Abstract
Background. The mechanisms leading to abnormal immune regulation in type 1 diabetes and allergic diseases may be partly overlapping. If so, these diseases should co-occur more often than expected. We investigated this phenomenon in two contrasting socio-economic environments, Finland and Russian Karelia.
Methods. We screened 413 Finnish children (of whom 147 had type 1 diabetes) and 244 Russian Karelian children (132 had type 1 diabetes) for total immunoglobulin E (IgE) levels and specific IgE against birch, cat, and egg albumen. In addition we analysed diabetes-related human leukocyte antigen (HLA) haplotypes and antibodies against hepatitis A virus (HAV) and recorded allergic diseases by a questionnaire in Russian Karelia.
Results. In Russian Karelia 15% of the patients with type 1 diabetes, but only 4% of the control subjects had allergen-specific IgE (P = 0.012). A similar difference was observed in the frequency of allergic symptoms. Co-occurrence of allergic sensitization and type 1 diabetes was associated with lack of HAV antibodies and was not seen in Finland where infections are less frequent than in Karelia.
Conclusion. Our findings support the idea of common mechanisms in the pathogenesis of allergic diseases and type 1 diabetes, which may be particularly important in an environment with low penetrance of these diseases.
Key messages
The frequency of allergic sensitization is three times higher than expected in Russian Karelian patients with type 1 diabetes. This suggests that common mechanisms may regulate the development of allergic diseases and type 1 diabetes.
It seems that a positive association between type 1 diabetes and allergy can be observed in countries with low income. This emphasizes the role of environmental factors, such as microbial infections, in the development of these diseases.
Introduction
According to the hygiene hypothesis microbial infections play an important role in the maturation of the immune system and regulate the risk of allergy. During recent years this hypothesis has been expanded to autoimmune diseases, and it has been suggested that infections may protect against both autoimmunity and allergy by a common mechanism (Citation1,Citation2). Such a mechanism could be related to the induction of immune regulation by a variety of infections in early childhood. Accordingly, a reduced exposure to microbes could lead to an over-reaction of immune responses involved in both autoimmune and allergic conditions. Consequently, autoimmune and allergic diseases should also coexist more frequently than expected.
Nonetheless, there is also a divergent view on the development of allergy and autoimmune diseases, namely the dichotomy of the immune responses resulting in either autoimmune or allergic responses. This dichotomy is represented by T helper 1 (Th1) and T helper 2 (Th2) cells. These cells represent distinct functional effectors of the T cell lineage. Th1 cells are responsible for cell-mediated immunity, and Th1-mediated immune responses are perceived to play a role in organ-specific autoimmunity such as type 1 diabetes. On the other hand, Th2 cells are involved in humoral immunity, and Th2-biased immune reactions are thought to guide the development of atopic diseases. Moreover, the characteristic cytokine products of Th1 (interferon[IFN]-γ, interleukin[IL]-2) and Th2 (IL-4, IL-5) cells are inhibitory towards differentiation and effector functions of the reciprocal cell type. According to this paradigm, autoimmune and allergic diseases should more likely occur in different than in the same individuals. It is worth noticing that the recent discovery of the Th17 cell lineage does not exclude this Th1/Th2 paradigm, but rather widens it to concern other T cells as well. Indeed, Th1 and Th2 cells have been shown to inhibit the expansion of Th17 cells, which in turn have reciprocal effects on regulatory T cells (Citation3).
Yet, in the light of the Th1/Th2 paradigm, a number of studies have evaluated whether allergic conditions are inversely related to organ-specific autoimmunity. Studies analysing the frequency of allergic manifestations in patients with type 1 diabetes have not shown a clear relationship (Citation4). Most of these surveys have focused on the prevalence of allergic symptoms or skin prick test results, but the few studies analysing the prevalence of allergen-specific immunoglobulin E (IgE) in patients with type 1 diabetes have not reported any definite relationship either (Citation5).
