Abstract
Introduction. Arterial calcification is a risk factor for atherosclerosis. Calumenin (CALU), a protein regulating proteins involved in coagulation and arterial calcification also has extracellular functions related to atherosclerosis. We recently described that CALU polymorphism A29809G was related to acenocoumarol requirements, and we wanted to evaluate its role in arterial calcification and prognosis.
Patients and methods. A total of 374 consecutive patients with non-ST-elevation acute coronary syndrome (nSTACS). In 175 of them, who underwent percutaneous coronary intervention, we assessed calcification in each main coronary artery. Follow-up at 1 and 6 months was performed for adverse end-points.
Results. CALU 29809G carriers were more frequent in the low calcium group (P = 0.037). The presence of ≥3 cardiovascular risk factors and CALU polymorphism were associated with arterial calcification (OR 2.34, P = 0.049; and OR 0.34, P = 0.019, respectively). CALU 29809G allele was the only variable associated with events at 1 month (HR 0.42; P = 0.042). Multivariate analysis showed that, at 6 months, age and severe anginal symptoms were associated with worse prognosis (HR 2.13, P = 0.023; and HR 2.01, P = 0.011, respectively), whereas CALU 29809G allele associated with good prognosis (HR 0.59, P = 0.044). Our results suggest that CALU A29809G is associated with arterial calcification and short-term prognosis of the outcome of patients with nSTACS.
| Abbreviations | ||
| 3′UTR | = | 3′-untranslated region |
| AC | = | arterial calcification |
| CALU | = | calumenin |
| CVRF | = | cardiovascular risk factors |
| HR | = | hazard ratio |
| hs-CRP | = | high-sensitivity C-reactive protein |
| nSTACS | = | non-ST-elevation acute coronary syndrome |
| NT-proBNP | = | N-terminal brain natriuretic propeptide |
| OR | = | odds ratio |
| PTCA | = | percutaneous coronary angioplasty |
| P | = | P value |
| SD | = | standard deviation |
| STEMI | = | ST-elevation myocardial infarction |
| VKOR | = | vitamin K1 2,3-epoxide reductase |
Key messages
Calumenin (CALU) A29809G polymorphism plays a role in arterial calcification.
CALU 29809G allele confers better prognosis of atherothrombotic processes.
Introduction
Non-ST-elevation acute coronary syndromes (nSTACS) have a complex and heterogeneous pathogenesis, where many pathophysiological systems have been implicated in the formation and destabilization of atherosclerotic plaques (Citation1–3). Atherosclerotic plaque calcification is a common phenomenon in nSTACS, usually associated with long-standing atherosclerotic disease. Histopathological studies have unequivocally shown that vulnerable atherosclerotic plaques contain calcium deposits, although the extent and radiographic appearance of the calcifications varied considerably among patients (Citation4). Calcification is localized within the thickened intima and media of blood vessel walls, increasing the risk of plaque rupture. Indeed, coronary artery calcification has been considered as a strong predictor of poor cardiovascular outcomes (Citation5,Citation6).
Endothelial dysfunction, an early event in the atherosclerotic process, leads to platelet adhesion as a primordial event with biological significance (Citation7). Platelets may mediate events such as leucocyte accumulation through products released following adhesion and activation. Thus, secreted platelet proteins act in an autocrine or paracrine fashion to modulate cell signalling. This release contains well known factors of major significance in the development of atherothrombosis, including prothrombotic and regulating cell proliferation proteins, immune modulators or adhesion proteins (Citation8). Thus, Coppinger et al. have characterized about 300 novel platelets proteins in human atherosclerotic lesions released from activated platelets (Citation8). Among these, it was observed that calumenin may have a pathophysiological role, since immunohistochemical analysis showed that it was localized in atherosclerotic plaques but it was absent from the normal artery (Citation8).
