Abstract
Atrial fibrillation (AF) is associated with a significant burden of morbidity and increased risk of mortality. Beyond outstanding advances in catheter ablation procedures, antiarrhythmic drug therapy remains a corner-stone to restore and maintain sinus rhythm. However, potentially life-threatening hazards (proarrhythmia) and significant non-cardiac organ toxicity have made new drug development of prominent relevance. Multichannel blocking, atrial selectivity, and the reduction of the risk of adverse events have all constituted the main theme of modern antifibrillatory drug development. Dronedarone, an analog of amiodarone, has the unique characteristic of being the first antiarrhythmic drug demonstrated to reduce hospitalizations in AF. Dronedarone is associated with less systemic toxicity than amiodarone, although being less effective for sinus rhythm maintenance. Atrial selective agents have been developed to target ion channels expressed selectively in the atria. Among the most promising drugs of this class is vernakalant, which has been shown effective for the acute conversion of AF with small risk of proarrhythmia. Finally, increasing evidences support antiarrhythmic effectiveness of traditional non-antiarrhythmic drugs, such as renin-angiotensin system blockers, statins, and omega-3 fatty acids.
In this article, we will focus on recent advances in antiarrhythmic therapy for AF, reviewing the possible clinical utility of novel antifibrillatory agents.
| Abbreviations | ||
| AF | = | atrial fibrillation |
| SR | = | sinus rhythm |
| RRR | = | relative risk reduction |
| ARR | = | absolute risk reduction |
| NNT | = | number needed to treat |
Key messages
Multichannel blocking, atrial selectivity, and the reduction of the risk of adverse events have all constituted the main theme of modern antifibrillatory drug development.
Dronedarone, a multichannel blocker analog of amiodarone, has been recently approved for sinus rhythm maintenance in AF, although its benefit across all the spectrum of AF patients is not definitely demonstrated.
Vernakalant, a promising atrial-selective agent that has recently been approved in Europe, appears an effective and safe drug for rapid termination of AF, although the real incremental value in this setting compared to other available antiarrhythmic agents merits further investigation.
Introduction
Atrial fibrillation (AF) is the most common sustained arrhythmia in Western countries, with an estimated 30 million patients affected by 2050 across the United States and Europe alone (Citation1). Atrial fibrillation has a significant impact on morbidity, mainly related to symptoms, heart failure and thromboembolic events, and is the most frequent arrhythmic cause of hospital admission in the US (Citation2,Citation3). In addition, AF is associated with excess mortality independently of thromboembolic complications (Citation4,Citation5).
The primary therapeutic goal in the management of AF is the restoration and maintenance of sinus rhythm. Recently, international societies have reconsidered the problems of trials of AF treatment and have suggested that additional end-points may be important in the assessment of different AF treatments (Citation6,Citation7). These include reduction of important clinical outcomes in AF, such as mortality, hospitalization, and stroke, as well as relevant economic analyses such as the cost of treatment (Citation6,Citation7).
To date, the most effective treatment for AF is catheter ablation (Citation8). On the other hand, antiarrhythmic drug therapy still remains the most widely adopted rhythmcontrol strategy and also constitutes an important component of treatment for many patients undergoing catheter ablation. Currently available antiarrhythmic drugs, however, have significant limitations. In particular, the use of class I antiarrhythmic agents is limited by increased risk of proarrhythmia, with a demonstrated increase in cardiovascular death in patients with structural heart disease (Citation9,Citation10). Among class III agents, sotalol has been demonstrated to increase mortality in high-risk patients (Citation11), and dofetilide is rarely used due to the substantial risk of torsade de pointes and the need for in-hospital initiation (Citation12). Ultimately, amiodarone, the most widely used antiarrhythmic agent, has multiple mechanisms of action including class III properties and has minimal proarrhythmic potential (Citation13). On the other hand, amiodarone has never been proven to reduce hospitalization or mortality in AF patients (Citation14,Citation15) and is flawed by high risk of non-cardiac toxicity, such as thyroid and hepatic toxicities, pulmonary fibrosis, neuropathy, corneal pigmentation, and skin discoloration (Citation13). As a result, the development of new antiarrhythmic drugs for AF is crucial. The main theme of modern antifibrillatory drug development has been the minimization of the risk of cardiac and systemic toxicities, while maintaining and, possibly, intensifying antiarrhythmic effectiveness (Citation16). To this aim, significant effort has been directed toward the development of drugs that act through multiple ion channels blockade, resulting in a net beneficial antiarrhythmic effect with minimal toxicity (Citation17–19). Moreover, the identification of ion channels selectively or preferentially located in the atria has led to the development of atrial selective antiarrhythmic agents, in order to minimize simultaneous unwanted effects on the ventricles (Citation20–25). Finally, there is increasing evidence supporting antiarrhythmic properties of traditionally non-antiarrhythmic agents (i.e. upstream therapies), such as inhibitors of the renin-angiotensin-aldosterone system (Citation25–28), statins (Citation29,Citation30), and omega-3 fatty acids (Citation31–33).
A large number of preclinical studies have evaluated these compounds, elucidating their pharmacokinetic and pharmacodynamic properties, which can be found summarized in recent elegant reviews (Citation16). However, it is not unusual in cardiology that what may appear a promise in preclinical studies actually reveals serious pitfalls in the clinical setting. For example, suppression of ventricular ectopy after myocardial infarction by antiarrhythmic drugs demonstrated very effective in preclinical studies led to an unexpected increase in mortality in clinical studies (Citation9).
Therefore, the purpose of this article is to review the current advances in antiarrhythmic drug therapy for AF, discussing the pharmacologic properties with a critical appraisal on potential clinical applications of antifibrillatory agents in the pipeline.
Antiarrhythmic drug analogs of amiodarone
Amiodarone is the most effective available drug for restoring and maintaining sinus rhythm (Citation14,Citation15,Citation34,Citation35) and is the most widely used antiarrhythmic drug for AF. Amiodarone is an action potential-prolonging agent, with little effects on depolarization and conduction velocity. From an electrophysiological standpoint, amiodarone blocks multiple ion channels, including Na+, K+, and Ca2+ channels (Citation36). Moreover, it has significant vasodilatatory and antiadrenergic properties (Citation36,Citation37). Vasodilatatory effects are mediated by the major metabolite of amiodarone, N-desethylamiodarone (Citation38), and may partially contribute to the clinical efficacy of amiodarone.
The major drawback of amiodarone consists in its high degree of lipophilicity, which, together with its long half-life, causes accumulation and toxicity in several organ systems (Citation13). In recent years, intense research has focused on the development of analogs of amiodarone, with the intent of replicating its antiarrhythmic effects while minimizing the risk of toxicity.
Dronedarone
Dronedarone has a structure similar to amiodarone, but with two important differences: dronedarone does not have iodine moieties, and has a methylsulfonamide group incorporated in the benzofuran ring. These two features result in a decreased potential of thyroid dysfunction and in decreased lipophilicity and half-life, thus reducing the tissue accumulation of the drug (Citation39). Dronedarone preserves the multichannel blockade features of amiodarone, together with its vasodilatatory and antiadrenergic effects (Citation40). In cardiomyocytes, dronedarone blocks inward currents, including rapid Na+, and L- and T-type Ca2+ currents, and also outward K+ currents, and the pacemaker current If (Citation40,Citation41). Translating such pharmacodynamic properties into clinically visible effects, dronedarone decreases heart rate and blood pressure and lengthens the PR and QT intervals (Citation40,Citation42). The pharmacokinetics of dronedarone differs significantly from that of amiodarone (Citation40,Citation41). After oral administration with meals, the bioavailability is about 15% due to extensive first-pass metabolism, which is mediated by the hepatic cytochrome CYP3A4. Peak plasma concentration is reached within 4 to 6 hours after oral ingestion, and steady-state levels are reached within 4 to 8 days. The elimination half-life of dronedarone is 13 to 24 hours and is significantly shorter than that of amiodarone, which requires up to several months for complete removal from adipose tissue stores. The extensive metabolism by the hepatic CYP3A4 results in significant interactions with many agents that either inhibit or induce the CYP3A4. Among the most notable cardiovascular drugs that act as inhibitors of the CYP3A4, thus increasing the plasma levels of dronedarone, are non-dihydropyridine calcium channel blockers (i.e. verapamil and diltiazem) and several statins (e.g. simvastatin, atorvastatin). Of note, at variance with amiodarone, dronedarone is not metabolized by CYP2C9 and CYP2C19, which are involved in the metabolism of warfarin. Furthermore, dronedarone is a direct inhibitor of the renal organic cation transport, resulting in an increase in serum creatinine that occurs early after treatment initiation and persists up to 1 week after treatment discontinuation (Citation42,Citation43). This increase in serum creatinine levels, however, does not reflect kidney damage, since the rate of glomerular filtration remains unaffected, when measured with the inulin clearance method (Citation44). Dronedarone is contra-indicated in patients with severe hepatic impairment, while no dosage adjustment is required for patients with renal insufficiency.
The antiarrhythmic effects of dronedarone, as well as its effects on morbidity and mortality in different patient populations, have been tested in multiple randomized clinical trials () (Citation42, Citation43,Citation45–49). The Dronedarone Atrial Fibrillation Study After Electrical Cardioversion (DAFNE) trial (Citation42) compared three doses of dronedarone (400, 600, and 800 mg twice daily) with placebo on the end-point of AF recurrence in 270 patients with persistent AF scheduled for elective cardioversion. The median time to first AF recurrence was 60 days in the dronedarone (400 mg twice daily) group, as compared to 5.3 days in the placebo groups (relative risk reduction (RRR) = 55%; 95% confidence interval (CI) 72% to 28%; P = 0.001; absolute risk reduction (ARR) at 6 months = 25%; number needed to treat (NNT) = 4). Surprisingly, no significant reduction in AF recurrence was reported with higher doses of dronedarone (i.e. 600 and 800 mg twice daily), and based on these findings the authors suggest the absence of a dose-response effect. In secondary analyses, dronedarone was shown to reduce ventricular rate in case of AF recurrence (P = 0.0001) as compared to placebo, a result further confirmed in an appropriately designed randomized trial () (Citation45).
