in the response to experimental endotoxemia and modulatory effects of exogenous melatonin. Chronobiol Int. 30:1174–80
- Albrecht U. (2012). Timing to perfection: The biology of central and peripheral clocks. Neuron. 74:246–60
- Aleandri V, Spina V, Ciardo A. (1997). [The role of the pineal body in the endocrine control of puberty]. Minerva Ginecol. 49:43–8
- Alikasifoglu A, Vuralli D, Gonc EN, et al. (2015). Changing etiological trends in male precocious puberty: Evaluation of 100 cases with central precocious puberty over the last decade. Horm Res Paediatr. 83:340–4
- Anderson SE, Must A. (2005). Interpreting the continued decline in the average age at menarche: Results from two nationally representative surveys of U.S. girls studied 10 years apart. J Pediatr. 147:753–60
- Appleton RE, Jones AP, Gamble C, et al. (2012). The use of melatonin in children with neurodevelopmental disorders and impaired sleep: A randomised, double-blind, placebo-controlled, parallel study (MENDS). Health Technol Assess. 16:i–000239
- Ardura J, Gutierrez R, Andres J, Agapito T. (2003). Emergence and evolution of the circadian rhythm of melatonin in children. Horm Res. 59:66–72
- Arendt J. (1992). The pineal. In: Touitou Y, Haus E. eds. Biologic rhythms in clinical and laboratory medicine. Berlin: Springer-Verlag, pp. 348–62
- Arendt J. (2011). The pineal gland and pineal tumours. [Updated 2011 Jan 1]. In: De Groot LJ, Beck-Peccoz P, Chrousos G, et al. eds. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc; 2000
- Arora T, Broglia E, Thomas GN, Taheri S. (2014). Associations between specific technologies and adolescent sleep quantity, sleep quality, and parasomnias. Sleep Med. 15:240–7
- Arslanoglu S, Bertino E, Nicocia M, Moro GE. (2011). WAPM Working Group on Nutrition: Potential chronobiotic role of human milk in sleep regulation. J Perinat Med. 40:1–8
- Ashkenazi IE, Reinberg A, Bicakova-Rocher A, Ticher A. (1993). The genetic background of individual variations of circadian-rhythm periods in healthy human adults. Am J Hum Genet. 52:1250–9
- Benstaali C, Mailloux A, Bogdan A, et al. (2001). Circadian rhythms of body temperature and motor activity in rodents their relationships with the light–dark cycle. Life Sci. 68:2645–56
- Berry DJ, Hyppönen E, Cortina-Borja M. (2013). Investigating the association of vitamin D seasonality on inflammatory and hemostatic markers. Chronobiol Int. 30:786–95
- Bidabadi E, Mashouf M. (2010). A randomized trial of propranolol versus sodium valproate for the prophylaxis of migraine in pediatric patients. Paediatr Drugs. 12:269–75
- Binkley N, Ramamurthy R, Krueger D. (2010). Low vitamin D status: Definition, prevalence, consequences, and correction. Endocrinol Metab Clin North Am. 39: 287–301
- Borges FNS, Fischer FM, Moreno CRC, et al. (2007). 6-sulfatoxymelatonin among night healthcare workers: Differences between more and less tolerant to night works (abstract). 2nd Congress of Applied Chronobiology and Chronomedicine; Tunis, Tunsia
- Borges FNS, Fischer FM, Moreno CRC, et al. (2008). Tolerance to night work among nursing personnel, effects on sleepiness, sleep duration and sleep quality, on work and off-days. In: Sznelwar L, Mascia F, Montedo U, eds. Human factors in organizational design and management. 1st ed. São Paulo: Editora Blucher e IEA Press, v. IX, pp. 657–62
- Bouchlariotou S, Liakopoulos V, Giannopoulou M, et al. (2014). Melatonin secretion is impaired in women with preeclampsia and an abnormal circadian blood pressure rhythm. Ren Fail. 36:1001–7
- Bouillon R, Carmeliet G, Verlinden L, et al. (2008). Vitamin D and human health: Lessons from vitamin D receptor null mice. Endocr Rev. 29:726–76
- Brainard GC, Hanifin JP, Greeson JM, et al. (2001). Action spectrum for melatonin regulation in humans: Evidence for a novel circadian photoreceptor. J Neurosci. 21:6405–12
- Bromundt V, Frey S, Odermatt J, Cajochen C. (2014). Extraocular light via the ear canal does not acutely affect human circadian physiology, alertness and psychomotor vigilance performance. Chronobiol Int. 31:343–8
- Brown SB, Hankinson SE, Eliassen AH, et al. (2015). Urinary melatonin concentration and the risk of breast cancer in Nurses' Health Study II. Am J Epidemiol. 181:155–62
- Bruni O, Alonso-Alconada D, Besag F, et al. (2015). Current role of melatonin in pediatric neurology: Clinical recommendations. Eur J Paediatr Neurol. 19:122–33
- Burgess HJ, Molina TA. (2014). Home lighting before usual bedtime impacts circadian timing: A field study. Photochem Photobiol. 90:723–6
- Bywalez W, Menegazzi P, Rieger D, et al. (2012). The dual-oscillator system of Drosophila melanogaster under natural-like temperature cycles. Chronobiol Int. 29:395–407
- Cajochen C, Zeitzer JM, Czeisler CA, Dijk DJ. (2000). Dose-response relationship for light intensity and ocular and electroencephalographic correlates of human alertness. Behav Brain Res. 115:75–83
- Cajochen C, Frey S, Anders D, et al. (2011). Evening exposure to a light emitting diodes (LED)-backlit computer screen affects circadian physiology and cognitive performance. J Appl Physiol. 110:1432–8
- Carter BD, Diver WR, Hildebrand JS, et al. (2014). Circadian disruption and fatal ovarian cancer. Am J Prev Med. 46:S34–41
- Cavallo A. (1993). Melatonin and human puberty: Current perspectives. J Pineal Res. 15:115–21
- Chahal H, Fung C, Kuhle S, Veugelers PJ. (2013). Availability and night-time use of electronic entertainment and communication devices are associated with short sleep duration and obesity among Canadian children. Pediatr Obes. 8:42–51
- Chang AM, Scheer FA, Czeisler CA. (2011). The human circadian system adapts to prior photic history. J Physiol. 589:1095–102