Calumenin belongs to a family of multiple EF-hand Ca2+-binding proteins originally localized to the secretory pathway and known as CREC family. Its functions are primarily connected with Ca2+-dependent processes in the secretory pathway, especially with γ-carboxylation of coagulation factors (Citation9). However, functional properties of calumenin are steadily emerging (Citation10). Thus, Wajih and co-workers (Citation11) have demonstrated that calumenin endogenously regulates the activity of the γ-carboxylation system, consisting of vitamin K1 2,3-epoxide reductase (VKOR), and γ-glutamyl carboxylase. This enzymatic system modifies vitamin K-dependent proteins, making them able to form a complex with Ca2+ (Citation12). Most recently, Honoré's group has demonstrated that calumenin forms a complex with thrombospondin-1 (Citation13), also released from thrombocytes and incorporated into fibrin clots.
At least 23 polymorphisms have been reported in the CALU gene (Citation14), but the solely published functional effects rely on 3 of them, all related with the coagulation system (Citation14–16). Our group found that the A29809G polymorphism located in the 3′-untranslated region (3′UTR) of the CALU gene might have some impact on the efficacy of acenocoumarol therapy (Citation15). However, we are unaware of any data on calumenin polymorphisms in nSTACS—neither the relationship to vascular calcification nor prognosis in such patients.
In this context we wanted to research the role of calumenin A29809G change on vascular calcification. For this purpose patients with nSTACS were consecutively included and followed up in a longitudinal study, to also ascertain the influence of this calumenin polymorphism on prognosis.
Methods
Patient group
Between June of 2003 and August of 2005 we prospectively recruited 374 patients admitted in the cardiology departments of two hospitals with the diagnosis of nSTACS. The inclusion criteria were patients presenting typical cardiac ischaemic chest pain, with ECG changes, including down-sloping ST-segment or inverted T-waves associated with the chest pain; and/or raised troponin T levels (defined as levels higher than 0.1 ng/mL in the first 12 hours from the beginning of cardiac ischaemic symptoms). A complete history, clinical examination, and variables related to TIMI risk score were performed at admission. Exclusion criteria were patients with concomitant neoplastic, infectious, or connective tissue diseases, anticoagulant treatment, or inflammatory diseases. All the patients received standard management as recommended by guidelines for acute coronary syndrome with regard to aspirin, clopidogrel, low-molecular weight heparin, glycoprotein IIbIIIa inhibitors, β-blockers, statins, and ACE inhibitors, as appropriate (Citation17). Patients subsequently proceeded to coronary angiography and/or revascularization (percutaneous coronary angioplasty (PTCA)), treadmill testing, or conservative approach in keeping with current management protocols (Citation18). The Research Ethics Committee of the two centres approved the study, and all the subjects gave written informed consent to participation.
Calcium assessment
In those patients who underwent cardiac catheterization from one of the two centres (175 patients), arterial calcification was assessed. The presence and extent of calcification at the target lesion (the stenosis that was about to undergo coronary intervention) were assessed in a blinded manner by two independent observers, using a four-point score as previously described (Citation19): 0 = no calcification, 1 = calcification barely visible on close examination, 2 = readily visible but mild degree of calcification, and 3 = obvious, heavy calcification. This determination was performed on four main coronary arteries: left coronary artery, descendent artery, and circumflex and right coronary artery. We classified arbitrarily calcification using a global score (resulting from the addition of the partial scores obtained in each coronary artery) into low calcification (total score 1–2 points) and high calcification (total score ≥2 points).
Laboratory
Blood samples were collected within the first 48 h after admission, the next morning at 08.00, and after 6 and 12 h, always in fasting conditions. Serum and DNA samples were stored at −80°C until batch analysis. Troponin T levels were determined in all three serum samples, using one-step enzyme immunoassay based on electrochemiluminescence technology (Elecsys; Roche Diagnostics, Basel, Switzerland). Serum samples were also used for measurement of N-terminal brain natriuretic propeptide (NT-proBNP), using a Roche Diagnostic proBNP assay on an Elecsys 2010 analyser (Roche Diagnostics, Mannheim, Germany). Total assay precision ranges from 1.8% at 800 pmol L−1 to 2.7% at 20.7 pmol L−1, and the detection limits are 0.6 and 4130 pmol L−1 (to convert pmol L−1 to pg mL−1, multiply pmol L−1 values by 8.457). Serum hs-CRP was quantified by kinetic nephelometry with an immunochemical system (IMMAGE®; Beckman, Magburg, Germany).