Table I. Randomized clinical trials of dronedarone.
With regard to safety, a gradual increase in the rate of premature drug discontinuation was observed with increasing doses of dronedarone, with 3.9%, 7.6%, and 22.6% discontinuation rates in the 400 mg b.i.d., 600 mg b.i.d., and 800 mg b.i.d.groups. The high rate of drug discontinuation associated with the 600 mg b.i.d.and 800 mg b.i.d.doses of dronedarone may also provide a more plausible explanation to the apparent lack of antiarrhythmic superiority of higher doses of dronedarone, since analyses were carried out on the intention-to-treat basis. The most frequent causes of premature drug discontinuation were gastrointestinal side-effects (diarrhea, nausea, and vomiting). Interestingly, although a progressive lengthening of the QT interval was observed with increasing doses of dronedarone, no torsade de pointes was reported. Again, this is an important similarity with amiodarone, which is known to cause prolongation of the QT interval with very small torsadogenic potential (Citation41). After the results of DAFNE, a dose of dronedarone of 400 mg twice daily was selected for subsequent studies.
Two large trials tested the efficacy of dronedarone for the maintenance of sinus rhythm in patients with history of AF, namely the European Trial in Atrial Fibrillation or Flutter Patients Receiving Dronedarone for the Maintenance of Sinus Rhythm (EURIDIS) and the American-Australian-African Trial with Dronedarone in Atrial Fibrillation or Flutter Patients for the Maintenance of Sinus Rhythm (ADONIS) (Citation43). EURIDIS and ADONIS shared the same enrollment criteria and end-points, and their results were ultimately published in a single paper (Citation43). Taken together, EURIDIS and ADONIS included a total of 1,239 patients that were in sinus rhythm for at least 1 hour at the time of randomization and had history of at least one documented episode of AF in the preceding 3 months. Patients were randomized to receive 400 mg of dronedarone twice daily or placebo for 1 year. The primary end-point of the study was time to first AF or atrial flutter recurrence, as assessed by transtelephonic ECG monitoring or 12-lead ECG. Dronedarone significantly prolonged the time to first AF/atrial flutter recurrence as compared to placebo both in the EURIDIS and in the ADONIS trials, with a median time to recurrence of 96 versus 41 days (P = 0.01) in the EURIDIS and of 158 versus 59 days (P = 0.002) in the ADONIS. These figures account for a statistically significant 25% RRR of AF/atrial flutter recurrence within 1 year associated with dronedarone use, although the ARR (11.1%) was quite small for a soft end-point such as AF/atrial flutter recurrence.
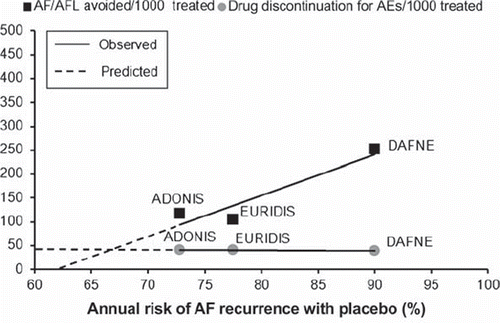
Despite such encouraging results, caution should be exercised when generalizing the results of randomized trials with dronedarone to the general population with AF. In fact, an analysis of treatment effects of dronedarone shows that the benefit of this compound is not the same across all the spectrum of patients with AF. Plotting the total number of AF/atrial flutter events avoided every 1,000 patients treated with dronedarone against the base-line risk of AF recurrence with placebo observed in each dronedarone trial, it appears that the antifibrillatory effectiveness of dronedarone is directly dependent on the base-line risk of AF recurrence (). Moreover, it is predictable that, when base-line annual risk of AF recurrence is lower than 60%–65%, dronedarone would have minimal—if any—clinically meaningful antiarrhythmic effectiveness. On the other hand, dronedarone is not a completely harmless agent. The yearly risk of drug discontinuation because of a serious adverse effect was quite similar across all dronedarone trials, and approximates 12%. Therefore, taking into account also the risk of adverse events, dronedarone predictably loses clinically meaningful antifibrillatory effectiveness—and is potentially harmful—when the base-line annual risk of AF recurrence is lower than 65%–70% (). The results of such analysis, notwithstanding the limitations inherent to statistical prediction models, call for a trial with dronedarone in a population of patients with annual risk of AF recurrence lower than that reported in published trials, before considering appropriate the use of dronedarone in the general population with AF.
Figure 1. Benefits and risks of dronedarone to prevent the occurrence of atrial fibrillation (AF) or atrial flutter (AFL). The number of AF/AFL events avoided (black squares) and the number dronedarone discontinuations for a serious drug-related adverse event (gray circles) every 1,000 patients treated are plotted against the annual risk of AF recurrence with placebo in each trial. Solid lines indicate linear regressions of treatment effects. Extrapolation of regression lines (dotted lines) toward their intersection suggests that the antiarrhythmic benefits of dronedarone may not outweigh the risks associated with its use when the patients' annual risk of AF recurrence is smaller than 65%–70% (intersection of dotted lines). AEs = adverse events; ADONIS (Citation43) = American-Australian-African Trial with Dronedarone in Atrial Fibrillation or Flutter Patients for the Maintenance of Sinus Rhythm; EURIDIS (Citation43) = European Trial in Atrial Fibrillation or Flutter Patients Receiving Dronedarone for the Maintenance of Sinus Rhythm; DAFNE (Citation42) = Dronedarone Atrial Fibrillation Study After Electrical Cardioversion.
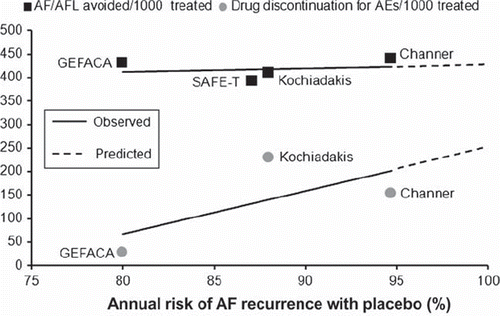
Of note, amiodarone displays a completely different antiarrhythmic behavior, with greater effectiveness than dronedarone, as demonstrated by a greater number of AF events avoided every 1,000 patients treated (), and consistency of treatment effect across different base-line risks of AF recurrence. The antiarrhythmic superiority of amiodarone compared with dronedarone has been recently confirmed in a randomized trial in patients with persistent AF () (Citation48). Moreover, the antifibrillatory effectiveness of amiodarone predictably outweighs the risks of serious adverse events across all AF patient populations ().
Figure 2. Benefits and risks of amiodarone to prevent the occurrence of atrial fibrillation (AF) or atrial flutter (AFL). The number of AF/AFL events avoided (black squares) and the number of amiodarone discontinuations for a serious drug-related adverse event (gray circles) every 1,000 patients treated are plotted against the annual risk of AF recurrence with placebo in each trial. Solid lines indicate linear regressions of treatment effects. Extrapolation of regression lines (dotted lines) does not show intersection, suggesting that the antiarrhythmic benefits of amiodarone outweigh the risks associated with its use across all patient populations. AEs = adverse events; GEFACA (Citation34) = Grupo de Estudio de Fibrilacion Auricular Con Amiodarona; SAFE-T (Citation15) = Sotalol Amiodarone Atrial Fibrillation Efficacy Trial; Kochiadakis et al. (Citation35); Channer et al. (Citation14).
Dronedarone has also been reported to reduce the risk of death or hospitalization for cardiovascular causes in patients with AF. Subgroup analyses of EURIDIS and ADONIS suggested a 20% lower rate of death or hospitalization in the dronedarone arm, largely related to a reduction in the risk of cardiovascular hospitalization. To further evaluate the effect of dronedarone on death or hospitalizations, a large trial on AF/atrial flutter patients with moderate to high risk of cardiovascular death was designed, that is, the ATHENA (A Placebo-Controlled, Double-Blind, Parallel Arm Trial to Assess the Efficacy of Dronedarone 400 mg b.i.d.for the Prevention of Cardiovascular Hospitalization or Death from Any Cause in Patients with Atrial Fibrillation/Atrial Flutter) (Citation47). The design of the ATHENA is the result of the modern ‘change of philosophy’ in AF treatment evaluation, which takes into account important clinical end-points beyond sinus rhythm restoration and maintenance, together with a peculiar focus on safety (Citation6,Citation7).
ATHENA randomized a total of 4,628 patients with AF/atrial flutter and at least one additional risk factor, including hypertension, diabetes, prior cerebrovascular accident, an enlarged left atrium (i.e. ≥50 mm), or a left ventricular ejection fraction <40%, to receive dronedarone or placebo. The trial was adequately powered to address the primary composite end-point of hospitalization for cardiovascular cause or death from any cause. After a mean follow-up of 21±5 months, primary end-point was reached in 31.9% of patients allocated to dronedarone and in 39.4% of patients receiving placebo (RRR 24.2%; 95% CI 16.5%–31.2%; P< 0.001; NNT = 13). Such reduction in primary end-point was largely driven by a reduction of cardiovascular hospitalizations, whereas no significant reduction in all-cause mortality was observed (HR 0.844; 95% CI 0.660–1.080; P = 0.17). Importantly, the upper bound of the 95% CI for the relative risk of all-cause mortality reasonably excluded any clinically significant increase in the risk of death associated with dronedarone use. A post-hoc analysis suggested also a protective effect of dronedarone on ischemic stroke, which appeared independent of concomitant use of antithrombotic therapy and was more pronounced in patients with higher base-line risk of thromboembolic complications, as assessed by the CHADS2 score (Citation50). As the latter would be the subset of patients with the worst prognosis for thromboembolic complications, the finding would be easy to account for by saying—as the ATHENA authors did—that dronedarone has its greatest advantage in patients at higher risk of thromboembolism (Citation50). From a methodological standpoint, this approach to the comparison of subgroups is highly flawed. Indeed, these results are based on a post-hoc subgroup analysis, not pre-specified in the original trial, with resulting P values not adjusted for multiple comparisons (Citation47,Citation50). Therefore, any reported result is very difficult to interpret, because if we go on testing long enough we will inevitably find something which is ‘significant’, and such a ‘significant’ result may be the one in a mass which we expect by chance alone (Citation51).