The aim of this study was to investigate the possible association between allergic sensitization and type 1 diabetes. As the socio-economic environment modulates the risk of these diseases, this study was carried out in two populations that live close to each other and share a similar genetic background, but are characterized by a conspicuous difference in the frequency of type 1 diabetes, allergy, and microbial infections, i.e. Finland and Russian Karelia (Citation6,Citation7).
Subjects and methods
Subjects
The study cohorts comprised children with type 1 diabetes and unaffected control subjects from both Finland and the Karelian Republic of the Russian Federation. In addition a separate group of children who had allergic symptoms was recruited in Russian Karelia. The study cohorts were recruited as a part of the type 1 diabetes-related EPIVIR project (EU INCO-Copernicus program, contract number IC15-CT98-0316, Co-ordinator Professor Hyöty), and their characteristics are presented in .
Table I. Characteristics of the study populations.
The series from Russian Karelia included a large cohort of non-diabetic school-children (n = 2070) and a separate cohort of 132 children with type 1 diabetes that were primarily identified from the records of the Republic Hospital in Petrozavodsk. The present study included all 132 patients diagnosed with type 1 diabetes in this hospital from 1990 to 1999. Non-diabetic control children represented a similar age and gender distribution, and season of blood sampling (no more than 2 months apart; year of the blood sampling may differ). Altogether 112 such control children who fulfilled these criteria were identified from the initial cohort of 2070 school-children. All of these children were included in the control group. Their serum samples were collected during the years 1997–2001. Neither diabetic children nor control children were selected in terms of atopic status or allergic symptoms. The diagnosis of diabetes had been made according to the World Health Organization criteria, and all patients were treated with daily insulin injections. The recruitment of these cohorts has been described in detail previously (Citation7,Citation8). In addition, a cohort of 33 allergic children with a history of allergic symptoms or asthma was enrolled in order to investigate the correlation of allergen-specific IgE and allergic symptoms. These children were identified from the initial cohort of school-children (n = 2070) using a questionnaire where diseases other than diabetes were recorded. Only 33 children reported allergic symptoms or asthma (16 children reported allergic dermatitis, 15 reported asthma, and 2 reported simply allergy), and all of these children were included in the group of allergic children. Serum samples from these children were collected in the years 1997–2001.
The Finnish cohort was recruited in the same way as the Karelian cohort and initially comprised 3654 school-children living in the Oulu region of Finland. A group of 266 non-diabetic school-children who had been previously analysed for allergen-specific IgE was included as the non-diabetic control group (Citation6,Citation8). We were able to identify 147 diabetic patients of a similar age and gender distribution, and season of sampling (no more than 2 months apart; year of the blood sampling may differ) from the outpatient Diabetes Clinic, Department of Paediatrics, University of Oulu. All of these diabetic patients were on daily insulin treatment. The Finnish samples were collected in the years 1991–1997. Unlike Russian Karelian children, no data were available on allergic symptoms in the Finnish children.
All of the children had written parental consent to participate in the study. The study plan was approved by the ethical committee of the Faculty of Medicine, University of Oulu, Finland, and by the Ministry of Health in the Karelian Republic of the Russian Federation. The reported investigations were carried out in accordance with the principles of the Declaration of Helsinki.
IgE assays
The levels of total IgE and allergen-specific IgE were measured using the ImmunoCAP® fluoroenzyme immunoassay (Phadia Diagnostics, Uppsala, Sweden). Specific IgE for two common inhalant allergens (birch and cat) and for egg albumin was analysed according to the manufacturer's instructions. For allergen-specific IgE, values of 0.35 kU/L or more were considered positive. Total IgE values of 100 kU/L or more were considered high, as values exceeding 100 kU/L have been considered as markers of atopic predisposition in previous studies (Citation6,Citation9).
Enzyme immunoassay (EIA) for hepatitis A virus antibodies
IgG class hepatitis A virus (HAV) antibodies were measured using the Enzygnost® Anti-HAV commercial EIA kit according to the manufacturer's instructions (Dade Behring, Marburg, Germany). HAV antibodies were analysed only in the Karelian children because previous studies have shown that the prevalence of HAV antibodies is very low (2%) in Finnish children (Citation6).