Calumenin genotyping
DNA was extracted by standard methods. Genotyping of the CALU A29809G genotype (rs1043550) was performed by real-time PCR reaction on a LC480 PCR system (Roche Pharma S.A., Madrid, Spain) by a validated assay (Applied Biosystems C_7564455_20, Life Technologies Inc., Madrid, Spain). Direct sequencing was performed for five randomly selected samples of each genotype to confirm genotyping results.
Follow-up
Patients were followed up for 6 months by out-patient clinic attendance, telephone contact, and review of the medical notes. We defined end-points as cardiovascular death (death in the context of ischaemic or other heart disease, or death with unexplained cause), recurrent acute coronary syndrome, non-elective revascularization (emergent or urgent revascularization in the context of new admittance), and/or admission for acute heart failure. We used two time points to assess the presence of clinical events, at 1 and 6 months.
Statistical analysis
Continuous variables were tested for normal distribution by Kolmogorov-Smirnov test. Continuous variables are presented as mean ± SD or median (interquartile range), as appropriate, and categorical variables as a percentage. Comparisons between groups were performed by Student's t test (or Mann-Whitney U test as appropriate). Categorical data were compared using the chi-square test, and a Fisher's exact test was performed, if relevant. Correlations between two continuous variables were performed using the Pearson correlation coefficient, or Spearman rank correlation, if the variables were not normally distributed. The independent effect of variables on vascular calcification was assessed by multiple logistic regression. All variables that showed P-value <0.15 in the univariate analysis were included in the model. The event-free survival curves were plotted using the Kaplan-Meier method, and the differences determined using the log rank test. The effect of variables in the prognosis was calculated using a Cox proportional hazards regression model, using those clinical, ECG, and biological markers, incorporating in the multivariate model only those values that showed P-value <0.15 in the univariate analysis. The cut-off point used for NT-proBNP values was the upper quartile in our population (Citation20). A P < 0.05 was accepted as statistically significant. Statistical analysis was performed using SPSS 15.0 for Windows (SPSS, Inc., Chicago, IL, USA).
Results
shows demographic and clinical characteristics of the 374 consecutive patients enrolled. As shown, more than half patients (49.7%) had raised troponin T levels, and 36.6% presented ST down-sloping. Thus, the median TIMI risk score was 3 (interquartile range 2–4). The calcium score was valuated in a subset of 175 patients that underwent coronary angiography (). Finally, the distribution of the CALU A29809G genotype is also shown in . About 50% of patients were heterozygous, 33% were homozygous for the CALU 29809A allele, and almost 16% were homozygous for the CALU 29809G allele (). This frequency was in agreement with previous data obtained by our group in a former study with a cohort of healthy controls, and the population was in Hardy-Weinberg equilibrium (Citation15). In every case, we grouped GG and GA carriers for statistical analysis due to the low frequency of G allele in the study population, so we only consider presence/absence of G-allele.
Table I. Demographic and clinical characteristics, biomarkers, and genetic profile in non-ST-elevation acute coronary syndrome patients.
Calcification, clinical variables, and CALU genotype
shows the association of high calcification score (≥2 points) estimated on 175 patients both in clinical variables and biomarkers. We observed a strong association between high calcification score and age ≥65 years (odds ratio (OR) 2.35, P = 0.009), previous ischaemic heart disease (OR 3.54, P < 0.001), and the presence of at least three cardiovascular risk factors (OR 2.96, P = 0.002) (). There was also significant association with diabetes mellitus (OR 2.23, P = 0.020), hypertension (OR 2.66, P = 0.004), ≥50% arterial stenosis in previous angiography (OR 2.72, P = 0.028), and NT-proBNP (OR 3.15, P = 0.012) (). Additionally, patients carrying the CALU 29809G allele had a lower risk of high calcification score (OR 0.45, P = 0.042) ().
Table II. Association of high calcification score (≥2 points) with clinical variables, biomarkers, and genetic profile in 175 patients with coronary angiography.
In the multivariate analysis, only two parameters maintained a significant association with high calcification score: at least three cardiovascular risk factors (CVRF) (OR 2.34, P = 0.049), and CALU 29809G allele (OR 0.34, P = 0.019) ().