Moreover, the beneficial effect of dronedarone on death or hospitalization reported in the ATHENA is not applicable to all patient populations. In fact, another trial that aimed at assessing the effect of dronedarone on cardiovascular hospitalization and mortality in a high-risk population led to disappointing results (Citation49). Such trial was the Antiarrhythmic Trial with Dronedarone in Moderate to Severe CHF Evaluating Morbidity Decrease (ANDROMEDA), which compared dronedarone to placebo in a population of patients with a recent episode (<1 month) of decompensated congestive heart failure on the primary end-point of death from any cause or hospitalization for worsening heart failure. The ANDROMEDA was designed with the purpose of formally testing the safety of dronedarone in a high-risk population of patients and, as such, was not carried out in patients likely to receive the drug in clinical practice (i.e. patients with AF/atrial flutter). ANDROMEDA had to enroll a total of 1,000 patients. However, 7 months after study initiation, the trial was stopped with only 627 patients randomized, due to an excess mortality in the dronedarone group (i.e. 25/310 deaths in the dronedarone group versus 12/317 in the placebo group; HR 2.13; 95% CI 1.07–4.25; P = 0.03). Although such excess in mortality is likely over-estimated by the small number of deaths occurring over a short period of time in an under-powered sample of patients, the results of the ANDROMEDA trial raised important safety concerns regarding dronedarone use.
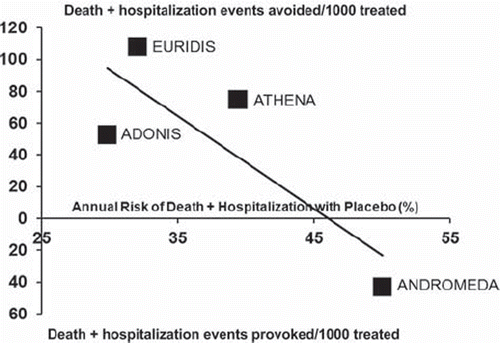
Putting together the results of dronedarone trials on the end-point of death or hospitalizations, dronedarone benefit appears inversely proportional to the base-line risk of the patient population, with a potential harm in high-risk patients, such as those included in the ANDROMEDA (). Accordingly, the American Food and Drug Administration (FDA) has contra-indicated dronedarone in high-risk patients, such as those enrolled in the ANDROMEDA (i.e. patients with class IV heart failure, or class II to III heart failure who had a recent decompensation) (Citation40,Citation49), and has recommended its use for sinus rhythm maintenance in patients with left ventricular ejection fraction greater than 35% (Citation40).
Figure 3. Effects of dronedarone on death or hospitalization for cardiovascular causes in different groups of patients. The number of death or hospitalization events (black squares) avoided (upper panel) or provoked (lower panel) every 1,000 patients treated is plotted against the annual risk of death or hospitalization with placebo in each trial. Solid line indicates linear regression of treatment effect and suggests an inverse relationship between dronedarone-associated benefits and base-line risk of patients, with harm for patients with an annual risk of death or hospitalization greater than 40%–45% (intersection of the regression line with the xaxis). The greatest benefit is observed in patients with low-to-moderate (25%–35%) annual risk of events. EURIDIS (Citation43) = European Trial in Atrial Fibrillation or Flutter Patients Receiving Dronedarone for the Maintenance of Sinus Rhythm; ADONIS (Citation43) = American-Australian-African Trial with Dronedarone in Atrial Fibrillation or Flutter Patients for the Maintenance of Sinus Rhythm; ATHENA (Citation47) = A Placebo-Controlled, Double-Blind, Parallel Arm Trial to Assess the Efficacy of Dronedarone 400 mg b.i.d.for the Prevention of Cardiovascular Hospitalization or Death from Any Cause in Patients with Atrial Fibrillation/Atrial Flutter; ANDROMEDA (Citation49) = Antiarrhythmic Trial with Dronedarone in Moderate to Severe CHF Evaluating Morbidity Decrease.
On the basis of the available evidence, however, we believe it reasonable to restrict the use of dronedarone to patients with moderate to high risk of AF recurrence—in whom the antiarrhythmic effectiveness clearly outweighs the risk of drug discontinuation because of adverse events ()—and low risk of death or hospitalization, in order to maximize the benefit on death and hospitalization (). Further studies on populations with lower risk of AF recurrence (<60% at 1 year) and moderate to high risk of death or hospitalization (40%–50% at 1 year), are warranted before considering appropriate the use of dronedarone across all the spectrum of AF patient populations.
Budiodarone
Budiodarone is an amiodarone analog that maintains iodine moieties but contains an ester modification that allows extensive metabolism by tissue esterases rather than by hepatic cytochromes (Citation19). As a result, the half-life of budiodarone is significantly shorter than that of amiodarone (7 hours), while electrophysiological properties are similar (Citation19). Only limited clinical data on budiodarone are available. Arya and colleagues evaluated the effect of budiodarone on 6 patients with paroxysmal AF and permanent pacemaker (Citation52). Patients underwent sequential 2-week periods of treatment with increasing doses of budiodarone (200 mg b.i.d., 400 mg b.i.d., 600 mg b.i.d., and 800 mg b.i.d.), preceded and followed by a week of drug wash-out. The primary end-point was reduction of AF burden, defined as percent of time in AF, as assessed by pacemaker interrogation. At base-line, mean AF burden was 20.3% ±14.6% and decreased significantly with all doses of budiodarone, with greater treatment effects with increasing budiodarone doses. Moreover, budiodarone decreased also the duration of AF episodes, again with a dose-effect response.
These encouraging results were confirmed in a recent phase II clinical trial, the Paroxysmal Atrial Fibrillation Study with Continuous Atrial Fibrillation Logging (PASCAL) (Citation53). In this trial, a total of 72 patients with paroxysmal AF and dual chamber permanent pacemaker with advanced rhythm-monitoring capabilities were randomized to placebo or increasing doses of budiodarone (200, 400, and 600 mg twice daily). Again, the primary end-point was decrease of AF burden as assessed by pacemaker interrogation. Increasing doses of budiodarone were associated with progressively greater reductions of AF burden (RRR 10%, P = 0.16 for the 200 mg b.i.d.; RRR = 54%, P = 0.015 for the 400 mg b.i.d.; and RRR = 75%, P = 0.005 for the 600 mg b.i.d.). With regard to safety, patients allocated to budiodarone experienced an increase of the thyroid-stimulating hormone, reversible after drug discontinuation. Moreover, an increase in serum creatinine levels was reported, likely due to a dronedarone-like inhibitory effect on renal organic cation transport. Interestingly, no QT prolongation was observed during the study course. On the other hand, the selection of patients with a permanent pacemaker may have limited the evaluation of some of the possible side-effects of budiodarone, such as depression of atrioventricular conduction velocity (Citation19).
In conclusion, budiodarone is a promising amiodarone analog. However, further studies on broader populations of patients and with longer follow-up durations are necessary to better evaluate the effectiveness and safety of this new drug in AF.
Celivarone
Celivarone is a non-iodinated amiodarone analog that is currently undergoing extensive evaluation in clinical trials as an antiarrhythmic agent for both supraventricular and ventricular arrhythmias (Citation17,Citation54–56). Experimental data have shown that celivarone exhibits electrophysiological and hemodynamic effects similar to amiodarone, with acute antifibrillatory efficacy after intravenous and oral loads (Citation54). A phase II dose-ranging study, the Maintenance of Sinus Rhythm in Patients with Recent Atrial Fibrillation or Flutter (MAIA), randomized 673 patients with a previous episode of AF or atrial flutter to four doses of celivarone (50, 100, 200, and 300 mg once daily) or placebo. The greater reduction of AF/atrial flutter recurrence was observed with the 50 mg dose of celivarone, as compared to placebo (52.1% versus 67.1%; P = 0.055) (Citation55). No enhanced efficacy was reported with higher doses of celivarone, an effect similar to that already observed with dronedarone (Citation42). With regard to safety, no torsade de pointes was reported, with a quite low rate of celivarone discontinuation because of adverse events (6.2% to 8% across different celivarone daily doses) (Citation55). Recently, another phase II trial, the Controlled Dose Ranging Study of the Efficacy and Safety (CORYFEE), tested the efficacy of 300 mg or 600 mg once daily doses of celivarone for the conversion to sinus rhythm in patients with AF/atrial flutter undergoing planned electrical cardioversion. The results of the CORYFEE are not yet available.
Atrial selective antiarrhythmic drugs
The development of agents with selective pharmacologic effect on ion channels expressed in the atria but not in the ventricles is a major focus of modern antifibrillatory drug development. Many different ionic currents contribute to the action potential of human atrial myocytes, and the majority are shared with ventricular myocytes (). However, several ionic currents are expressed predominantly or selectively in the atria () and constitute attractive targets for the development of atrial selective antiarrhythmic agents. To date, research on atrial selective compounds has focused mainly on two repolarization currents: the ultra-rapid potassium outward current (IKur) and the inwardly rectifying acetylcholine-sensitive current (IKACh) (). The latter is constitutively activated in an agonist-independent manner in patients with chronic AF (Citation57), and blockade of its activity has been suggested to contribute to the antiarrhythmic effectiveness of several older antiarrhythmic compounds, such as flecainide (Citation58).
Figure 4. Ionic currents involved in the action potential of atrial myocytes. Atrial selective currents are highlighted in gray.