- Chang AM, Aeschbach D, Duffy JF, Czeisler CA. (2015). Evening use of light-emitting eReaders negatively affects sleep, circadian timing, and next-morning alertness. Proc Natl Acad Sci USA. 112:1232–7
- Chau YM, West S, Mapedzahama V. (2014). Night work and the reproductive health of women: An integrated literature review. J Midwifery Womens Health. 59:113–26
- Chaumont A-J, LaPorte A, Nicolai A, Reinberg A. (1979). Adjustment of shift workers to a weekly rotation (study 1). Chronobiologia. 6:27–34
- Chen WY, Bertone-Johnson ER, Hunter DJ, et al. (2005). Associations between polymorphisms in the vitamin D receptor and breast cancer risk. Cancer Epidemiol Biomarkers Prev. 14:2335–9
- Chen YC, Tain YL, Sheen JM, Huang LT. (2012). Melatonin utility in neonates and children. J Formos Med Assoc. 111:57–66
- Chon JK, Borkowski A, Partin AW, et al. (1999). Alpha 1-adrenoceptor antagonists terazosin and doxazosin induce prostate apoptosis without affecting cell proliferation in patients with benign prostatic hyperplasia. J Urol. 161:2002–8
- Cinar N, Harmanci A, Yildiz BO, Bayraktar M. (2014). Vitamin D status and seasonal changes in plasma concentrations of 25-hydroxyvitamin D in office workers in Ankara, Turkey. Eur J Intern Med. 25:197–201
- Cole RJ, Kripke DF, Wisbey J, et al. (1995). Seasonal variation in human illumination exposure at two different latitudes. J Biol Rhythms. 10:324–34
- Collado Mateo MJ, Díaz-Morales JF, Escribano Barreno C, et al. (2012). Morningness–eveningness and sleep habits among adolescents: Age and gender differences. Psicothema. 24:410–15
- Commentz JC, Helmke K. (1995). Precocious puberty and decreased melatonin secretion due to a hypothalamic hamartoma. Horm Res. 44:271–5
- Cubero J, Valero V, Sánchez J, et al. (2005). The circadian rhythm of tryptophan in breast milk affects the rhythms of 6-sulfatoxymelatonin and sleep in newborn. Neuro Endocrinol Lett. 26:657–61
- Cubero J, Narciso D, Aparicio S, et al. (2006). Improved circadian sleep–wake cycle in infants fed a special day/night dissociated formula milk. Neuro Endocrinol Lett. 27:373–80
- Dai H, Zhang L, Cao M, et al. (2011). The role of polymorphisms in circadian pathway genes in breast tumorigenesis. Breast Cancer Res Treat. 127:531–40
- Daly RM, Gagnon C, Lu ZX, et al. (2012). Prevalence of vitamin D deficiency and its determinants in Australian adults aged 25 years and older: A national, population based study. Clin Endocrinol. (Oxf.) 77:26–35
- Dauchy RT, Xiang S, Mao L, et al. (2014). Circadian and melatonin disruption by exposure to light at night drives intrinsic resistance to tamoxifen therapy in breast cancer. Cancer Res. 74:4099–110
- Dawodu A, Tsang RC. (2012). Maternal vitamin D status: Effect on milk vitamin D content and vitamin D status of breastfeeding infants. Adv Nutr. 3:353–61
- deHaro D, Kines KJ, Sokolowski M, et al. (2014). Regulation of L1 expression and retrotransposition by melatonin and its receptor: Implications for cancer risk associated with light exposure at night. Nucleic Acids Res. 42:7694–707
- De Leersnyder H, De Blois MC, Claustrat B, et al. (2001). Inversion of the circadian rhythm of melatonin in the Smith-Magenis syndrome. J Pediatr. 139:111–16
- De Leersnyder H, Claustrat B, Munnich A, Verloes A. (2006). Circadian rhythm disorder in a rare disease: Smith-Magenis syndrome. Mol Cell Endocrinol. 252:88–91
- Diffey BL. (2011). An overview analysis of the time people spend outdoors. Br J Dermatol. 164:848–54
- DiNicolantonio JJ, Fares H, Niazi AK, et al. (2015). β-Blockers in hypertension, diabetes, heart failure and acute myocardial infarction: A review of the literature. Open Heart. 2:e000230
- Dumont M, Paquet J. (2014). Progressive decrease of melatonin production over consecutive days of simulated night work. Chronobiol Int. 31:1231–8
- Ekirch AR. (2001). Sleep we have lost: Pre-industrial slumber in the British Isles. Am Hist Rev. 106:343–86
- Ekirch AR. (2005). At day's close: Night in times past. New York: W. W. Norton & Company, 447pp
- Ekmekcioglu C. (2006). Melatonin receptors in humans: Biological role and clinical relevance. Biomed Pharmacother. 60:97–108
- Emens JS, Laurie AL, Songer JB, Lewy AJ. (2013). Non-24-hour disorder in blind individuals revisited: Variability and the influence of environmental time cues. Sleep. 36:1091–100
- Erren TC. (2005). Could visible light contribute to the development of leukaemia and other cancers in children? Med Hypotheses. 64:864–71
- Espiritu RC, Kripke DF, Ancoli-Israel S, et al. (1994). Low illumination experienced by San Diego adults: Association with atypical depressive symptoms. Biol Psychiatry. 35:403–7
- Euling SY, Selevan SG, Pescovitz OH, Skakkebaek NE. (2008). Role of environmental factors in the timing of puberty. Pediatrics. 121:S167–71
- Fagioli I, Barbato G, Wehr TA. (2001). Dynamics of electroencephalographic slow wave activity and body temperature during monophasic and biphasic human sleep. Neurosci Lett. 298:83–6
- Falbe J, Davison KK, Franckle RL, et al. (2015). Sleep duration, restfulness, and screens in the sleep environment. Pediatrics. 135:e367–75
- Feychting M, Osterlund B, Ahlbom A. (1998). Reduced cancer incidence among the blind. Epidemiology. 9:490–4
- Figueiro MG, Rea MS. (2012). Preliminary evidence that light through the eyelids can suppress melatonin and phase shift dim light melatonin onset. BMC Res Notes. 5:221
- Figueiro MG, Plitnick B, Rea MS. (2014). Pulsing blue light through closed eyelids: Effects on acute melatonin suppression and phase shifting of dimlight melatonin onset. Nat Sci Sleep. 6:149–56