CALU genotype, clinical characteristics, and biomarkers
As CALU 29809G seemed to exert a protective role in calcification degree, we further analysed the relationship between this genotype and clinical parameters and biomarkers. In accordance with this, we found within the low calcium score group a higher proportion of CALU 29809G carriers than among the CALU 29809A carriers (37.9% versus 21.6%, P = 0.037) (). CALU A29809G genotype was also related to NT-proBNP levels, as 29809G carriers had lower NT-proBNP levels (422.9 (110.7–1783.0) pg/mL) than did homozygous CALU 29809A patients (806.2 (205.2–2402.0) pg/mL) (P = 0.026) ().
Figure 1. A: Relationship between CALU A29809G genotype and calcification score: high calcification score patients (empty bars), low calcification score patients (full bars). B: NT-proBNP levels according with CALU A29809G genotype.
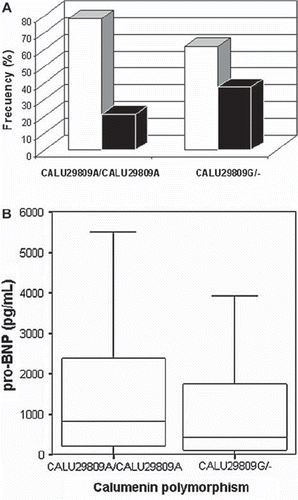
There were no significant associations between CALU genotype and the rest of clinical variables and/or biomarkers considered (data not shown).
Longitudinal analysis
Complete follow-up data at 1 month was available in 353 patients (94.4%) and in 350 patients (93.6%) at 6 months.
One-month follow-up. A total of 30 patients (8.5%) presented adverse events. Registered events were: 18 nSTACS, 10 cardiovascular deaths, and 3 heart failures. One patient suffered from more than one event simultaneously.
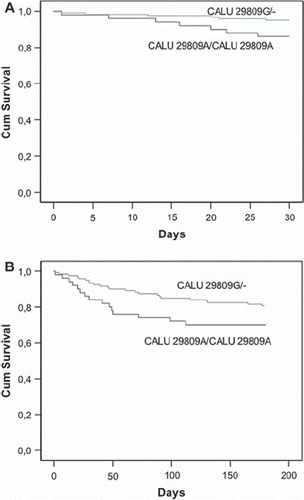
Cox regression analysis showed statistical association for down-sloping ST (hazard ratio (HR) 2.61 (1.59–5.42), P = 0.011), the presence of severe anginal symptoms variable (HR 2.88 (1.32–6.27), P = 0.008), and CALU 29809G allele (HR 0.42 (0.20–0.85), P = 0.017) as independent prognosis factors for adverse events (). Only the CALU 29809G allele variable remained significant in the multivariate analysis (HR 0.42 (0.18–0.97), P = 0.042) (). Moreover, carriers of the CALU 29809G allele had a better cumulative event-free survival at 1-month follow-up than did CALU 29809A homozygous patients (log rank P = 0.013) ().
Table III. Cox regression analysis at 1- and 6-month follow-up in non-ST-elevation acute coronary syndrome patients.
Figure 2. Kaplan-Meier curves showing the relationship between presence of CALU 29809G allele and cumulative event-free survival after nSTACS. A: Cumulative survival at 1-month follow-up. Log rank test, P = 0.013. B: Cumulative survival at 6-months follow-up. Log rank test, P = 0.041.
Six-month follow-up. A total of 76 patients (21.7%) presented adverse events at 6 months. Registered events were: 39 nSTACS, 20 cardiovascular deaths, 11 heart failures, 4 non-elective coronary artery by-pass grafts, and 2 ST-elevation myocardial infarctions (STEMI).