Assays for diabetes-associated antibodies
All diabetes-associated antibodies were analysed in the Research Laboratory, Department of Paediatrics, University of Oulu. Antibodies to glutamic acid decarboxylase (GADA) and to islet antigen 2 (IA-2A) were analysed with specific radiobinding assays (Citation10,Citation11). The cut-off values for GADA and IA-2A positivity were 5.36 relative units (RU) and 0.43 RU, respectively, based on the 99th percentile in more than 370 non-diabetic children and adolescents. According to the 2005 Diabetes Autoantibody Standardization Program (DASP) workshop the disease sensitivity was 82% and the disease specificity 96% for the GADA assay, while the corresponding figures for the IA-2A assay were 72% and 100%. Insulin antibodies were also measured with a specific radiobinding microassay with a cut-off value of 3.48 RU representing the 99th percentile in 370 non-diabetic children and adolescents (Citation12). The disease sensitivity of this assay was 58% and the disease specificity 98% in the 2005 DASP workshop.
HLA typing
HLA class II risk alleles (DQA1*05, DQB1*02, DQB1*0301, DQB1*0302, DQB1*0602, and DQB1*0603) were typed by polymerase chain reaction and microtitre well plate-based hybridization with lanthanide-labelled oligonucleotide probes as previously described (Citation13). The presence of HLA-DQB1*0302 allele was used as a marker of DR4-DQ8 haplotype, and the combination of HLA-DQA1*05 and -DQB1*02 alleles (in the absence of DQA1*0201 and DQB1*0301) as a marker of DR3-DQ2 haplotype. For the analyses, the presence of diabetes-related HLA class II genotypes was categorized as follows: DR3-DQ2/DR4-DQ8, DR3-DQ2/x (x≠DR4-DQ8), DR4-DQ8/y (y≠DR3-DQ2), and z/z (z≠DR3-DQ2 and DR4-DQ8). Samples from 131 (99%) Russian Karelian patients with diabetes, 114 (78%) Finnish patients with diabetes, 111 (99%) Russian Karelian controls, 266 (100%) Finnish controls, and 33 (100%) Russian Karelian children from the separate cohort with allergic symptoms were available for the analyses.
Statistical analyses
Statistical analyses were performed with the SPSS program version 14.0 (SPSS Inc., Chicago, IL, USA) using the tests listed below. The prevalence of allergen-specific IgE was compared between the groups using cross-tabulation and the chi-square test or the Fisher exact test. To identify the independent effect of diabetes on allergic sensitization, we used logistic regression as a multivariate technique. In addition, the Mann-Whitney U test was used to compare antibody levels between various groups, whereas base-10 logarithmic values of these levels were compared using the t test. A linear regression model was applied in the further analyses of insulin antibody levels between the two countries. The results are supported by the assessment of odds ratios (OR) and 95% confidence intervals (CI). All analyses were two-sided, and P-values <0.05 were considered statistically significant.
Results
The co-occurrence of allergic sensitization and type 1 diabetes
Allergic sensitization was more common in Finnish children than in Karelian children both among patients with type 1 diabetes and control subjects (25% of all Finnish children but only 10% of all Russian Karelian children had allergen-specific IgE against at least one of the three allergens; P < 0.001). Among the Finnish children allergic sensitization was equally frequent in diabetic and control children (). In contrast to Finnish children, the prevalence of allergen-specific IgE was conspicuously higher in Karelian patients with type 1 diabetes than in Karelian control subjects. Furthermore, Karelian children with type 1 diabetes reported also more allergic symptoms than control children (most frequently allergic eczema and asthma) (). The mean total IgE levels or the prevalence of high IgE levels (≥100 kU/L) did not differ between patients and control subjects in either of the countries.
Table II. Prevalence of allergen-specific immunoglobulin E (IgE) (>0.35 kU/L) against at least one out of three allergens tested (cat, birch, and egg albumen) and high levels (≥100 kU/L) of total IgE in patients with type 1 diabetes and in control subjects in Finland.