- Flynn-Evans EE, Stevens RG, Tabandeh H, et al. (2009). Total visual blindness is protective against breast cancer. Cancer Causes Control. 20:1753–6
- Flynn-Evans EE, Mucci L, Stevens RG, Lockley SW. (2013). Shiftwork and prostate-specific antigen in the National Health and Nutrition Examination Survey. J Natl Cancer Inst. 105:1292–7
- Flynn-Evans EE, Tabandeh H, Skene DJ, Lockley SW. (2014). Circadian rhythm disorders and melatonin production in 127 blind women with and without light perception. J Biol Rhythms. 29:215–24
- Gallagher JC, Sai AJ. (2010). Vitamin D insufficiency, deficiency, and bone health. J Clin Endocrinol Metab. 95:2630–3
- Gamble AL, D'Rozario AL, Bartlett DJ, et al. (2014). Adolescent sleep patterns and night-time technology use: Results of the Australian Broadcasting Corporation's Big Sleep Survey. PLoS One. 9:e111700
- Gamble KL, Motsinger-Reif AA, Hida A, et al. (2011). Shiftwork in nurses: Contribution of phenotypes and genotypes to adaptation. PLoS One. 6:e18395
- Gandini S, Gnagnarella P, Serrano D, et al. (2014). Vitamin D receptor polymorphisms and cancer. Adv Exp Med Biol. 810:69–105
- Giebel O, Wirtz A, Nachreiner F. (2008). The interference of flexible working times with the circadian temperature rhythm--a predictor of impairment to health and well-being? Chronobiol Int. 25:263–70
- Giménez MC, Beersma DG, Bollen P, et al. (2014). Effects of a chronic reduction of short-wavelength light input on melatonin and sleep patterns in humans: Evidence for adaptation. Chronobiol Int. 31:690–7
- Gitto E, Marseglia L, Manti S, et al. (2013). Protective role of melatonin in neonatal diseases. Oxid Med Cell Longev. 2013:980374
- Glickman G, Levin R, Brainard GC. (2002). Ocular input for human melatonin regulation: Relevance to breast cancer. Neuro Endocrinol Lett. 23:17–22
- Goel N. (2005). Late-night presentation of an auditory stimulus phase delays human circadian rhythms. Am J Physiol Regul Integr Comp Physiol. 289:R209–16
- Goodwin PJ, Ennis M, Pritchard KI, et al. (2009). Prognostic effects of 25-hydroxyvitamin D levels in early breast cancer. J Clin Oncol. 27:3757–63
- Gooley JJ, Chamberlain K, Smith KA, et al. (2011). Exposure to room light before bedtime suppresses melatonin onset and shortens melatonin duration in humans. J Clin Endocrinol Metab. 96:E463–72
- Gradisar M, Wolfson AR, Harvey AG, et al. (2013). The sleep and technology use of Americans: Findings from the National Sleep Foundation's 2011 Sleep in America poll. J Clin Sleep Med. 9:1291–9
- Grant WB. (2012). Ecological studies of the UVB-vitamin D-cancer hypothesis. Anticancer Res. 32:223–36
- Gronfier C, Wright KP Jr, Kronauer RE, et al. (2004). Efficacy of a single sequence of intermittent bright light pulses for delaying circadian phase in humans. Am J Physiol Endocrinol Metab. 287:E174–81
- Grundy A, Sanchez M, Richardson H, et al. (2009). Light intensity exposure, sleep duration, physical activity, and biomarkers of melatonin among rotating shift nurses. Chronobiol Int. 26:1443–61
- Gutierrez-Monreal MA, Cuevas-Diaz Duran R, Moreno-Cuevas JE, Scott SP. (2014). A role for 1α,25-dihydroxyvitamin d3 in the expression of circadian genes. J Biol Rhythms. 29:384–8
- Hahn RA. (1991). Profound bilateral blindness and the incidence of breast cancer. Epidemiology. 2:208–10
- Haim A & Portnov BA. (2013). Light pollution as a new risk factor for human breast and prostate cancers. Dordrecht: Springer, 168 pp
- Haim A, Zubidat AE. (2015). Artificial light at night: Melatonin as a mediator between the environment and epigenome. Philos Trans R Soc Lond B Biol Sci. 370(1667). pii:20140121
- Halloran BP, Portale AA, Castro M, et al. (1985). Serum concentration of 1,25-dihydroxyvitamin D in the human: Diurnal variation. J Clin Endocrinol Metab. 60:1104–10
- Hanchette CL, Schwartz GG. (1992). Geographic patterns of prostate cancer mortality. Evidence for a protective effect of ultraviolet radiation. Cancer. 70:2861–9
- Hartz I, Furu K, Bratlid T, et al. (2012). Hypnotic drug use among 0–17 year olds during 2004–2011: A nationwide prescription database study. Scand J Public Health. 40:704–11
- Hashimoto S, Kohsaka M, Nakamura K, et al. (1997). Midday exposure to bright light changes the circadian organization of plasma melatonin rhythm in humans. Neurosci Lett. 221:89–92
- Haus E, Smolensky MH. (2013). Shift work and cancer risk: Role of circadian disruption, sleep deprivation, and light at night as potential mechanisms. Sleep Med Rev. 17:273–84
- Hayes DK. (1989). Insects as novel models for research in chronobiology. Chronobiologia. 16:417–20
- Hébert M, Dumont M, Paquet J. (1998). Seasonal and diurnal patterns of human illumination under natural conditions. Chronobiol Int. 15:59–70
- Hébert M, Martin SK, Lee C, Eastman CI. (2002). The effects of prior light history on the suppression of melatonin by light in humans. J Pineal Res. 33:198–203
- Hermida RC, Ayala DE, Fernández JR, et al. (2011). Circadian rhythms in blood pressure regulation and optimization of hypertension treatment with ACE inhibitor and ARB medications. Am J Hypertens. 24:383–91
- Hermida RC, Ayala DE, Fernández JR, et al. (2013). Administration-time differences in effects of hypertension medications on ambulatory blood pressure regulation. Chronobiol Int. 30:280–314
- Higuchi S, Nagafuchi Y, Lee SI, Harada T. (2014). Influence of light at night on melatonin suppression in children. J Clin Endocrinol Metab. 99:3298–303
- Hilger J, Friedel A, Herr R, et al. (2014). A systematic review of vitamin D status in populations worldwide. Br J Nutr. 111:23–45
- Hill SM, Belancio VP, Dauchy RT, et al. (2015). Melatonin: An inhibitor of breast cancer. Endocr Relat Cancer. 22:R183–204