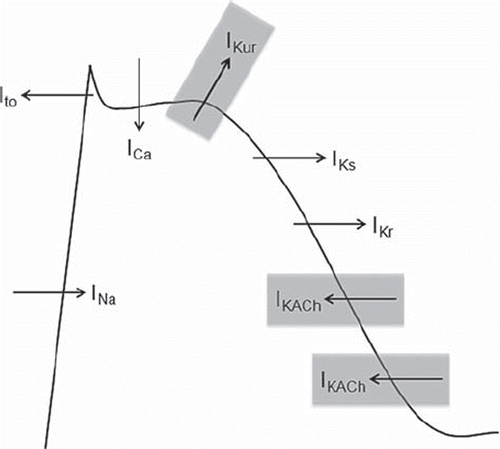
Table II. Summary of atrial selective agents.
Other atrial-selective molecular targets under active investigation include atrial-selective Na+ channel (Citation59), small-conductance K+ channels (Citation60), and other repolarization channels, such as the two-pore K+ channel TASK (Citation61).
To date, truly selective modulators of atrial ion channels have not yet been developed, and most of the currently available ‘atrial selective’ agents are actually multichannel blockers with higher affinity to atrial ion channels.
Vernakalant
Vernakalant is among the most promising and most investigated atrial selective agents for the treatment of AF (Citation62–72). Vernakalant acts through multichannel blockade, including atrial selective repolarization currents (IKur and IKACh), but also several other currents, such as Ito and INa with a frequency-dependent effect () (Citation62). On the other hand, blockade of IKr and IKs repolarization currents, which are highly expressed in the ventricles, is minimal. As a result, vernakalant slows the conduction velocity and prolongs refractoriness within the atria, with small effects on the ventricles (Citation64). The atrial-specific repolarization-delaying effect appears more prominent in patients with paroxysmal rather than chronic AF (Citation73), which is possibly related to a higher degree of electrical remodeling in the latter group.
Due to the short half-life (2 h), vernakalant has been mostly investigated as an intravenous agent (Citation62,Citation66,Citation67), although an oral slow-release preparation has been developed and is undergoing phase III clinical evaluation (Citation67). After intravenous administration, vernakalant undergoes metabolism by the hepatic cytochrome CYP2D6 (Citation66). The extent of such metabolism is unclear, since plasma concentrations of vernakalant have been reported unaffected by the administration of CYP2D6 inducers or inhibitors (Citation66,Citation67).
Six randomized studies have evaluated the effect of intravenous vernakalant for the acute cardioversion of persistent AF (Citation65,Citation68–72) (). These studies led to the recent (September 2010) approval of vernakalant for the acute conversion of AF by the European Medicines Agency (EMEA). In a phase II multicenter placebo-controlled trial, the Randomized, Controlled Trial of RSD1235, a Novel Antiarrhythmic Agent, in the Treatment of Recent Onset Atrial Fibrillation (CRAFT) (Citation69), 56 patients with persistent AF were randomly assigned to one of two vernakalant intravenous doses (i.e. 0.5 mg/kg or 2 mg/kg in 10 minutes) or to placebo. A repeat infusion of vernakalant (or placebo) at a rate of either 2 mg/kg (for patients initially treated with the dose of 0.5 mg/kg), or 3 mg/kg (for patients initially treated with the dose of 2 mg/kg), was permitted if cardioversion was not reached within 30 minutes from the first infusion. The primary end-point was cardioversion of AF during or within 30 minutes after drug infusion. Patients allocated to the higher intravenous dose of vernakalant (i.e. 2 mg/kg followed by 3 mg/kg) had a significantly higher rate of conversion to sinus rhythm than those receiving placebo (61% versus 5%; P< 0.0005; RRR 59%; ARR 56%; NNT 1.8), together with a shorter median time-to-conversion (14 versus 162 minutes; P = 0.016). The encouraging results of the CRAFT led to four other clinical trials, the Atrial Arrhythmia Conversion Trials (ACTs) () (Citation68,Citation71,Citation72,Citation74). The ACT 1 randomized a total of 336 patients with persistent AF of either short (≤7 days) or long duration (8–45 days), to vernakalant (3 mg/kg in 10 minutes, followed by another 2 mg/kg if cardioversion was not reached within 15 minutes), or placebo (Citation68). The primary end-point was conversion to sinus rhythm within 90 minutes of drug initiation. The conversion rate with vernakalant in patients with short-duration persistent AF was 51.7%, compared to 4% with placebo (P< 0.001). On the other hand, only 7.9% of patients with long-duration persistent AF converted to sinus rhythm with vernakalant, compared to 0% in the placebo arm (P = 0.09). The drug was well tolerated, with only transient minor side-effects (mainly dysgeusia and sneezing), and no torsade de pointes was reported. Such results were confirmed in two other trials, the ACT 3, which included a cohort of patients similar to that of the ACT 1, and the ACT 2, that focused on patients after cardiac surgery (Citation65,Citation72). Of note, ACT 3 and 2 included also a small subgroup of patients with atrial flutter, in whom vernakalant consistently failed to show an antiarrhythmic efficacy (Citation65,Citation72) (). Finally, the ACT 4 was designed to further evaluate the safety of intravenous vernakalant in recent-onset AF patients, with results quite similar to the previous ACT studies. Of note, patients with decompensated heart failure were excluded from all ACT trials.
Table III. Randomized clinical trials of vernakalant.
The relative effectiveness of vernakalant compared to other available antiarrhythmic agents is unknown, and studies of direct comparison with agents currently recommended for AF cardioversion are warranted. However, since currently available antiarrhythmic drugs have been evaluated extensively against placebo, an indirect comparison analysis of effectiveness can be performed. On indirect comparison, vernakalant appears one of the most effective antiarrhythmic agents for the acute conversion of AF, with effectiveness comparable to intravenous flecainide (). Amiodarone, on the other hand, is the least effective antiarrhythmic agent in this setting, with a clearly smaller effectiveness compared to vernakalant ().
Figure 5. Indirect comparison of treatment effects of different intravenous antiarrhythmic agents for the acute conversion of atrial fibrillation (AF). SR = sinus rhythm.
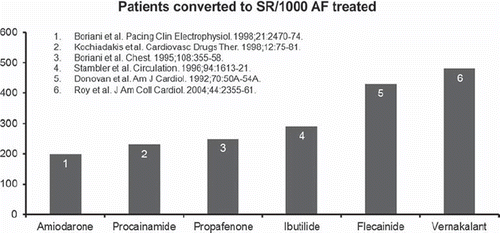
Surprisingly, amiodarone has been the only antiarrhythmic agent directly compared with vernakalant in a randomized study. This study was the A phase III prospective, Randomized, double-blind, active-controlled, multicenter, superiority study of Vernakalant injection versus amiodarone in subjects with Recent Onset atrial fibrillation (AVRO) (Citation75), and the results were presented at the last Heart Rhythm Society annual meeting (Citation70). In total, 254 patients with AF were enrolled in the study, with 116 patients in each group receiving either a 10-minute infusion of 3 mg/kg of vernakalant, eventually followed by another 10-minute infusion of 2 mg/kg if conversion to sinus rhythm was not reached within 15 minutes after the first infusion, or a 60-minute infusion of 5 mg/kg of amiodarone followed by a maintenance infusion of 50 mg over another 60 minutes. At 90 minutes after the first infusion, the proportion of patients converted to sinus rhythm was higher in the vernakalant arm (51.7%) than in the amiodarone arm (5.2%; P< 0.0001). Moreover, treatment with vernakalant resulted in a more rapid conversion to sinus rhythm, with a median conversion time of 11 minutes, and was associated with a higher rate of symptom relief compared to amiodarone (53.4% versus 32.8%, respectively; P = 0.0012). Also in this study, there was no reported case of drug-induced ventricular arrhythmias.
As previously mentioned, the results of the AVRO study were largely predictable by an accurate analysis of the available evidence (). Further direct comparison studies with other available and more effective antiarrhythmic agents, such as intravenous flecainide, are certainly warranted before considering vernakalant of clear incremental therapeutic value for the acute conversion of AF.
In conclusion, vernakalant appears a new effective and safe drug for rapid termination of AF, although the real incremental value in this setting compared to other available antiarrhythmic agents merits further investigation. On-going studies with oral vernakalant will also reveal whether this agent may be useful for sinus rhythm maintenance.
Ranolazine
Ranolazine is predominantly a late Na+ current (INa) blocker, with effects also on the ultra-rapid delayed rectifier K+ current (IKur) (Citation76,Citation77). The effect on late INa accounts for significant anti-ischemic properties, which have been demonstrated both in preclinical and clinical studies (Citation76,Citation78). Late INa is up-regulated during myocardial ischemia, which leads to increased cytosolic calcium concentration due to enhanced activity of Na+/Ca2+ exchanger, and increased susceptibility to delayed after-depolarization (Citation76,Citation79). Recent evidences suggest that late INa is increased also in patients with AF (Citation80), and ranolazine has been shown capable of reversing such late INa current up-regulation (Citation80).
As an anti-ischemic agent, up to 1,000 mg twice daily of ranolazine is administered orally, with variable bioavailability due to extensive first-pass metabolism, mainly CYP3A4-dependent (Citation23,Citation76,Citation79,Citation81). At concentrations within the therapeutic range for anti-ischemic activity (2–6 μM) (Citation23,Citation79), ranolazine produces a much greater depression of atrial versus ventricular INa, which is of potential interest in the treatment of AF. Interestingly, in experimental models of isolated canine pulmonary vein sleeve, ranolazine has been shown to suppress excitability and triggered activity, to terminate AF induced by acetylcholine and isoproterenol, and to prevent AF reinduction (Citation23,Citation82).