In this case, Cox regression analysis revealed that age ≥65 years (HR 2.04 (1.23–3.37), P = 0.006), the presence of down-sloping ST (HR 1.80 (1.16–2.77), P = 0.009), elevated troponin T (TnT) level (HR 1.88 (1.18–2.97), P = 0.008), the presence of severe anginal symptoms (HR 2.34 (1.45–3.77), P = 0.001), NT-proBNP (HR 2.38 (1.49–3.78), P < 0.001), hs-CRP (HR 1.81 (1.39–2.88), P = 0.012), and CALU 29809G allele (HR 0.63 (0.41–0.99), P = 0.043) were independent prognosis factors of adverse events at 6-month follow-up (). In the multivariate Cox regression analysis only age ≥65 years (HR 2.13 (1.12–4.07), P = 0.023), severe anginal symptoms (HR 2.01 (1.17–3.46), P = 0.011), and CALU 29809G allele (HR 0.59 (0.35–0.99), P = 0.044) remained as significant predictors of adverse outcomes (). Again, CALU 29809G carriers had a better event-free survival at 6-month follow-up (log rank P = 0.041) ().
Discussion
Coronary artery calcium is currently recognized as an independent and incremental predictor of events in patients at intermediate risk of coronary artery disease, and preliminary evidence also adds prognostic significance to risk factors in high-risk patients (Citation21). However, arterial calcification is a complex process whose precise molecular and cellular mechanisms are unclear (Citation22).
Our study underlines the already known association between clinical variables such as age, diabetes mellitus, or ischaemic heart disease with coronary calcification as part of the complex process of atherosclerosis (Citation5,Citation6). We also observe an association between NT-proBNP and calcification within nSTACS patients, supporting similar findings previously described for population-based studies (Citation23). But the most novel finding of our study is the possible implication of calumenin in the aetiopathogenia of coronary calcification. We here analysed the association between the A29809G polymorphism of the CALU gene, coronary calcification, and prognosis in patients with nSTACS. Our results revealed that carriers of the CALU 29809G allele had a significantly reduced risk of coronary arterial calcification and almost half the risk of cardiovascular events at 6-month follow-up.
The link between calumenin and artery calcification is double, because calumenin has been related to the two predominant mechanisms supporting the pathobiology of vascular calcification: structural and circulating proteins that regulate this process (Citation10), and induction of osteogenesis (Citation22). Firstly, calumenin has been found extracellularly in the core of atherosclerotic lesions from activated thrombocytes (Citation8) and seems to modulate the protein expression of fibroblasts in vivo (Citation24). Secondly, calumenin is an endogenous regulator of the γ-carboxylation system harboured in the endoplasmic reticulum that makes fully functional matrix-gla protein, a vitamin K-dependent protein that participates in both cell differentiation and calcification through mechanisms not yet well elucidated (Citation25,Citation26).
To date, the information about the functional effect of CALU A29809G polymorphism, located at 3′UTR of the CALU gene, is scarce. Our group has previously reported that the clear effect of vitamin-K epoxide reductase complex-1 (VKORC1) genotype was exacerbated in carriers of the 29809G allele, as such patients needed a higher dose of acenocoumarol for a steady oral anticoagulation (Citation15). Although we cannot discard that the functional effect is due to any other polymorphism shown to be in linkage disequilibrium and located in regulatory regions of the CALU gene (Citation27), it has recently been recognized that 3′UTRs contain genetic information for post-transcriptional control (Citation28). Thus, the natural functions of 3′UTR that would regulate mRNA stability, controlling mRNA subcellular localization and/or mRNA translation efficiency, might be disturbed in the CALU gene by the A29809G change. This hypothesis, which needs further verification, would affect both extracellular and intracellular functions of calumenin. Alternatively, the better prognosis found in CALU 29809G carriers could be caused by calumenin's biological activities other than calcification (Citation10). Interestingly, the CALU A29809G polymorphism maintained its independent prognostic value, even after adjusting for other consistent variables. In this context, whether calumenin per se could be implicated in myocardial damage, inflammation, or left ventricular overload, behaving as other recognized markers (Citation29–31) and then contributing to the patient prognosis, remains to be further investigated.
The lack of apparent implication of the calcification process in prognosis may be due to two limitations in our study: firstly, there is a population size limitation, with a low number of patients who underwent cardiac catheterization and assessment of arterial calcification (n = 175). Additionally, the method that we used for calcification quantification, although previously validated (Citation19), is, however, not quantitatively accurate, and the evaluation of data was observer-dependent.