- Holick MF. (2007). Vitamin D deficiency. N Engl J Med. 357:266–81
- Hoogerwerf WA, Hellmich HL, Cornelissen G, et al. (2007). Clock gene expression in the murine gastrointestinal tract: Endogenous rhythmicity and effects of a feeding regimen. Gastroenterology. 133:1250–60
- Hossein-nezhad A & Holick MF. (2012). Optimize dietary intake of vitamin D: An epigenetic perspective. Curr Opin Clin Nutr Metab Care. 15:567–79
- Hossein-nezhad A, Holick MF. (2013). Vitamin D for health: A global perspective. Mayo Clin Proc. 88:720–55
- Hoteit M, Al-Shaar L, Yazbeck C, et al. (2014). Hypovitaminosis D in a sunny country: Time trends, predictors, and implications for practice guidelines. Metabolism. 63:968–78
- Hysing M, Pallesen S, Stormark KM, et al. (2015). Sleep and use of electronic devices in adolescence: Results from a large population-based study. BMJ Open. 5:e006748
- I.A.R.C. Monograph 98. (2010). I.A.R.C. monographs on evaluation of carcinogenic risks to humans: Painting, firefighting, and shiftwork – section on mechanisms. Lyon, France: IARC Publication, pp. 818
- Illnerová H, Buresová M, Presl J. (1993). Melatonin rhythm in human milk. J Clin Endocrinol Metab. 77:838–41
- Imtiaz S, Siddiqui N. (2014). Vitamin-D status at breast cancer diagnosis: Correlation with social and environmental factors and dietary intake. J Ayub Med Coll Abbottabad. 26:186–90
- Jaldo-Alba F, Muñóz-Hoyos A, Molina-Carballo A, et al. (1993). Light deprivation increases plasma levels of melatonin during the first 72 h of life in human infants. Acta Endocrinol (Copenh). 129:442–5
- Jan Mohamed HJ, Rowan A, Fong B, Loy SL. (2014). Maternal serum and breast milk vitamin D levels: Findings from the Universiti Sains Malaysia Pregnancy Cohort Study. PLoS One. 9:e100705
- Jaruratanasirikul S, Sriplung H. (2014). Secular trends of growth and pubertal maturation of school children in Southern Thailand. Ann Hum Biol. 18:1–8
- John EM, Schwartz GG, Dreon DM, Koo J. (1999). Vitamin D and breast cancer risk: The NHANES I Epidemiologic follow-up study, 1971–1975 to 1992. National Health and Nutrition Examination Survey. Cancer Epidemiol Biomarkers Prev. 8:399–406
- Juniku-Shkololli A, Manxhuka-Kerliu S, Ahmetaj H, et al. (2015). Expression of immunohistochemical markers of progression in pre-cancerous and cancerous human colon: Correlation with serum vitamin D levels. Anticancer Res. 35:1513–20
- Jurvelin H, Takala T, Heberg L, et al. (2014). Transcranial bright light exposure via ear canals does not suppress nocturnal melatonin in healthy adults – a single-blind, sham-controlled, crossover trial. Chronobiol Int. 31:855–60
- Kanabrocki EL, Sothern RB, Scheving LE, et al. (1990). Circadian reference data for men in fifth decade of life. Prog Clin Biol Res. 341A:771–81
- Kennaway DJ. (2014). Light at night, melatonin and breast cancer. Chronobiol Int. 31:297–8
- Kennaway DJ, Stamp GE, Goble FC. (1992). Development of melatonin production in infants and the impact of prematurity. J Clin Endocrinol Metab. 75:367–9
- Kennaway DJ, Lushington K, Dawson D, et al. (1999). Urinary 6-sulfatoxymelatonin excretion and aging: New results and a critical review of the literature. J Pineal Res. 27:210–20
- Klein K, Wegman H-M. (1979). Circadian rhythms in air operations. In: Nicholson AN, ed. Sleep, wakefulness, and circadian rhythm. Neuilly sur Seine, France: AGARD Series No. 105. NATO/OTAN, pp. 10–25
- Klepeis NE, Nelson WC, Ott WR, et al. (2001). The National Human Activity Pattern Survey (NHAPS): A resource for assessing exposure to environmental pollutants. Expo Anal Environ Epidemiol. 11:231–52
- Kliukiene J, Tynes T, Andersen A. (2001). Risk of breast cancer among Norwegian women with visual impairment. Br J Cancer. 84:397–9
- Kloog I, Haim A, Stevens RG, Portnov BA. (2009). Global co-distribution of light at night (LAN) and cancers of prostate, colon, and lung in men. Chronobiol Int. 26:108–25
- Kloog I, Stevens RG, Haim A, Portnov BA. (2010). Nighttime light level co-distributes with breast cancer incidence worldwide. Cancer Causes Control. 21:2059–68
- Kloog I, Portnov BA, Rennert HS, Haim A. (2011). Does the modern urbanized sleeping habitat pose a breast cancer risk? Chronobiol Int. 28:76–80
- Knauth P, Rutenfranz J. (1981). Duration of sleep related to type of shift work. In: Reinberg A, Vieux N, Andlauer P. eds. Night and shift work: Biological and social aspects. Oxford. Pergamon Press, pp. 161–7
- Koroshi A, Idrizi A. (2011). Renoprotective effects of Vitamin D and renin–angiotensin system. Hippokratia. 15:308–11
- Kubo T, Ozasa K, Mikami K, et al. (2006). Prospective cohort study of the risk of prostate cancer among rotating-shift workers: Findings from the Japan collaborative cohort study. Am J Epidemiol. 164:549–55
- Leech JA, Wilby K, McMullen E, Laporte K. (1996). The Canadian Human Activity Pattern Survey: Report of methods and population surveyed. Chronic Dis Can. 17:118–23
- Lewy AJ. (2007). Melatonin and human chronobiology. Cold Spring Harb Symp Quant Biol. 72: 623–36
- Lewy AJ, Cutler NL, Sack RL. (1999). The endogenous melatonin profile as a marker for circadian phase position. J Biol Rhythms. 14:227–36
- Li JC, Xu F. (1997). Influences of light-dark shifting on the immune system, tumor growth and life span of rats, mice and fruit flies as well as on the counteraction of melatonin. Biol Signals. 6:77–89
- Li YC, Qiao G, Uskokovic M, et al. (2004). Vitamin D: A negative endocrine regulator of the renin-angiotensin system and blood pressure. J Steroid Biochem Mol Biol. 89–90:387–92
- Lockley SW, Skene DJ, Tabandeh H, et al. (1997a). Relationship between napping and melatonin in the blind. J Biol Rhythms. 12:16–25