In a post-hoc analysis of the Metabolic Efficiency With Ranolazine for Less Ischemia in Non-ST-Elevation Acute Coronary Syndrome (MERLIN)-Thrombolysis in Myocardial Infarction (TIMI) 36 trial (Citation78), which randomized more than 6,000 patients with acute coronary syndrome to ranolazine or placebo, treatment with ranolazine was associated with lower rate of new-onset AF (3.1% versus 4.3%; P = 0.01) (Citation81). With regard to safety, ranolazine resulted in up to 6 ms mean increase in the QTc interval, which is likely due to its effects on the IKur current, although no case of drug-induced torsade de pointes was reported (Citation78,Citation81).
The potential usefulness of ranolazine in AF has been recently confirmed in a consecutive series of seven patients with recurrent AF after at least one failed antiarrhythmic agent (dofetilide, sotalol, propafenone, flecainide, or amiodarone) (Citation83). In this series, ranolazine controlled the arrhythmia and maintained sinus rhythm at 27±11 weeks of follow-up in 5/7 patients (Citation83).
Based on these preliminary findings, ranolazine may represent a safe atrial selective agent to treat AF, although appropriate randomized trials are warranted to evaluate its potential therapeutic role against AF.
Other atrial selective agents
Xention (XEN-D0101) is an atrial repolarization delaying agent, with a selective blockade of IKur(Citation84). Xention has been demonstrated to prolong atrial refractoriness and suppress AF in canine models (Citation84), with similar electrophysiological effects also on isolated human atrial myocytes (Citation85). The phase I clinical development of Xention was initiated in October 2006.
AZD7009 exhibits mixed potassium and sodium ion channel-blocking properties and exerts potent effects on atrial refractoriness (Citation86–88), without affecting significantly the ventricular refractoriness and QT interval (Citation24). In a randomized study on 122 patients with persistent AF/atrial flutter undergoing planned cardioversion, intravenous AZD7009 increased the proportion of patients who converted to sinus rhythm in a concentration-dependent manner (Citation25). Only one case of asymptomatic non-sustained polymorphic ventricular tachycardia was reported after AZD7009 infusion (Citation25). Research on AZD7009 was stopped in 2006 for undisclosed side-effects (Citation89).
AVE0118 is an atrial-selective multichannel blocker, with effects on IKur, IKACh, and Ito (Citation90). In animal models of AF, AVE0118 prolongs atrial refractoriness and repolarization in a dose-dependent manner without affecting ventricular repolarization (Citation91,Citation92). Such electrophysiological effects translate into effective conversion of persistent AF (Citation91) and prevention of AF induction (Citation92). Of note, the atrial repolarization delaying effect of AVE0118 appears specific for AF, whereas it actually shortens the atrial action potential duration during sinus rhythm (Citation93). Clinical studies on AVE0118 have stopped in the IIa phase for unspecified reasons (Citation94).
AVE1231 is a molecular congener of AVE0118, with similar unselective blockade of IKur (Citation95). AVE1231 is currently in phase I clinical development (Citation94).
NIP-141 is a multiple channel blocker with atrial selective action potential duration prolonging profile (Citation96). In animal models, NIP-141 has been reported to inhibit atrial action potential shortening induced by rapid atrial pacing, with a possible anti-remodeling effect mediated by inhibition of L-type Ca2+ channel (Citation97) ().
In conclusion, modulation of ionic currents selectively expressed in the atria represents a main focus of modern antifibrillatory drug development. The main target of this novel drug category is the atrial selective repolarization current IKur (Citation98). The clinical efficacy and safety of these compounds warrant adequate investigation in phase III studies.
Selective adenosine receptor agonists
The clinical effects of adenosine are mediated by four purinergic receptors, all with different functions (Citation99,Citation100). The stimulation of A1 receptors accounts for the negative dromotropic properties of adenosine (Citation99), due to prolonged refractoriness of the atrioventricular nodal conduction system. Such effect has important therapeutic applications in many supraventricular arrhythmias (Citation101). In non-specialized myocytes, however, A1 receptor stimulation determines a shortening of refractoriness, with possible induction of atrial or ventricular fibrillation (Citation101).
In the setting of chronic AF, in which a rate-control strategy is often attempted (Citation102), the negative dromotropic effects of A1 receptor stimulation may be of interest. Several selective A1 receptor agonists are in the active phase of development. Tecadenoson is the compound of this class in the most advanced phase of development. The selectivity of tecadenoson on A1 receptor appeared very high in preclinical studies (Citation103,Citation104), with significant effect on atrioventricular nodal conduction and refractoriness without causing hypotension or negative inotropic effects. A dose-ranging study in 32 healthy humans confirmed a dose-dependent effect on atrioventricular nodal conduction, without affecting the infra-nodal conduction (Citation105). Four patients had drug-induced second- or third-degree atrioventricular block, and AF was induced in three patients.
A phase II clinical study with tecadenoson for rate control of chronic AF was completed in 2009, but results have not yet been published (Citation106).
Upstream therapies for atrial fibrillation
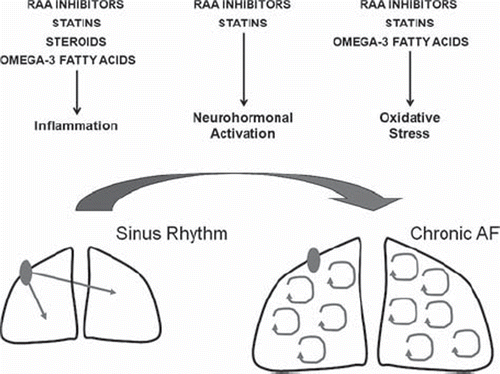
The value of upstream therapies for AF has become increasingly appreciated in recent years, as the pivotal role of different non-electrophysiological triggers and substrates for AF has been elucidated (Citation107). In particular, the exploration of the left atrial substrate has suggested that AF is a self-perpetuating disease, characterized by progressive electrical and tissue structural remodeling, which ultimately leads to loss of atrial myocytes and fibrosis (Citation108,Citation109).
The prevention of atrial structural remodeling through inhibition of biologic pathways leading to tissue fibrosis constitutes the rationale for up-stream therapies for AF. Several traditionally non-antiarrhythmic agents have been suggested for this purpose (), including inhibitors of the renin-angiotensin-aldosterone system (Citation28,Citation110,Citation111) and anti-inflammatory agents (Citation29,Citation30,Citation33,Citation112).
Figure 6. Putative molecular effects of upstream therapies for atrial fibrillation. RAA = renin-angiotensin-aldosterone.
Inhibitors of the renin-angiotensin-aldosterone system
Atrial tissue remodeling is greatly enhanced by angiotensin II. Angiotensin II promotes growth of cardiac myocytes, vascular smooth muscle cells, and fibroblasts (Citation113), increases atrial oxidative stress (Citation114), mediates unwanted increase in ventricular oxidative stress induced by rapid atrial rate (Citation115), and modifies electrophysiological properties by indirect actions on ion channels (Citation116). Angiotensin II is also a potent pressor agent and directly stimulates aldosterone secretion, which further promotes atrial fibrosis (Citation117). Moreover, overstimulation of the renin-angiotensin-aldosterone system chronically increases the left ventricular afterload, which ultimately leads to diastolic dysfunction, increased left atrial dimension, and higher risk of developing AF (Citation111,Citation116–118).
With these premises, inhibition of the renin-angiotensin-aldosterone system appears a reasonable strategy to prevent the occurrence of AF. Retrospective analyses of studies with renin-angiotensin system blockers in different cohorts of patients have reported a lower incidence or recurrence of AF in patients treated with renin-angiotensin inhibitors (Citation26,Citation119–122). In a recent large meta-analysis, the greatest benefit of renin-angiotensin inhibitors has been reported for secondary prevention of AF, especially after electrical cardioversion (Citation28). Among primary prevention studies, a benefit was observed among patients with heart failure and those with hypertension and left ventricular hypertrophy, thus suggesting a possible benefit in patients with structural heart disease (Citation123). On the other hand, renin-angiotensin inhibitors did not appear to prevent the occurrence of AF in patients after myocardial infarction (Citation28). Despite these encouraging findings, a trial specifically aimed at assessing the effect of renin-angiotensin system blockade on AF recurrences reported negative results (Citation110). This trial was the Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto Miocardico-Atrial Fibrillation (GISSI-AF) (Citation110), which randomized 1,442 patients with history of AF to either valsartan (an angiotensin receptor blocker) or matching placebo. At 1-year follow-up, AF recurred in 51.4% of the patients in the valsartan group versus 52.1% in the placebo group (HR 0.98; 96% CI 0.85–1.14; P = 0.83) (Citation110). The results of the GISSI-AF question the effectiveness of renin-angiotensin system blockers for the secondary prevention of AF, although the wide use of other ACE inhibitors in the control group and the short duration of the follow-up may have confounded the results (Citation110). Of note, we also found no improvement in the success rate of radiofrequency catheter ablation of AF in patients treated with renin-angiotensin system inhibitors (Citation124), further supporting a lack of benefit in secondary prevention of AF.
With regard to aldosterone receptor antagonists, preclinical data suggest a potential role of these agents in AF management (Citation111,Citation117), although adequate clinical evidences in this setting are lacking.
In conclusion, inhibitors of the renin-angiotensin-aldosterone system appear an emerging treatment option for AF, mainly due to their significant anti-remodeling properties. Further clinical studies are necessary to identify the population of AF patients who may earn the greatest benefit from these agents.
Anti-inflammatory and anti-oxidant agents
Inflammation and oxidative stress contribute to electrical and structural remodeling of the atria (Citation125–127) and are intimately linked to initiation and perpetuation of AF (Citation127). Several anti-inflammatory and anti-oxidant agents have been tested against AF (Citation29,Citation33,Citation112,Citation128).
Steroids, the paradigm of anti-inflammatory agents, have been associated with a lower incidence of AF in preclinical and clinical studies (Citation112,Citation129), although the high rate of side-effects limits their usefulness as antiarrhythmics.