Table III. Prevalence of allergen-specific IgE (>0.35 kU/L) against at least one out of three allergens tested (cat, birch, and egg albumen), reported frequency of allergic symptoms, and high levels (≥100 kU/L) of total IgE in patients with type 1 diabetes and in control subjects in Russian Karelia. Logistic regression model adjustment for the effect of age and gender was applied in the analyses.
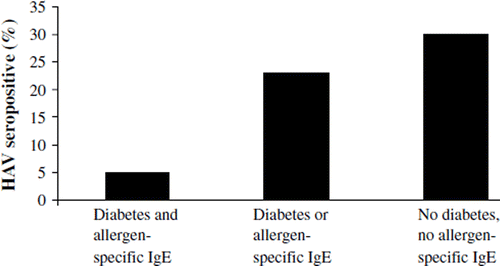
The prevalence of HAV antibodies in relation to allergic sensitization and type 1 diabetes in Russian Karelia
Only 5% (1/20) of the subjects with both diabetes and allergen-specific IgE were seropositive for HAV, while 23% (26/115) of the subjects who had either diabetes or allergen-specific IgE and 30% (32/105) of subjects with neither of these conditions were HAV-positive (; P = 0.016 for trend). There was also an inverse association between allergen-specific IgE and HAV antibodies, as 4% (1/25) of the IgE-positive children compared to 27% (58/215) of the IgE-negative children were HAV-seropositive (P = 0.012; OR = 0.11, 95% CI: 0.02–0.85). On the other hand, HAV antibodies did not differ between patients with type 1 diabetes and controls: 21% (27/130) versus 29% (32/110) were HAV-seropositive, respectively. Overall, 25% (59/240) of all Russian Karelian children were HAV-positive.
The association of allergic symptoms and allergen-specific IgE
There was a strong association between allergic symptoms and allergen-specific IgE. When all Russian Karelian children were included in the analyses, 45% (21/47) of those reporting allergic symptoms had allergen-specific IgE compared to 8% (19/230) of those without allergic symptoms (P < 0.001; OR = 8.97, 95% CI: 4.27–18.84). Similarly, among patients with type 1 diabetes, 39% (5/13) and 13% (15/119) of the children with or without allergic symptoms, respectively, were sensitized to at least one allergen (P = 0.014; OR = 4.33, 95% CI: 1.25–15.00). Again, among non-diabetic control children the only child who reported symptoms was also sensitized, while only 4% (4/111) of the children without allergic symptoms were sensitized (P < 0.001).
Type 1 diabetes-associated antibodies in Finnish and Russian Karelian children
IA-2 and insulin antibodies were more frequent in Finnish patients with type 1 diabetes than in Russian Karelian patients (). Furthermore, when antibody levels of antibody-positive patients were compared, Finnish patients had significantly higher insulin antibody levels (median 170 RU, range 4–1532 RU) than Russian Karelian patients (median 61 RU, range 4–2001 RU; P = 0.001), while the levels of GADA and IA-2A antibodies did not differ. Linear regression model adjusting for the effect of age and duration of diabetes showed also significantly higher insulin antibody levels in Finnish patients (P < 0.001).
Diabetes-related HLA haplotypes in different cohorts and their relation to allergic sensitization
Diabetes-associated HLA haplotypes were conspicuously more frequent among type 1 diabetic patients than among controls in both countries. The DR3-DQ2/DR4-DQ8 genotype was more frequent in Russian Karelian patients with type 1 diabetes than in their Finnish peers. The frequency of HLA genotypes did not differ between the non-diabetic control groups in the two countries ().
Diabetes-associated HLA genotypes had no effect on allergic sensitization. In the whole study population, the DR3-DQ2/DR4-DQ8, DR3-DQ2/x, or DR4-DQ8/y genotypes were present in 57% of the study subjects. About 19% of these subjects were sensitized against at least one of the tested allergens, while 20% of the subjects without risk genotypes (z/z) were sensitized. The prevalence of allergen-specific IgE in Russian Karelian patients with diabetes and control subjects matched by diabetes-associated HLA haplotypes is shown in .