- Lockley SW, Skene DJ, Arendt J, et al. (1997b). Relationship between melatonin rhythms and visual loss in the blind. J Clin Endocrinol Metab. 82:3763–70
- Lockley SW, Skene DJ, Butler LJ, Arendt J. (1999). Sleep and activity rhythms are related to circadian phase in the blind. Sleep. 22:616–23
- Lockley SW, Brainard GC, Czeisler CA. (2003). High sensitivity of the human circadian melatonin rhythm to resetting by short wavelength light. J Clin Endocrinol Metab. 88:4502–5
- Lockley SW, Evans EE, Scheer FA, et al. (2006). Short-wavelength sensitivity for the direct effects of light on alertness, vigilance, and the waking electroencephalogram in humans. Sleep. 29:161–8
- Looker AC, Johnson CL, Lachner DA, et al. (2011). Vitamin D Status: United States, 2001–2006. NCHS Data Brief. 56:1–8
- Luscombe CJ, Fryer AA, French ME, et al. (2001). Exposure to ultraviolet radiation: Association with susceptibility and age at presentation with prostate cancer. Lancet. 358:641–2
- Mahlberg R, Tilmann A, Salewski L, Kunz D. (2006). Normative data on the daily profile of urinary 6-sulfatoxymelatonin in healthy subjects between the ages of 20 and 84. Psychoneuroendocrinology. 31:634–41
- Mandarino NR, Júnior Fd, Salgado JV, et al. (2015). Isvitamin D deficiency a new risk factor for cardiovascular disease? Open Cardiovasc Med J. 9:40–9
- Mann NP, Haddow R, Stokes L, et al. (1986). Effect of night and day on preterm infants in a newborn nursery: Randomised trial. Br Med J (Clin Res Ed). 293:1265–7
- Martin-du-Pan R. (1970). Le róle du rythme circadian dans l'alimentation du nourrisson. La femme et l'enfant. 4:23–30
- Martinez-Nicolas A, Madrid JA, Rol MA. (2014). Day-night contrast as source of health for the human circadian system. Chronobiol Int. 31:382–93
- Marwaha RK, Tandon N, Garg MK, et al. (2011a). Vitamin D status in healthy Indians aged 50 years and above. J Assoc Physicians India. 59:706–9
- Marwaha RK, Tandon N, Chopra S, et al. (2011b). Vitamin D status in pregnant Indian women across trimesters and different seasons and its correlation with neonatal serum 25-hydroxyvitamin D levels. Br J Nutr. 106:1383–9
- Masood T, Kushwaha RS, Singh R, et al. (2015). Circadian rhythm of serum 25 (OH) vitamin D, calcium and phosphorus levels in the treatment and management of type-2 diabetic patients. Drug Discov Ther. 9:70–4
- Matz CJ, Stieb DM, Davis K, et al. (2014). Effects of age, season, gender and urban-rural status on time-activity: Canadian Human Activity Pattern Survey 2 (CHAPS 2). Int J Environ Res Public Health. 11:2108–24
- McMillen IC, Kok JS, Adamson TM, et al. (1991). Development of circadian sleep–wake rhythms in preterm and full-term infants. Pediatr Res. 29:381–4
- McMillen IC, Mulvogue HM, Kok JS, et al. (1993). Circadian rhythms in sleep and wakefulness and in salivary melatonin and cortisol concentrations in mothers of term and preterm infants. Sleep. 16:624–31
- Millen AE, Wactawski-Wende J, Pettinger M, et al. (2010). Predictors of serum 25-hydroxyvitamin D concentrations among postmenopausal women: The Women's Health Initiative Calcium plus Vitamin D clinical trial. Am J Clin Nutr. 91:1324–35
- Min B. (2013). Effects of vitamin d on blood pressure and endothelial function. Korean J Physiol Pharmacol. 17:385–92
- Mishima K, Okawa M, Shimizu T, Hishikawa Y. (2001). Diminished melatonin secretion in the elderly caused by insufficient environmental illumination. Clin Endocrinol Metab. 86:129–34
- Molina Carballo A, Muñoz Hoyos A, Uberos Fernández J, et al. (1996). [Pineal functioning (melatonin levels) in healthy children of different ages: An update and the value of pineal gland study in pediatrics]. An Esp Pediatr. 45:33–44
- Montagnese S, Middleton B, Corrias M, et al. (2015). Assessment of 6-sulfatoxymelatonin rhythms and melatonin response to light in disease states: Lessons from cirrhosis. Chronobiol Int. 32:187–94
- Morag I, Ohlsson A. (2013). Cycled light in the intensive care unit for preterm and low birth weight infants. Cochrane Database Syst Rev. 8:CD006982
- Morris DH, Jones ME, Schoemaker MJ, et al. (2011). Secular trends in age at menarche in women in the UK born 1908–93: Results from the Breakthrough Generations Study. Paediatr Perinat Epidemiol. 25: 394–400
- Motohashi Y, Reinberg A, Ashkenazi IE, Bicakova-Rocher A. (1995). Genetic aspects of circadian dyschronism: Comparison between Asiatic-Japanese and Caucasian-French populations. Chronobiol Int. 12:324–32
- Mozos I, Marginean O. (2015). Links between Vitamin D deficiency and cardiovascular diseases. Biomed Res Int. 2015:109275
- Munter G, Levi-Vineberg T, Sylvetsky N. (2015). Vitamin D deficiency among physicians: A comparison between hospitalists and community-based physicians. Osteoporos Int. 26:1673–6
- Nakamura K, Kitamura K, Takachi R, et al. (2015). Impact of demographic, environmental, and lifestyle factors on vitamin D sufficiency in 9084 Japanese adults. Bone. 74:10–17
- Nelson W, Halberg F. (1986). Schedule-shifts, circadian rhythms and lifespan of freely-feeding and meal-fed mice. Physiol Behav. 38:781–8
- Ng M, Knuth C, Weisbrod C, Murthy A. (2015). Propranolol therapy for problematic infantile hemangioma. Ann Plast Surg. 2015 May 22. [Epub ahead of print]
- Obaidi J, Musallam E, Al-Ghzawi HM, et al. (2014). Vitamin D and its relationship with breast cancer: An evidence based practice paper. Glob J Health Sci. 7:261–6
- Obayashi K, Saeki K, Iwamoto J, et al. (2014a). Independent associations of exposure to evening light and nocturnal urinary melatonin excretion with diabetes in the elderly. Chronobiol Int. 31:394–400