Inhibitors of the 3-hydroxy-3-methylglutaryl coenzyme A reductase (i.e. statins) are among the most promising anti-inflammatory and anti-oxidant agents with demonstrated antifibrillatory effectiveness (Citation29,Citation31,Citation128,Citation130,Citation131). Multiple clinical studies have suggested an antiarrhythmic effect of statins against AF, especially when this condition is associated with increased inflammation, such as after cardiac surgery or acute coronary syndromes (Citation128). Furthermore, we recently reported that the antifibrillatory effectiveness of statins may extend also to patients with sinus node dysfunction and a permanent pacemaker, a condition characterized by a high incidence of AF (Citation29). Also in this condition, inflammation and oxidative stress have been associated with both structural changes in the atria leading to atrial fibrosis and dilatation, as well as with biochemical and electrophysiological changes in the individual atrial myocytes (Citation29).
A limited amount of evidence supports the usefulness of omega-3 fatty acids against AF (Citation32,Citation33,Citation127,Citation132–134). In a randomized trial on 160 patients undergoing coronary artery bypass surgery, treatment with omega-3 fatty acids reduced the occurrence of postoperative AF by 46% compared to placebo (P = 0.013) (Citation33). Similar findings were confirmed in another study on postoperative cardiac surgery patients (Citation132), while a recent randomized trial failed to show a beneficial effect of omega-3 fatty acids on postoperative AF (Citation133). Pooling together these results, there is inconclusive evidence supporting the use of omega-3 fatty acids to prevent the occurrence of postoperative AF ().
Figure 7. Forest plot showing the individual and pooled hazard ratios of postoperative atrial fibrillation comparing therapy with omega-3 fatty acids versus placebo. Square boxes denote hazard ratio; horizontal lines represent 95% confidence interval (CI).
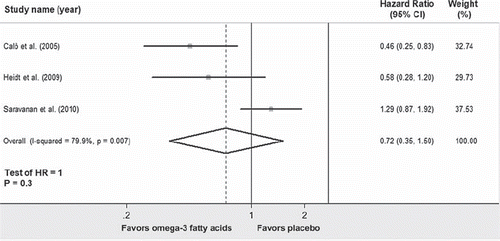
The benefit of omega-3 fatty acids in other populations of patients with AF is undefined and is being actively investigated in adequate randomized trials (Citation135,Citation136). To this regard, we recently reported, in a retrospective analysis of 1,500 patients with AF referred to our institution for pulmonary vein antrum isolation, that treatment with omega-3 fatty acids was associated with lower rate of early AF recurrences (Citation134), thus suggesting a benefit also in patients eligible for AF catheter ablation.
In conclusion, statins represent the most promising anti-inflammatory and anti-oxidant agents with antiarrhythmic effectiveness against AF. Omega-3 fatty acids supplementation has been suggested to lower the incidence of AF, particularly in the postoperative period, although further studies are necessary to verify this finding.
Conclusions
In the upcoming years, plenty of novel pharmacologic strategies will become available to treat AF. Beyond demonstrating their antiarrhythmic effectiveness, research should also focus on identifying the subset of patients who may earn the greatest benefit from these compounds. Moreover, the role of these therapies in relationship to established curative approaches to AF, such as catheter ablation, should also be investigated.
Dronedarone is the first of these novel compounds approved for AF treatment. Its antiarrhythmic effectiveness seems lower in comparison to amiodarone, and the benefit across all the spectrum of AF patients is not clearly demonstrated. Intense research for atrial selectivity has led to the development of vernakalant, which appears very effective for the acute conversion of AF. Finally, upstream therapies have the important advantage of targeting AF substrates. Among these, statins are certainly the most promising ones, although further randomized studies are warranted to verify their real benefit in AF.
Declaration of interest: Dr. Natale has received compensation for belonging to the speakers’ bureau for St Jude Medical, Boston Scientific, Medtronic, and Biosense Webster and has received a research grant from St Jude Medical. The other authors declare no conflicts of interest.
References
- Miyasaka Y, Barnes ME, Gersh BJ, Cha SS, Bailey KR, Abhayaratna WP, . Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114:119–25.
- Friberg J, Buch P, Scharling H, Gadsbphioll N, Jensen GB. Rising rates of hospital admissions for atrial fibrillation. Epidemiology. 2003;14:666–72.
- Bialy D, Lehmann H, Schumacher DN, Steinman RT, Meissner MD. Hospitalization for arrhythmias in the United States: importance of atrial fibrillation. J Am CollCardiol. 1992;19:41A–1.
- Benjamin EJ, Wolf PA, D'Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;98:946–52.
- Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22:983–8.
- Benjamin EJ, Chen PS, Bild DE, Mascette AM, Albert CM, Alonso A, . Prevention of atrial fibrillation: report from a national heart, lung, and blood institute workshop. Circulation. 2009;119:606–18.
- Kirchhof P, Auricchio A, Bax J, Crijns H, Camm J, Diener HC, . Outcome parameters for trials in atrial fibrillation: recommendations from a consensus conference organized by the German Atrial Fibrillation Competence NETwork and the European Heart Rhythm Association. Europace. 2007;9:1006–23.
- Cappato R, Calkins H, Chen SA, Davies W, Iesaka Y, Kalman J, . Updated worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circ Arrhythm Electrophysiol. 2010;3:32–8.
- Preliminary report: effect of encainide and flecainide on mortality in a randomized trial of arrhythmia suppression after myocardial infarction. The Cardiac Arrhythmia Suppression Trial (CAST) Investigators. N Engl J Med. 1989; 321:406–12.
- Roden DM. Risks and benefits of antiarrhythmic therapy. N Engl J Med. 1994;331:785–91.
- Waldo AL, Camm AJ, deRuyter H, Friedman PL, MacNeil DJ, Pauls JF, . Effect of d-sotalol on mortality in patients with left ventricular dysfunction after recent and remote myocardial infarction. The SWORD Investigators. Survival With Oral d-Sotalol. Lancet. 1996;348:7–12.
- Pedersen OD, Bagger H, Keller N, Marchant B, Kober L, Torp-Pedersen C. Efficacy of dofetilide in the treatment of atrial fibrillation-flutter in patients with reduced left ventricular function: a Danish investigations of arrhythmia and mortality on dofetilide (diamond) substudy. Circulation. 2001;104:292–6.
- Zimetbaum P. Amiodarone for atrial fibrillation. N Engl J Med. 2007;356:935–41.
- Channer KS, Birchall A, Steeds RP, Walters SJ, Yeo WW, West JN, . A randomized placebo-controlled trial of pre-treatment and short- or long-term maintenance therapy with amiodarone supporting DC cardioversion for persistent atrial fibrillation. Eur Heart J. 2004;25:144–50.
- Singh BN, Singh SN, Reda DJ, Tang XC, Lopez B, Harris CL, . Amiodarone versus sotalol for atrial fibrillation. N Engl J Med. 2005;352:1861–72.
- Dobrev D, Nattel S. New antiarrhythmic drugs for treatment of atrial fibrillation. Lancet. 375:1212–23.
- Gautier P, Guillemare E, Djandjighian L, Marion A, Planchenault J, Bernhart C, . In vivo and in vitro characterization of the novel antiarrhythmic agent SSR149744C: electrophysiological, anti-adrenergic, and anti-angiotensin II effects. J Cardiovasc Pharmacol. 2004;44:244–57.
- Kathofer S, Thomas D, Karle CA. The novel antiarrhythmic drug dronedarone: comparison with amiodarone. Cardiovasc Drug Rev. 2005;23:217–30.
- Morey TE, Seubert CN, Raatikainen MJ, Martynyuk AE, Druzgala P, Milner P, . Structure-activity relationships and electrophysiological effects of short-acting amiodarone homologs in guinea pig isolated heart. J Pharmacol ExpTher. 2001;297:260–6.
- Fedida D, Orth PM, Chen JY, Lin S, Plouvier B, Jung G, . The mechanism of atrial antiarrhythmic action of RSD1235. J Cardiovasc Electrophysiol. 2005;16: 1227–38.
- Orth PM, Hesketh JC, Mak CK, Yang Y, Lin S, Beatch GN, . RSD1235 blocks late INa and suppresses early afterdepolarizations and torsades de pointes induced by class III agents. Cardiovasc Res. 2006;70:486–96.
- Antzelevitch C, Belardinelli L, Wu L, Fraser H, Zygmunt AC, Burashnikov A, . Electrophysiologic properties and antiarrhythmic actions of a novel antianginal agent. J Cardiovasc Pharmacol Ther. 2004;9 Suppl1:S65–83.
- Burashnikov A, Di Diego JM, Zygmunt AC, Belardinelli L, Antzelevitch C. Atrium-selective sodium channel block as a strategy for suppression of atrial fibrillation: differences in sodium channel inactivation between atria and ventricles and the role of ranolazine. Circulation. 2007;116:1449–57.
- Carlsson L, Chartier D, Nattel S. Characterization of the in vivo and in vitro electrophysiological effects of the novel antiarrhythmic agent AZD7009 in atrial and ventricular tissue of the dog. J Cardiovasc Pharmacol. 2006;47:123–32.
- Crijns HJ, Van Gelder IC, Walfridsson H, Kulakowski P, Ronaszeki A, Dedek V, . Safe and effective conversion of persistent atrial fibrillation to sinus rhythm by intravenous AZD7009. Heart Rhythm. 2006;3:1321–31.
- Vermes E, Tardif JC, Bourassa MG, Racine N, Levesque S, White M, . Enalapril decreases the incidence of atrial fibrillation in patients with left ventricular dysfunction: insight from the Studies Of Left Ventricular Dysfunction (SOLVD) trials. Circulation. 2003;107:2926–31.
- Madrid AH, Escobar C, Rebollo JM, Marin I, Bernal E, Nannini S, . Angiotensin receptor blocker as adjunctive therapy for rhythm control in atrial fibrillation: results of the irbesartan-amiodarone trial. Card Electrophysiol Rev. 2003;7:243–6.