Table IV. Prevalence of allergen-specific IgE (>0.35 kU/L) against at least one out of three allergens tested (cat, birch, and egg albumen) in patients with type 1 diabetes and in control subjects in Russian Karelia in relation to diabetes-related HLA genotypes.
Discussion
The present study suggests that allergic sensitization and type 1 diabetes co-occur more often than expected in the Russian Karelian population with a low prevalence of these diseases and a high frequency of infections, but not in the Finnish population with a high prevalence of both type 1 diabetes and allergies and a low frequency of infections.
Previous studies carried out in Russian Karelia have shown somewhat similar frequencies of allergic sensitization to cat and birch (both about 2%–4%) as those observed in this study in the control cohort (Citation6,Citation9,Citation14). In contrast, in the group of children with type 1 diabetes, allergic sensitization, especially to cat, shows a prevalence clearly higher than in control subjects or that previously reported in Russian Karelia. In addition to the increased frequency of allergen-specific IgE responses, the patients affected by type 1 diabetes also reported more allergic symptoms. Together with the observed association of allergen-specific IgE and allergic symptoms, this indicates that the former phenomenon has clinical relevance.
In addition, our results suggest that diabetes-associated autoantibodies and insulin antibodies are more common in Finnish patients with type 1 diabetes than in Russian Karelian peers. This may indicate more powerful immunoregulation in the Russian Karelian children affected by type 1 diabetes. The exogenous insulin formulations used in the two study areas did differ at the time of this study, with a higher proportion of non-human insulin used in Russian Karelia. One should expect that such a practice would result in a higher frequency of insulin antibodies in patients living in Russian Karelia, whereas the actual status was the opposite. Thus, the results suggest that, in addition to allergic sensitization, autoimmune responses are stronger in Finnish children. This is in line with our previous observations showing that a series of autoimmune diseases and phenomena (such as type 1 diabetes, coeliac disease, and thyroid autoimmunity) are 4–6-fold more common in Finland than in Russian Karelia (Citation7,Citation8,Citation15,Citation16). Similarly, higher prevalence of antibodies to GAD and insulin has been reported in Swedish compared to Lithuanian children with type 1 diabetes (Citation17).
The prevalence of diabetes-associated HLA haplotypes did not differ between Finnish and Russian Karelian unaffected school-children. This further emphasizes that genetic differences between the two study populations are an unlikely explanation for differences. However, the highest-risk DR3-DQ2/DR4-DQ8 genotype was clearly more frequent in Russian Karelian patients with type 1 diabetes than in their Finnish peers. This may indicate that a higher proportion of all subjects with genetic susceptibility are affected by type 1 diabetes in Finland. Indeed, previous studies have shown that subjects with the highest-risk genotype have become less frequent over time among Finnish patients with type 1 diabetes, while subjects with lower-risk genotypes have become more frequent (Citation18). This phenomenon has been also found in other Western countries with high living standards, suggesting increasing environmental pressure (Citation19–21).
Our observation of increased allergic sensitization in patients with type 1 diabetes runs counter to some previous findings. A meta-analysis in 2003 suggested that there is a small reduction in the prevalence of asthma among children with diabetes, while the association between other atopic diseases and type 1 diabetes was less conclusive (Citation4). However, when previous studies are reviewed in more detail, a pattern that is consistent with our observations can be seen. In other words, an inverse correlation or no association between type 1 diabetes and allergic symptoms has been reported mainly from countries characterized by a high standard of living, such as Italy (Citation22), the Netherlands (Citation23), Finland (Citation24), Germany (Citation25), Norway (Citation26), Denmark (Citation27), Sweden (Citation5), Austria (Citation28), and the UK (Citation28). In addition, an extensive Israeli study reported an inverse relationship between type 1 diabetes and asthma (Citation29). A large Canadian study reported a positive relationship between asthma and type 1 diabetes, but this was seen only in those aged over 40 years and not in younger age groups (Citation30). The EURODIAB Substudy 2 Study Group reported an inverse association between atopic diseases and the development of type 1 diabetes when eight European centres were combined, but individually only Austria and the UK showed a significant inverse relation. In contrast, Bulgaria was the only country where there was a significant positive association (Citation28).