- Obayashi K, Saeki K, Iwamoto J, et al. (2014b). Effect of exposure to evening light on sleep initiation in the elderly: A longitudinal analysis for repeated measurements in home settings. Chronobiol Int. 31:461–7
- Obayashi K, Saeki K, Kurumatani N. (2014c). Association between light exposure at night and insomnia in the general elderly population: The HEIJO-KYO cohort. Chronobiol Int. 31:976–82
- Obayashi K, Saeki K, Kurumatani N. (2015). Light exposure at night is associated with subclinical carotid atherosclerosis in the general elderly population: The HEIJO-KYO cohort. Chronobiol Int. 32:310–17
- Oda GA, Menaker M, Friesen WO. (2000). Modeling the dual pacemaker system of the tau mutant hamster. J Biol Rhythms. 15:246–64
- Ordóñez Mena JM, Brenner H. (2014). Vitamin D and cancer: An overview on epidemiological studies. Adv Exp Med Biol. 810:17–32
- Pandi-Perumal SR, Smits M, Spence W, et al. (2007). Dim light melatonin onset (DLMO): A tool for the analysis of circadian phase in human sleep and chronobiological disorders. Prog Neuropsychopharmacol Biol Psychiatry. 31:1–11
- Park SJ, Tokura H. (1999). Bright light exposure during the daytime affects circadian rhythms of urinary melatonin and salivary immunoglobulin A. Chronobiol Int. 16:359–71
- Pechanova O, Paulis L, Simko F. (2014). Peripheral and central effects of melatonin on blood pressure regulation. Int J Mol Sci. 15:17920–37
- Peixoto CA, da Silva AG, Carskadon MA, Louzada FM. (2009). Adolescents living in homes without electric lighting have earlier sleep times. Behav Sleep Med. 7:73–80
- Penev PD, Kolker DE, Zee PC, Turek FW. (1998). Chronic circadian desynchronization decreases the survival of animals with cardiomyopathic heart disease. Am J Physiol. 275:H2334–7
- Porojnicu AC, Robsahm TE, Dahlback A, et al. (2007). Seasonal and geographical variations in lung cancer prognosis in Norway. Does Vitamin D from the sun play a role? Lung Cancer. 55:263–70
- Portaluppi F & Smolensky MH. (2010). Perspectives on the chronotherapy of hypertension based on the results of the MAPEC study. Chronobiol Int. 27:1652–67
- Portaluppi F, Montanari L, Ferlini M, Gilli P. (1990). Altered circadian rhythms of blood pressure and heart rate in non-hemodialysis chronic renal failure. Chronobiol Int. 7:321–7
- Portaluppi F, Vergnani L, Manfredini R, Fersini C. (1996). Endocrine mechanisms of blood pressure rhythms. Ann N Y Acad Sci. 783:113–31
- Portaluppi F, Boari B, Manfredini R. (2004). Oxidative stress in essential hypertension. Curr Pharm Des. 10:1695–8
- Pramyothin P & Holick MF. (2012). Vitamin D supplementation: Guidelines and evidence for subclinical deficiency. Curr Opin Gastroenterol. 28:139–50
- Pukkala E, Ojamo M, Rudanko SL, et al. (2006). Does incidence of breast cancer and prostate cancer decrease with increasing degree of visual impairment. Cancer Causes Control. 17: 573–6
- Rabstein S, Harth V, Justenhoven C, et al, GENICA Consortium. (2014). Polymorphisms in circadian genes, night work and breast cancer: Results from the GENICA study. Chronobiol Int. 31:1115–22
- Rahman SA, Shapiro CM, Wang F, et al. (2013). Effects of filtering visual short wavelengths during nocturnal shiftwork on sleep and performance. Chronobiol Int. 30:951–62
- Reinberg A, Vieux N, Ghata J, et al. (1979). Consideration of the circadian rhythm amplitude in relation to the ability to phase shift circadian rhythms of shift workers. Chronobiologia. 6:57–63
- Reinberg AE, Ashkenazi I, Smolensky MH. (2007). Euchronism, allochronism and dyschronism: Is internal desynchronization of human circadian rhythms a sign of illness? Chronobiol Int. 24:553–8
- Reinberg A, Riedel M, Brousse E, et al. (2013). Circadian time organization of professional firemen: Desynchronization-tau differing from 24.0 hours-documented by longitudinal self-assessment of 16 variables. Chronobiol Int. 30:1050–65
- Reinberg A, Smolensky MH, Riedel M, et al. (2015). Chronobiologic perspectives of Black Time – Accident risk is greatest at night: An opinion paper. Chronobiol Int. 32:1005–18
- Reiter RJ, Tan DX, Korkmaz A, Ma S. (2012). Obesity and metabolic syndrome: Association with chronodisruption, sleep deprivation, and melatonin suppression. Ann Med. 44:564–77
- Reiter RJ, Tamura H, Tan DX, Xu XY. (2014a). Melatonin and the circadian system: Contributions to successful female reproduction. Fertil Steril. 102:321–8
- Reiter RJ, Tan DX, Korkmaz A, Rosales-Corral SA. (2014b). Melatonin and stable circadian rhythms optimize maternal, placental and fetal physiology. Hum Reprod Update. 20:293–307
- Rejnmark L, Lauridsen AL, Vestergaard P, et al. (2002). Diurnal rhythm of plasma 1,25-dihydroxyvitamin D and vitamin D-binding protein in postmenopausal women: Relationship to plasma parathyroid hormone and calcium and phosphate metabolism. Eur J Endocrinol. 146:635–42
- Reppert SM. (1985). Maternal entrainment of the developing circadian system. In: Wurtman RJ, Baum MJ, Potts JT. eds. The medical and biological effects of light. Ann NY Acad Sci. 453:162–9
- Reppert SM, Weaver DR. (2002). Coordination of circadian timing in mammals. Nature. 418:935–41
- Reszka E, Peplonska B, Wieczorek E, et al. (2013). Rotating night shiftwork and polymorphism of genes important for the regulation of circadian rhythm. Scand J Work Environ Health. 39:178–86
- Rivkees SA, Mayes L, Jacobs H, Gross I. (2004). Rest-activity patterns of premature infants are regulated by cycled lighting. Pediatrics. 113:833–9
- Rose AA, Elser C, Ennis M, Goodwin PJ. (2013). Blood levels of vitamin D and early stage breast cancer prognosis: A systematic review and meta-analysis. Breast Cancer Res Treat. 14:331–9
- Rossignol DA, Frye RE. (2011). Melatonin in autism spectrum disorders: A systematic review and meta-analysis. Dev Med Child Neurol. 53:783–92