In summary, our data suggest that the CALU A29809G polymorphism has a significant role both in coronary calcification and prognosis in patients with nSTACS. Thus, the CALU 29809G allele might have a protective role in arterial calcification, contributing (by means of its intracellular and/or extracellular functions) to a better prognosis of carriers. Although based on a selected patient population, these results would, if they were further confirmed, open a new outlook in the field of vascular calcification, one of the major complications of cardiovascular disorders (Citation32).
Acknowledgements
This work was partially supported by 04515/GERM/06 from Fundación Séneca, SAF2009-08993 (MCYT & FEDER), RD06/0014/039 (RECAVA) from ISCIII, and PI081531 from ISCIII. Dr Hernández-Romero holds a post-doctoral position funded by Instituto de Salud Carlos III.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Ross R. Atherosclerosis-an inflammatory disease. N Engl J Med. 1999;340:115–26.
- Tousoulis D, Davies G, Stefanadis C, Toutouzas P, Ambrose JA. Inflammatory and thrombotic mechanisms in coronary atherosclerosis. Heart. 2003;89:993–7.
- Falk E. Pathogenesis of atherosclerosis. J Am Coll Cardiol. 2006;47 Suppl 8:C7–12.
- Cheng GC, Loree HM, Kamm RD, Lee RT. Distribution of circumferential stress in ruptured and stable atherosclerotic lesions. A structural analysis with histopathological correlation. Circulation. 1993;87:1179–87.
- Wexler L, Brundage B, Crouse J, Detrano R, Fuster V, Maddahi J, . Coronary artery calcification: pathophysiology, epidemiology, imaging methods, and clinical implications. A statement for heath professional from the American Heart Association. Circulation. 1996;94:1175–92.
- Tanimura A, McGregor DH, Anderson HC. Calcification in atherosclerosis, I: human studies. J Exp Pathol. 1986;2: 261–73.
- Massberg S, Brand K, Gruner S, Page S, Müller E, Müller I, . A critical role of platelet adhesion in the initiation of atherosclerotic lesion formation. J Exp Med. 2002;196: 887–96.
- Coppinger JA, Cagney G, Toomey S, Kislinger T, Belton O, McRedmond JP, . Characterization of the proteins released from activated platelets leads to localization of novel platelet proteins in human atherosclerotic lesions. Blood. 2004;103:2096–104.
- Jung DH, Mo SH, Kim DH. Calumenin, a multiple EF-hands Ca2+-binding protein, interacts with ryanodine receptor-1 in rabbit skeletal sarcoplasmic reticulum. Biochem Biophys Res Comm. 2006;343:34–42.
- Honoré B. The rapidly expanding CREC protein family: members, localization, function, and role in disease. BioEssays. 2009;31:262–77.
- Wajih N, Sane DC, Hutson SM, Tallin R. The inhibitory effect of calumenin on the vitamin K-dependent γ-carboxylation system. Characterization of the system in normal and warfarin-resistant rats. J Biol Chem. 2004;24: 25279–83.
- Furie B, Furie BC. Molecular basis of vitamin K-dependent gamma-carboxylation. Blood. 1990;75:1753–62.
- Hansen GA, Vorum H, Jacobsen C, Honoré B. Calumenin but not reticulocalbin forms a Ca+2-dependent complex with thrombospondin-1. A potential role in haemostasis and thrombosis. Moll Cell Biochem. 2009;320:25–33.
- Kimura R, Kokubo Y, Miyashita K, Otsubo R, Nagatsuka K, Otsuki T, . Polymorphisms in vitamin K-dependent gamma-carboxylationrelated genes influence interindividual variability in plasma protein C and protein S activities in the general population. Int J Hematol. 2006;84:387–97.
- González-Conejero R, Corral J, Roldán V, Ferrer F, Sánchez-Serrano I, Sánchez-Blanco JJ, . The genetic interaction between VKORC1 c1173t and calumenin a29809g modulates the anticoagulant response of acenocoumarol. J Throm Haemost. 2007;5:1701–6.
- Vecsler M, Loebstein R, Almog S, Kurnik D, Goldman B, Halkin H, . Combined genetic profiles of components and regulators of the vitamin K-dependent gamma-carboxylation system affect individual sensitivity to warfarin. Thromb Haemost. 2006;95:205–11.