When considering the results of the EURODIAB Substudy 2 in terms of prosperity, four of the study areas (two of which were located in the UK and one in Austria) clearly represent countries of high income, whereas the other four represent countries with much lower average income, Bulgaria having the lowest gross national income (GNI) per capita, i.e. US$3,990 (Citation31). In addition, an association between a positive family history for allergic diseases and risk of type 1 diabetes has been reported from former Yugoslavia (Citation32). Thus, our observation of a positive relationship between type 1 diabetes and allergen-specific IgE responses in Russian Karelia, where the GNI per capita is US$5,780, but not in Finland, with a GNI per capita of US$40,650, is in line with the trend seen in the EURODIAB Substudy 2 (Citation31).
Taken together, it appears likely that a positive association between type 1 diabetes and allergy can be observed in countries with low income. The reason for this is not clear. Nevertheless, gene– environment interactions have been shown to play a role in the pathogenesis of allergic diseases (Citation33,Citation34) as well as type 1 diabetes (Citation35). It is therefore possible that the clean and urban environment in prosperous countries (such as Finland) predisposes to the development of allergic diseases and type 1 diabetes, leading to the development of these diseases in a large proportion of those who are genetically susceptible. This may well be the case in Finland, since type 1 diabetes may now develop in subjects with weaker HLA-conferred susceptibility than a few decades ago (Citation18). In this scenario the prevalence of these diseases would merely reflect the proportion of those who are genetically predisposed to these diseases. In contrast, in regions with lower income (such as Russian Karelia) environmental factors may offer some protection against the development of these diseases, leaving a larger proportion of those genetically susceptible unaffected. In this scenario on the other hand, the coexistence of allergy and type 1 diabetes would reflect the impact of protective environmental factors (e.g. infections) in the pathogenesis of both of these diseases.
In line with the hygiene hypothesis, a strong candidate for such protective environmental factors are microbes which stimulate toll-like receptors and activate regulatory T cells leading to bystander suppression of autoimmune and allergic responses. Our finding that subjects with both type 1 diabetes and allergic sensitization had lower frequency of HAV antibodies supports this hypothesis. HAV may not be the causative agent underlying this effect, but as a virus with a faecal–oral transmission route it can rather be a marker of a life-style that is associated with a high frequency of many different viral and bacterial infections.
On the other hand, if impaired regulatory mechanisms were solely responsible for the development of type 1 diabetes and allergic sensitization, it would be expected that these diseases co-occurred also in an environment with high hygiene. Therefore the protective factors for these diseases may not be the same in high and low-hygiene environments. Indeed, it may be that the disease-modulating factors are not as strongly related to microbial exposures when the general microbial pressure on the immune system is low.
In conclusion, the present study shows a strong association between allergy and type 1 diabetes in Russian Karelia and therefore implies that allergic diseases and diabetes may have common pathogenic mechanisms that may be linked to the role of microbes in immune regulation. Nevertheless, the disease mechanisms may differ in populations characterized by different standards of hygiene. Further studies are needed to uncover these mechanisms, as this may open up possibilities for clinical interventions aimed at preventing the development of these diseases.
Acknowledgements
This study has received support from the EU as a part of the EPIVIR project (INCO-Copernicus Program, contract number IC15-CT98-0316) and has been supported by grants from the Päivikki and Sakari Sohlberg Foundation, the Academy of Finland, the Tuberculosis Foundation in Tampere, the University of Tampere, and the Medical Research Fund of Tampere University Hospital.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Bach JF. The effect of infections on susceptibility to autoimmune and allergic diseases. N Engl J Med. 2002;347: 911–20.
- Bach JF. Infections and autoimmune diseases. J Autoimmun. 2005;25:74–80.