- Rybnikova N, Haim A, Portnov BA. (2015). Artificial light at night (ALAN) and breast cancer incidence worldwide: A revisit of an earlier findings with analysis of current trends. Chronobiol Int. 32:757–73
- Sánchez-Barceló EJ, Mediavilla MD, Reiter RJ. (2011). Clinical uses of melatonin in pediatrics. Int J Pediatr. 2011:892624
- Santoro D, Caccamo D, Lucisano S, et al. (2015). Interplay of vitamin D, erythropoiesis, and the renin-angiotensin system. Biomed Res Int. 2015:145828
- Schernhammer ES, Hankinson SE. (2009). Urinary melatonin levels and postmenopausal breast cancer risk in the Nurses' Health Study cohort. Cancer Epidemiol Biomarkers Prev. 18:74–9
- Schernhammer ES, Laden F, Speizer FE, et al. (2001). Rotating night shifts and risk of breast cancer in women participating in the nurses' health study. J Natl Cancer Inst. 93:1563–8
- Schernhammer ES, Rosner B, Willett WC, et al. (2004). Epidemiology of urinary melatonin in women and its relation to other hormones and night work. Cancer Epidemiol Biomarkers Prev. 13:936–43
- Schernhammer ES, Berrino F, Krogh V, et al. (2008). Urinary 6-sulfatoxymelatonin levels and risk of breast cancer in postmenopausal women. J Natl Cancer Inst. 100:898–905
- Schibler U. (2006). Circadian time keeping: The daily ups and downs of genes, cells, and organisms. Prog Brain Res. 153:271–82
- Schwartz GG, Hulka BS. (1990). Is vitamin D deficiency a risk factor for prostate cancer? (Hypothesis). Anticancer Res. 10:1307–11
- Schwimmer H, Metzer A, Pilosof Y, et al. (2014). Light at night and melatonin have opposite effects on breast cancer tumors in mice assessed by growth rates and global DNA methylation. Chronobiol Int. 31:144–50
- Seron-Ferre M, Valenzuela GJ, Torres-Farfan C. (2007). Circadian clocks during embryonic and fetal development. Birth Defects Res C Embryo Today. 81:204–14
- Shui I, Giovannucci E. (2014). Vitamin D status and cancer incidence and mortality. Adv Exp Med Biol. 810:33–51
- Singh M, Jadhav HR. (2014). Melatonin: Functions and ligands. Drug Discov Today. 19:1410–18
- Skene DJ, Lockley SW, Arendt J. (1999). Melatonin in circadian sleep disorders in the blind. Biol Signals Recept. 8:90–5
- Smith KA, Schoen MW, Czeisler CA. (2004). Adaptation of human pineal melatonin suppression by recent photic history. J Clin Endocrinol Metab. 89:3610–14
- Smolensky MH, Halberg F, Sargent II F. (1972). Chronobiology of the life sequence. In: Ogata SIK, Yoshimura H., eds. Advances in climatic physiology. Tokyo, Japan: Igaku Shoin, Chapter 18, pp. 281–319
- Smolensky MH, Siegel RA, Haus E, et al. (2012). Biological rhythms, drug delivery, and chronotherapeutics. In: Siepmann J, Siegel RA, Rathbone MJ. eds. Fundamentals and applications of controlled release drug delivery.Advances in delivery science and technology. Heidelberg: Springer, Chapter 13, pp. 359–443
- Song Y, Ma J, Wang HJ, et al. (2014). Trends of age at menarche and association with body mass index in Chinese school-aged girls, 1985–2010. J Pediatr. 165:1172–7
- Stevens RG. (2006). Artificial lighting in the industrialized world: Circadian disruption and breast cancer. Cancer Causes Control. 17:501–7
- Stevens RG. (2012). Does electric light stimulate cancer development in children? Cancer Epidemiol Biomarkers Prev. 21:701–4
- Stevens RG, Hansen J, Costa G, et al. (2011). Considerations of circadian impact for defining 'shift work' in cancer studies: IARC Working Group Report. Occup Environ Med. 68:154–62
- Stevens RG, Brainard GC, Blask DE, et al. (2014). Breast cancer and circadian disruption from electric lighting in the modern world. CA Cancer J Clin. 64:207–18
- Takasu NN, Hashimoto S, Yamanaka Y, et al. (2006). Repeated exposures to daytime bright light increase nocturnal melatonin rise and maintain circadian phase in young subjects under fixed sleep schedule. Am J Physiol Regul Integr Comp Physiol. 291:R1799–807
- Talma H, Schönbeck Y, van Dommelen P, et al. (2013). Trends in menarcheal age between 1955 and 2009 in the Netherlands. PLoS One. 8:e60056
- Tamura H, Nakamura Y, Terron MP, et al. (2008). Melatonin and pregnancy in the human. Reprod Toxicol. 25:291–303
- Tauman R, Zisapel N, Laudon M, et al. (2002). Melatonin production in infants. Pediatr Neurol. 26:379–82
- Thomas L, Drew JE, Abramovich DR, Williams LM. (1998). The role of melatonin in the human fetus (review). Int J Mol Med. 1:539–43
- Ticher A, Ashkenazi IE, Reinberg A. (1995). Preservation of the functional advantage of human time structure. FASEB J. 9:269–72
- Tordjman S, Najjar I, Bellissant E, et al. (2013). Advances in the research of melatonin in autism spectrum disorders: Literature review and new perspectives. Int J Mol Sci. 14:20508–42
- Tordjman S, Davlantis KS, Georgieff N, et al. (2015). Autism as a disorder of biological and behavioral rhythms: Toward new therapeutic perspectives. Front Pediatr. 3:1. doi: 10.3389/fped.2015.00001. eCollection 2015. Review
- Torres-Farfan C, Rocco V, Monsó C, et al. (2006). Maternal melatonin effects on clock gene expression in a nonhuman primate fetus. Endocrinology. 147:4618–26
- Tosini G, Owino S, Guillaume JL, Jockers R. (2014). Understanding melatonin receptor pharmacology: Latest insights from mouse models, and their relevance to human disease. Bioessays. 36:778–87
- Touitou Y. (2013). Adolescent sleep misalignment: A chronic jet lag and a matter of public health. J Physiol (Paris). 107:323–6
- Truong T, Liquet B, Menegaux F, et al. (2014). Breast cancer risk, night work, and circadian clock gene polymorphisms. Endocr Relat Cancer. 21:629–38