- Schneider MP, Hua TA, Bohm M, Wachtell K, Kjeldsen SE, Schmieder RE. Prevention of atrial fibrillation by renin-angiotensin system inhibition a meta-analysis. J Am Coll Cardiol. 2010;55:2299–307.
- Santangeli P, Ferrante G, Pelargonio G, Dello Russo A, Casella M, Bartoletti S, . Usefulness of statins in preventing atrial fibrillation in patients with permanent pacemaker: a systematic review. Europace. 2010;12:649–54.
- Adam O, Neuberger HR, Bohm M, Laufs U. Prevention of atrial fibrillation with 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors. Circulation. 2008;118:1285–93.
- Savelieva I, Camm J. Statins and polyunsaturated fatty acids for treatment of atrial fibrillation. Nat Clin Pract Cardiovasc Med. 2008;5:30–41.
- Sakabe M, Shiroshita-Takeshita A, Maguy A, Dumesnil C, Nigam A, Leung TK, . Omega-3 polyunsaturated fatty acids prevent atrial fibrillation associated with heart failure but not atrial tachycardia remodeling. Circulation. 2007; 116:2101–9.
- Calo L, Bianconi L, Colivicchi F, Lamberti F, Loricchio ML, de Ruvo E, . N-3 fatty acids for the prevention of atrial fibrillation after coronary artery bypass surgery: a randomized, controlled trial. J Am Coll Cardiol. 2005;45:1723–8.
- Galperin J, Elizari MV, Chiale PA, Molina RT, Ledesma R, Scapin AO, . Efficacy of amiodarone for the termination of chronic atrial fibrillation and maintenance of normal sinus rhythm: a prospective, multicenter, randomized, controlled, double blind trial. J Cardiovasc Pharmacol Ther. 2001;6:341–50.
- Kochiadakis GE, Igoumenidis NE, Marketou ME, Kaleboubas MD, Simantirakis EN, Vardas PE. Low dose amiodarone and sotalol in the treatment of recurrent, symptomatic atrial fibrillation: a comparative, placebo controlled study. Heart. 2000;84:251–7.
- Zipes DP, Prystowsky EN, Heger JJ. Amiodarone: electrophysiologic actions, pharmacokinetics and clinical effects. J Am Coll Cardiol. 1984;3:1059–71.
- Grossman M, Dobrev D, Kirch W. Amiodarone causes endothelium-dependent vasodilation in human hand veins in vivo. Clin Pharmacol Ther. 1998;64:302–11.
- Grossmann M, Dobrev D, Himmel HM, Kirch W. Local venous response to N-desethylamiodarone in humans. Clin Pharmacol Ther. 2000;67:22–31.
- Zimetbaum PJ. Dronedarone for atrial fibrillation—an odyssey. N Engl J Med. 2009;360:1811–3.
- Center for Drug Evaluation and Research. Briefing information for the March 18, 2009 meeting of the Cardiovascular and Renal Drugs Advisory Committee [Online]. February 19, 2009. Available from: URL: http://www.fda.gov/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/CardiovascularandRenalDrugsAdvisoryCommittee/ucm134972.htm. (Accessed date: August 2010).
- Center for Drug Evalution and Research. Cordarone (amiodarone) prescribing information [Online]. April, 21 2009. Available from: URL: http://www.accessdata.fda.gov/drugsatfda˙docs/label/2009/018972s038s039lbl.pdf. (Accessed date: August 2010).
- Touboul P, Brugada J, Capucci A, Crijns HJ, Edvardsson N, Hohnloser SH. Dronedarone for prevention of atrial fibrillation: a dose-ranging study. Eur Heart J. 2003;24:1481–7.
- Singh BN, Connolly SJ, Crijns HJ, Roy D, Kowey PR, Capucci A, . Dronedarone for maintenance of sinus rhythm in atrial fibrillation or flutter. N Engl J Med. 2007;357:987–99.
- Tschuppert Y, Buclin T, Rothuizen LE, Decosterd LA, Galleyrand J, Gaud C, . Effect of dronedarone on renal function in healthy subjects. Br J Clin Pharmacol. 2007;64:785–91.
- Davy JM, Herold M, Hoglund C, Timmermans A, Alings A, Radzik D, . Dronedarone for the control of ventricular rate in permanent atrial fibrillation: the Efficacy and safety of dRonedArone for the cOntrol of ventricular rate during atrial fibrillation (ERATO) study. Am Heart J. 2008;156:527,e1–9.
- Hohnloser SH, Connolly SJ, Van Eickels M, Radzik D, Davy JM, Singh BN. Effect of dronedarone on cardiovascular outcomes: a meta-analysis of five randomized controlled trials in 6157 patients with atrial fibrillation/flutter. J Am Coll Cardiol. 2009;53:A113.
- Hohnloser SH, Crijns HJ, van Eickels M, Gaudin C, Page RL, Torp-Pedersen C, . Effect of dronedarone on cardiovascular events in atrial fibrillation. N Engl J Med. 2009;360:668–78.
- Le Heuzey JY, De Ferrari GM, Radzik D, Santini M, Zhu J, Davy JM. A short-term, randomized, double-blind, parallel-group study to evaluate the efficacy and safety of dronedarone versus amiodarone in patients with persistent atrial fibrillation: The DIONYSOS Study. J Cardiovasc Electrophysiol. 2010;21:597–605.
- Kober L, Torp-Pedersen C, McMurray JJ, Gotzsche O, Levy S, Crijns H, . Increased mortality after dronedarone therapy for severe heart failure. N Engl J Med. 2008;358:2678–87.
- Connolly SJ, Crijns HJ, Torp-Pedersen C, van Eickels M, Gaudin C, Page RL, . Analysis of stroke in ATHENA: a placebo-controlled, double-blind, parallel-arm trial to assess the efficacy of dronedarone 400 mg BID for the prevention of cardiovascular hospitalization or death from any cause in patients with atrial fibrillation/atrial flutter. Circulation. 2009;120:1174–80.
- Lee KL, McNeer JF, Starmer CF, Harris PJ, Rosati RA. Clinical judgment and statistics. Lessons from a simulated randomized trial in coronary artery disease. Circulation. 1980;61:508–15.
- Arya A, Silberbauer J, Teichman SL, Milner P, Sulke N, Camm AJ. A preliminary assessment of the effects of ATI-2042 in subjects with paroxysmal atrial fibrillation using implanted pacemaker methodology. Europace. 2009;11:458–64.
- Ezekowitz MD, Hohnloser SH, Lubinski A, Bandman O, Canafax D, Ellis DJ, . PASCAL: a randomized doubleblind,placebo-controlled study of budiodarone (ATI-2042) in patients with paroxysmal atrial fibrillation and pacemakers with atrial fibrillation data logging capabilities [abstract]. Heart Rhythm. 2009;6:Supplement.
- Gautier P, Serre M, Cosnier-Pucheu S, Djandjighian L, Roccon A, Herbert JM, . In vivo and in vitro antiarrhythmic effects of SSR149744C in animal models of atrial fibrillation and ventricular arrhythmias. J Cardiovasc Pharmacol. 2005;45:125–35.
- Kowey PR, Aliot E, Cappucci A, Connolly SJ, Crijns H, Hohnloser SH, . Placebo-controlled,double-blind dose-ranging study of the efficacy and safety of SSR149744C in patients with recent atrial fibrillation/flutter [abstract]. Heart Rhythm. 2007;5:S72.
- Kowey PR, Aliot E, Cappucci A, Connolly SJ, Hohnloser SH, Kulakowski P, . Placebo-controlled double-blind dose ranging study of the efficacy and safety of celivarone for the prevention of ventricular arrhythmia-triggered ICD interventions [abstract]. J Am Coll Cardiol. 2008;51:A2.
- Dobrev D, Graf E, Wettwer E, Himmel HM, Hala O, Doerfel C, . Molecular basis of downregulation of G-protein-coupled inward rectifying K(+) current (I(K,ACh) in chronic human atrial fibrillation: decrease in GIRK4 mRNA correlates with reduced I(K,ACh) and muscarinic receptor-mediated shortening of action potentials. Circulation. 2001;104:2551–7.
- Voigt N, Rozmaritsa N, Trausch A, Zimniak T, Christ T, Wettwer E, . Inhibition of IK, ACh current may contribute to clinical efficacy of class I and class III antiarrhythmic drugs in patients with atrial fibrillation. Naunyn Schmiedebergs Arch Pharmacol. 381:251–9.
- Antzelevitch C, Burashnikov A. Atrial-selective sodium channel block as a novel strategy for the management of atrial fibrillation. J Electrocardiol. 2009;42:543–8.
- Das S, Makino S, Melman YF, Shea MA, Goyal SB, Rosenzweig A, . Mutation in the S3 segment of KCNQ1 results in familial lone atrial fibrillation. Heart Rhythm. 2009;6:1146–53.
- Harleton E, Besana A, Argenziano M, Robinson RB, Feinmark SJ. Inhibitory phosphorylation of TASK-1 is associated with atrial fibrillation. FASEB J. 2008;22: 721.3.
- Nattel S, De Blasio E, Wang WQ, Beatch GN. RSD1235: A novel antiarrhythmic agent with a unique electrophysiological profile that terminates AF in dogs. Eur Heart J. 2001;22:448.
- Comtois P, Sakabe M, Vigmond EJ, Munoz M, Texier A, Shiroshita-Takeshita A, . Mechanisms of atrial fibrillation termination by rapidly unbinding Na+ channel blockers: insights from mathematical models and experimental correlates. Am J Physiol Heart Circ Physiol. 2008;295: H1489–504.
- Dorian P, Pinter A, Mangat I, Korley V, Cvitkovic SS, Beatch GN. The effect of vernakalant (RSD1235), an investigational antiarrhythmic agent, on atrial electrophysiology in humans. J Cardiovasc Pharmacol. 2007;50:35–40.