- Dardalhon V, Korn T, Kuchroo V, Anderson A. Role of Th1 and Th2 cells in organ-specific autoimmunity. J Autoimmun. 2008;31:252–6.
- Cardwell CR, Shields MD, Carson DJ, Patterson CC. A meta-analysis of the association between childhood type 1 diabetes and atopic disease. Diabetes Care. 2003;26: 2568–74.
- Stromberg LG, Ludvigsson GJ, Bjorksten B. Atopic allergy and delayed hypersensitivity in children with diabetes. J Allergy Clin Immunol. 1995;96:188–92.
- Seiskari T, Kondrashova A, Viskari H, Kaila M, Haapala AM, Aittoniemi J, . Allergic sensitization and microbial load—a comparison between Finland and Russian Karelia. Clin Exp Immunol. 2007;148:47–52.
- Kondrashova A, Reunanen A, Romanov A, Karvonen A, Viskari H, Vesikari T, . A six-fold gradient in the incidence of type 1 diabetes at the eastern border of Finland. Ann Med. 2005;37:67–72.
- Kondrashova A, Viskari H, Kulmala P, Romanov A, Ilonen J, Hyöty H, . Signs of beta-cell autoimmunity in nondiabetic schoolchildren: a comparison between Russian Karelia with a low incidence of type 1 diabetes and Finland with a high incidence rate. Diabetes Care. 2007;30:95–100.
- Vartiainen E, Petays T, Haahtela T, Jousilahti P, Pekkanen J. Allergic diseases, skin prick test responses, and IgE levels in North Karelia, Finland, and the republic of Karelia, Russia. J Allergy Clin Immunol. 2002;109:643–8.
- Savola K, Bonifacio E, Sabbah E, Kulmala P, Vähäsalo P, Karjalainen J, . IA-2 antibodies—a sensitive marker of IDDM with clinical onset in childhood and adolescence. Childhood diabetes in Finland study group. Diabetologia. 1998;41:424–9.
- Savola K, Sabbah E, Kulmala P, Vahasalo P, Ilonen J, Knip M. Autoantibodies associated with type I diabetes mellitus persist after diagnosis in children. Diabetologia. 1998;41:1293–7.
- Ronkainen M, Hämäläinen A-M, Koskela P, Åkerblom HK, Knip M; the Finnish TRIGR Study Group. Pregnancy induces non-immunoglobulin insulin-binding activity in both maternal and cord blood serum. Clin Exp Immunology. 2001;124:190–6.
- Nejentsnev S, Sjöroos M, Soukka T, Knip M, Simell O, Lövgren T, . Population-based genetic screening for the estimation of type 1 diabetes mellitus risk in Finland: selective genotyping of markers in the HLA-DQB1, HLA-DQA1 and HLA-DRB1 loci. Diabet Med. 1999;16:985–92.
- Pekkarinen PT, von Hertzen L, Laatikainen T, Mäkelä MJ, Jousilahti P, Kosunen TU, . A disparity in the association of asthma, rhinitis, and eczema with allergen-specific IgE between Finnish and Russian Karelia. Allergy. 2007;62: 281–7.
- Kodrashova A, Mustalahti K, Kaukinen K, Viskari H, Volodicheva V, Haapala AM, . Lower economic status and inferior hygienic environment may protect against celiac disease. Ann Med. 2008;40:223–31.
- Kondrashova A, Viskari H, Haapala AM, Seiskari T, Kulmala P, Ilonen J, . Serological evidence of thyroid autoimmunity among schoolchildren in two different socioeconomic environments. J Clin Endocrinol Metab. 2008;93:729–34.
- Holmberg H, Vaarala O, Sadauskaite-Kuehne V, Ilonen J, Zilvinas P, Ludvigsson J. Higher prevalence of autoantibodies to insulin and GAD65 in Swedish compared to Lithuanian children with type 1 diabetes. Diabetes Res Clin Pract. 2006;72:308–14.