- Tsai SY, Barnard KE, Lentz MJ, Thomas KA. (2009). Twenty-four hours light exposure experiences in postpartum women and their 2-10-week-old infants: An intensive within-subject design pilot study. Int J Nurs Stud. 46:181–8
- Turner PL, Mainster MA. (2008). Circadian photoreception: Ageing and the eye's important role in systemic health. Br J Ophthalmol. 92:1439–44
- Valdés-Tovar M, Escobar C, Solís-Chagoyán H, et al. (2015). Constant light suppresses production of Met-enkephalin-containing peptides in cultured splenic macrophages and impairs primary immune response in rats. Chronobiol Int. 32:164–77
- van de Werken M, Giménez MC, de Vries B, et al. (2013). Short-wavelength attenuated polychromatic white light during work at night: Limited melatonin suppression without substantial decline of alertness. Chronobiol Int. 30:843–54
- Van den Bulck J. (2004). Television viewing, computer game playing, and Internet use and self-reported time to bed and time out of bed in secondary-school children. Sleep. 27:101–4
- Vásquez-Ruiz S, Maya-Barrios JA, Torres-Narváez P, et al. (2014). A light/dark cycle in the NICU accelerates body weight gain and shortens time to discharge in preterm infants. Early Hum Dev. 90:535–40
- Vidacek S, Radosević-Vidacek B, Kaliterna L, Prizmić Z. (1993). Individual differences in circadian rhythm parameters and short-term tolerance to shiftwork: A follow-up study. Ergonomics. 36:117–23
- Vieux N, Ghata J, LaPorte A, et al. (1979). Adjustment of shift workers adhering to a three-to four-day rotation (study 2). Chronobiologia. 6:37–42
- Voiculescu SE, Zygouropoulos N, Zahiu CD, Zagrean AM. (2014). Role of melatonin in embryo fetal development. J Med Life. 7:488–92
- Wacker M, Holick MF. (2013a). Sunlight and Vitamin D: A global perspective for health. Dermatoendocrinol. 5:51–108
- Wacker M, Holick MF. (2013b). Vitamin D - effects on skeletal and extraskeletal health and the need for supplementation. Nutrients. 5:111–48
- Wahnschaffe A, Haedel S, Rodenbeck A, et al. (2013). Out of the lab and into the bathroom: Evening short-term exposure to conventional light suppresses melatonin and increases alertness perception. Int J Mol Sci. 14:2573–89
- Waldhauser F, Boepple PA, Schemper M, et al. (1991). Serum melatonin in central precocious puberty is lower than in age-matched prepubertal children. J Clin Endocrinol Metab. 73:793–6
- Ward M, Berry DJ, Power C, Hyppönen E. (2011). Working patterns and vitamin D status in mid-life: A cross-sectional study of the 1958 British birth cohort. Occup Environ Med. 68:902–7
- Wehr TA. (1992). In short photoperiods, human sleep is biphasic. J Sleep Res. 1:103–7
- Wehr TA, Schwartz PJ, Turner EH, et al. (1995). Bimodal patterns of human melatonin secretion consistent with a two-oscillator model of regulation. Neurosci Lett. 194:105–8
- Wehr TA, Aeschbach D, Duncan WC Jr. (2001). Evidence for a biological dawn and dusk in the human circadian timing system. J Physiol. 535:937–51
- WHO scientific group on the prevention and management of osteoporosis. (2003). Prevention and management of osteoporosis: Report of a WHO scientific group. Geneva: World Health Organization
- Wittmann M, Dinich J, Merrow M, Roenneberg T. (2006). Social jetlag: Misalignment of biological and social time. Chronobiol Int. 23:497–509
- Working Group of the Australian and New Zealand Bone and Mineral Society; Endocrine Society of Australia; Osteoporosis Australia. (2005). Vitamin D and adult bone health in Australia and New Zealand: A position statement. Med J Aust. 182:281–5
- Wu AH, Wang R, Koh WP, et al. (2008). Sleep duration, melatonin and breast cancer among Chinese women in Singapore. Carcinogenesis. 29:1244–8
- Xiang F, Jiang J, Li H, et al. (2013). High prevalence of vitamin D insufficiency in pregnant women working indoors and residing in Guiyang, China. J Endocrinol Invest. 36:503–7
- Xiang S, Dauchy RT, Hauch A, et al. (2015). Doxorubicin resistance in breast cancer is driven by light at night-induced disruption of the circadian melatonin signal. J Pineal Res. 59:60–9
- Yoshii T, Rieger D, Helfrich-Förster C. (2012). Two clocks in the brain: An update of the morning and evening oscillator model in Drosophila. Prog Brain Res. 199:59–82
- Yu EA, Weaver DR. (2011). Disrupting the circadian clock: Gene-specific effects on aging, cancer, and other phenotypes. Aging (Albany NY). 3:479–93
- Yu S, Fang H, Han J, et al. (2015). The high prevalence of hypovitaminosis D in China: A multicenter vitamin D status survey. Medicine (Baltimore). 94:e585
- Zeitzer JM, Dijk D-J, Kronauer RE, et al. (2000). Sensitivity of the human circadian pacemaker to nocturnal light: Melatonin phase resetting and suppression. J Physiology. 526:695–702
- Zhang LT, Lee SW, Park K, et al. (2015). Multicenter, prospective, comparative cohort study evaluating the efficacy and safety of alfuzosin 10 mg with regard to blood pressure in men with lower urinary tract symptoms suggestive of benign prostatic hyperplasia with or without antihypertensive medications. Clin Interv Aging. 10:277–86
- Zhang Q, Shi LX, Peng NC, et al. (2012). Survey of vitamin D status in 700 healthy adult males in Guiyang, China. Zhonghua Yi Xue Za Zhi. 92:397–400
- Zhao ZY, Xie Y, Fu YR, et al. (2002). Aging and the circadian rhythm of melatonin: A cross-sectional study of Chinese subjects 30-110 yr of age. Chronobiol Int. 19:1171–82
- Zhu Y, Zheng T, Stevens RG, et al. (2006). Does "clock" matter in prostate cancer? Cancer Epidemiol Biomarkers Prev. 15:3–5
- Zienolddiny S, Haugen A, Lie JA, et al. (2013). Analysis of polymorphisms in the circadian-related genes and breast cancer risk in Norwegian nurses working night shifts. Breast Cancer Res. 15:R53