- Lip GY, Blann AD. Thrombogenesis, atherogenesis and angiogenesis in vascular disease: a new ‘vascular triad’. Ann Med. 2004;36:119–25.
- Bertrand ME, Simoons ML, Fox KA, Wallentin LC, Hamm CW, McFadden E, . Task Force on Management of Acute Coronary Syndromes of the European Society of Cardiology. Management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 2002;23:1809–40.
- Tuzcu EM, Berkalp B, De Franco A, Ellis SG, Goormastic M, Whitlow PL, . The dilemma of diagnosing coronary calcification: angiography versus intravascular ultrasound. J Am Coll Cardiol. 1996;27:832–8.
- Tello-Montoliu A, Marín F, Roldán V, Mainar L, López MT, Sogorb F, . A multimarker risk stratification approach to non-ST elevation acute coronary syndrome: implications of troponin T, CRP, NT pro-BNP and fibrin D-dimer levels. J Int Med. 2007;262:651–8.
- Greenland P, Bonow RO, Brundage BH, Budoff MJ, Eisenberg MJ, Grundy SM, . ACCF/AHA 2007 clinical expert consensus document on coronary artery calcium scoring by computed tomography in global cardiovascular risk assessment and in evaluation of patients with chest pain: a report of the American College of Cardiology Foundation Clinical Expert Consensus Task Force (ACCF/AHA Writing Committee to Update the 2000 Expert Consensus Document on Electron Beam Computed Tomography). J Am Coll Cardiol. 2007;49:378–402.
- Johnson RC, Leopold LA, Loscalzo J. Vascular calcification: pathobiological mechanisms and clinical implications. Circ Res. 2006;99:1044–59.
- Abdullah SM, Khera A, Das SR, Stanek HG, Canham RM, Chung AK, . Relation of coronary atherosclerosis determined by electron beam computed tomography and plasma levels of N-terminal pro-brain natriuretic peptide in a multiethnic population-based sample (The Dallas Heart Study). Am J Cardiol. 2005;96:1284–9.
- Østergaard M, Hansen GA, Vorum H, Honoré B. Proteomic profiling of fibroblasts reveals a modulating effect of extracellular calumenin on the organization of the actin cytoskeleton. Proteomics. 2006;6:3509–19.
- Zebboudj AF, Imura M, Boström K. Matrix gla protein, a regulatory protein for bone morphogenetic protein-2. J Biol Chem. 2002;277:4388–94.
- Newman B, Gigout LI, Sudre L, Grant ME, Wallis GA. Coordinated expression of matrix Gla protein is required during endochondral ossification for chondrocyte survival. J Cell Biol. 2001;154:659–66.
- Calumenin polymorphisms, last accessed 15-Jan-2010. Available at: http://hapmap.ncbi.nlm.nih.gov/.
- Pesole G, Grillo G, Larizza A, Liuni S. The untranslated regions of eukaryotic mRNAs: Structure, function, evolution and bioinformatic tools for their analysis. Brief Bioinform. 2000;1:236–49.
- Lindahl B, Toss H, Siegbahn A, Venge P, Wallentin L. for the FIRSC Study Group. Markers of myocardial damage and inflammation in relation to long-term mortality in unstable coronary artery disease. J Am Coll Cardiol. 1997;29:43–8.
- Sabatine MS, Morrow DA, de Lemos JA, Gibson M, Murphy SA, Rifaiet N, . Multimarker approach to risk stratification in non-ST elevation acute coronary syndromes. Simultaneous assessment of troponin-I, C-reactive protein, and B-type natriuretic peptide. Circulation. 2002;105:1760–3.
- Eggers KM, Lagerqvist B, Venge P, Wallentin L, Lindahl B. Prognostic value of biomarkers during and after non-ST-segment elevation acute coronary syndrome. J Am Coll C-*ardiol. 2009;54:357–64.
- Schurgers LJ, Aebert H, Vermeer C, Bültmann B Janzen J. Oral anticoagulant treatment: friend or foe in cardiovascular disease? Blood. 2004;104:3231–2.