- Hermann R, Knip M, Veijola R, Simell O, Laine AP, Åkerblom HK, . Temporal changes in the frequencies of HLA genotypes in patients with type 1 diabetes—indication of an increased environmental pressure? Diabetologia. 2003; 46:420–5.
- Gillespie K, Bain S, Barnett A, Bingley P, Christie M, Gill G, . The rising incidence of childhood type 1 diabetes and reduced contribution of high-risk HLA haplotypes. Lancet. 2004;364:1699–700.
- Resic-Lindehammer S, Larsson K, Ortqvist E, Carlsson A, Cederwall E, Cilio CM, . Temporal trends of HLA genotype frequencies of type 1 diabetes patients in Sweden from 1986 to 2005 suggest altered risk. Acta Diabetol. 2008;45: 231–5.
- Fourlanos S, Varney MD, Tait BD, Morahan G, Honeyman MC, Colman PG, . The rising incidence of type 1 diabetes is accounted for by cases with lower-risk human leukocyte antigen genotypes. Diabetes Care. 2008;31:1546–9.
- Caffarelli C, Cavagni G, Pierdomenico R, Chiari G, Spattini A, Vanelli M. Coexistence of IgE-mediated allergy and type 1 diabetes in childhood. Int Arch Allergy Immunol. 2004;134: 288–94.
- Meerwaldt R, Odink RJ, Landaeta R, Aarts F, Brunekreef B, Gerritsen J, . A lower prevalence of atopy symptoms in children with type 1 diabetes mellitus. Clin Exp Allergy. 2002;32:254–5.
- Mattila PS, Tarkkanen J, Saxen H, Pitkaniemi J, Karvonen M, Tuomilehto J. Predisposition to atopic symptoms to inhaled antigens may protect from childhood type 1 diabetes. Diabetes Care. 2002;25:865–8.
- Rosenbauer J, Herzig P, Giani G. Atopic eczema in early childhood could be protective against type 1 diabetes. Diabetologia. 2003;46:784–8.
- Stene LC, Joner G; Norwegian Childhood Diabetes Study Group. Atopic disorders and risk of childhood-onset type 1 diabetes in individuals. Clin Exp Allergy. 2004;34:201–6.
- Olesen AB, Juul S, Birkebaek N, Thestrup-Pedersen K. Association between atopic dermatitis and insulin-dependent diabetes mellitus: A case-control study. Lancet. 2001;357: 1749–52.
- The EURODIAB Substudy 2 Study Group. Decreased prevalence of atopic diseases in children with diabetes. J Pediatr. 2000;137:470–4.
- Tirosh A, Mandel D, Mimouni FB, Zimlichman E, Shochat T, Kochba I. Autoimmune diseases in asthma. Ann Intern Med. 2006;144:877–83.
- Dales R, Chen Y, Lin M, Karsh J. The association between allergy and diabetes in the Canadian population: Implications for the Th1-Th2 hypothesis. Eur J Epidemiol. 2005;20: 713–7.
- geo.worldbank.org | The World Bank, Mapped. Available from: http://geo.worldbank.org/ (accessed 9 November 2007).
- Sipeti S, Vlajinac H, Kocev N, Marinkovi J, Radmanovi S, Deni L. Family history and risk of type 1 diabetes mellitus. Acta Diabetol. 2002;39:111–5.
- Zhang G, Khoo S, Laatikainen T, Pekkarinen P, Vartiainen E, von Hertzen L, . Opposite gene by environment interactions in Karelia for CD14 and CC16 single nucleotide polymorphisms and allergy. Allergy. 2009;64:1333–41.
- Virta M, Pessi T, Helminen M, Seiskari T, Kondrashova A, Knip M, . Interaction between CD14-159C>T polymorphism and Helicobacter pylori is associated with serum total immunoglobulin E. Clin Exp Allergy. 2008;38: 1929–34.
- Lempainen J, Vaarala O, Mäkelä M, Veijola R, Simell O, Knip M, . Interplay between PTPN22 C1858T polymorphism and cow's milk formula exposure in type 1 diabetes. J Autoimmun. 2009;33:155–64.