- Kowey PR, Dorian P, Mitchell LB, Pratt CM, Roy D, Schwartz PJ, . Vernakalant hydrochloride for the rapid conversion of atrial fibrillation after cardiac surgery: a randomized, double-blind, placebo-controlled trial. Circ Arrhythm Electrophysiol. 2009;2:652–9.
- Mao ZL, Wheeler JJ, Clohs L, Beatch GN, Keirns J. Pharmacokinetics of novel atrial-selective antiarrhythmic agent vernakalant hydrochloride injection (RSD1235): influence of CYP2D6 expression and other factors. J Clin Pharmacol. 2009;49:17–29.
- Naccarelli GV, Wolbrette DL, Samii S, Banchs JE, Penny-Peterson E, Stevenson R, . Vernakalant—a promising therapy for conversion of recent-onset atrial fibrillation. Expert Opin Investig Drugs. 2008;17:805–10.
- Roy D, Pratt CM, Torp-Pedersen C, Wyse DG, Toft E, Juul-Moller S, . Vernakalant hydrochloride for rapid conversion of atrial fibrillation: a phase 3, randomized, placebo-controlled trial. Circulation. 2008;117:1518–25.
- Roy D, Rowe BH, Stiell IG, Coutu B, Ip JH, Phaneuf D, . A randomized, controlled trial of RSD1235, a novel anti-arrhythmic agent, in the treatment of recent onset atrial fibrillation. J Am Coll Cardiol. 2004;44:2355–61.
- Camm AJ. Clinical efficacy and safety of vernakalant compared with amiodarone for intravenous conversion of recent onset atrial fibrillation. Late Break Clinical Trials Session II. Presented at Heart Rhythm Society 2010 Scientific Sessions; 14 May 2010; Denver, CO, USA.
- Kowey PR, Roy D, Pratt CM, Schwartz PJ, Dorian P, Mitchell JB, . Efficacy and safety of vernakalant hydrochloride injection for the treatment of atrial fibrillation after valvular or coronary artery bypass surgery [abstract]. Circulation. 2007;114:636–37.
- Pratt CM, Roy D, Juul-Moller S, Torp-Pedersen C, Toft E, Nielsen T. Efficacy and tolerance of RSD1235 in the treatment of atrial fibrillation or atrial flutter: results of a phase III, randomized, placebo-controlled, multicenter trial [abstract]. J Am Coll Cardiol. 2006;47:10A.
- Wettwer E, Bormann S, Christ T, Dobrev D, Pourrier M, Gibson JK, . Effects of the novel antiarrhythmic agent vernakalant (RSD1235) on human atrial action potentials in chronic atrial fibrillation. Eur Heart J. 2007;28:400.
- Cheng JW. Vernakalant in the management of atrial fibrillation. Ann Pharmacother. 2008;42:533–42.
- Cardiome Pharma Corp. Available from: URL: http://www.cardiome.com/VernakalantIntravenous.php. (Accessed date: August 2010).
- Keating GM. Ranolazine: a review of its use in chronic stable angina pectoris. Drugs. 2008;68:2483–503.
- Zaza A, Belardinelli L, Shryock JC. Pathophysiology and pharmacology of the cardiac ‘late sodium current’. Pharmacol Ther. 2008;119:326–39.
- Morrow DA, Scirica BM, Karwatowska-Prokopczuk E, Murphy SA, Budaj A, Varshavsky S, . Effects of ranolazine on recurrent cardiovascular events in patients with non-ST-elevation acute coronary syndromes: the MERLIN-TIMI 36 randomized trial. JAMA. 2007;297: 1775–83.
- Antzelevitch C, Belardinelli L, Zygmunt AC, Burashnikov A, Di Diego JM, Fish JM, . Electrophysiological effects of ranolazine, a novel antianginal agent with antiarrhythmic properties. Circulation. 2004;110:904–10.
- Sossalla S, Kallmeyer B, Wagner S, Mazur M, Maurer U, Toischer K, . Altered Na(+) currents in atrial fibrillation effects of ranolazine on arrhythmias and contractility in human atrial myocardium. J Am Coll Cardiol. 2010;55: 2330–42.
- Scirica BM, Morrow DA, Hod H, Murphy SA, Belardinelli L, Hedgepeth CM, . Effect of ranolazine, an antianginal agent with novel electrophysiological properties, on the incidence of arrhythmias in patients with non ST-segment elevation acute coronary syndrome: results from the Metabolic Efficiency With Ranolazine for Less Ischemia in Non ST-Elevation Acute Coronary Syndrome Thrombolysis in Myocardial Infarction 36 (MERLIN-TIMI 36) randomized controlled trial. Circulation. 2007; 116:1647–52.
- Sicouri S, Glass A, Belardinelli L, Antzelevitch C. Antiarrhythmic effects of ranolazine in canine pulmonary vein sleeve preparations. Heart Rhythm. 2008;5:1019–26.
- Murdock DK, Overton N, Kersten M, Kaliebe J, Devecchi F. The effect of ranolazine on maintaining sinus rhythm in patients with resistant atrial fibrillation. Indian Pacing Electrophysiol J. 2008;8:175–81.
- Rivard L, Shiroshita-Takeshita A, Maltais C, Ford J, Pinnock R, Madge D, . Electrophysiological and atrial antiarrhythmic effects of a novel IKur/Kv1.5 blocker in dogs. Heart Rhythm. 2005;2:S180.
- Milnes JT, Louis L, Rogers M, Madge D, Ford J. The atrial antiarrhythmic drug XEN-D0101 selectively inhibits the human ultra-rapid delayed-rectifier potassium current (IKur) over other cardiac ion channels. Circulation. 2008;118:S342.
- Duker G, Björkman JA, Linhardt G, Carlsson L. AZD—7009—a new atrial antifibrillatory compound that markedly delays atrial refractoriness in the anaesthetised dog. Heart Rhythm. 2004;1:S159.
- Goldstein RN, Khrestian C, Carlsson L, Waldo AL. Azd7009: a new antiarrhythmic drug with predominant effects on the atria effectively terminates and prevents reinduction of atrial fibrillation and flutter in the sterile pericarditis model. J Cardiovasc Electrophysiol. 2004;15: 1444–50.
- Persson F, Carlsson L, Duker G, Jacobson I. Blocking characteristics of hERG, hNav1.5, and hKvLQT1/hminK after administration of the novel anti-arrhythmic compound AZD7009. J Cardiovasc Electrophysiol. 2005;16:329–41.
- Astra Zeneca Clinical Trials. Available from: URL: http://www.astrazenecaclinicaltrials.com/other-drug-products/discontinued-products/AZD7009/. (Accessed date: August 2010).
- Gogelein H, Brendel J, Steinmeyer K, Strubing C, Picard N, Rampe D, . Effects of the atrial antiarrhythmic drug AVE0118 on cardiac ion channels. NaunynSchmiedebergs Arch Pharmacol. 2004;370:183–92.
- Wirth KJ, Paehler T, Rosenstein B, Knobloch K, Maier T, Frenzel J, . Atrial effects of the novel K(+)-channel-blocker AVE0118 in anesthetized pigs. Cardiovasc Res. 2003;60:298–306.
- Blaauw Y, Gogelein H, Tieleman RG, van Hunnik A, Schotten U, Allessie MA. ‘Early’ class III drugs for the treatment of atrial fibrillation: efficacy and atrial selectivity of AVE0118 in remodeled atria of the goat. Circulation. 2004;110:1717–24.
- Wettwer E, Hala O, Christ T, Heubach JF, Dobrev D, Knaut M, . Role of IKur in controlling action potential shape and contractility in the human atrium: influence of chronic atrial fibrillation. Circulation. 2004; 110:2299–306.
- Sanofi-Aventis Website. Sanofi-Aventis R&D Portfolio. Sept 2007. Available from: URL: http://en.sanofi-aventis.com/rd/portfolio/p_rd_portfolio_ cardio.asp. (Accessed date: August 2010).
- Wirth KJ, Brendel J, Steinmeyer K, Linz DK, Rutten H, Gogelein H. In vitro and in vivo effects of the atrial selective antiarrhythmic compound AVE1231. J Cardiovasc Pharmacol. 2007;49:197–206.
- Matsuda T, Takeda K, Ito M, Yamagishi R, Tamura M, Nakamura H, . Atria selective prolongation by NIP-142, an antiarrhythmic agent, of refractory period and action potential duration in guinea pig myocardium. J Pharmacol Sci. 2005;98:33–40.
- Hashimoto N, Yamashita T, Fujikura N, Tsuruzoe N. NIP-141, a multiple ion channel blocker, terminates aconitine-induced atrial fibrillation and prevents the rapid pacing-induced atrial effective refractory period shortening in dogs. Europace. 2007;9:246–51.
- Ehrlich JR. Inward rectifier potassium currents as a target for atrial fibrillation therapy. J Cardiovasc Pharmacol. 2008;52:129–35.
- Fredholm BB, Abbracchio MP, Burnstock G, Dubyak GR, Harden TK, Jacobson KA, . Towards a revised nomenclature for P1 and P2 receptors. Trends Pharmacol Sci. 1997;18:79–82.
- Gao ZG, Jacobson KA. Emerging adenosine receptor agonists. Expert Opin Emerg Drugs. 2007;12:479–92.
- Blomstrom-Lundqvist C, Scheinman MM, Aliot EM, Alpert JS, Calkins H, Camm AJ, . ACC/AHA/ESC guidelines for the management of patients with supraventricular arrhythmias—executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Supraventricular Arrhythmias). Circulation. 2003;108:1871–909.
- Fuster V, Ryden LE, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, . ACC/AHA/ESC 2006 Guidelines for the Management of Patients with Atrial Fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation): developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation. 2006;114:e257–354.
- Snowdy S, Liang HX, Blackburn B, Lum R, Nelson M, Wang L, . A comparison of an A1 adenosine receptor agonist (CVT-510) with diltiazem for slowing of AV nodal conduction in guinea-pig. Br J Pharmacol. 1999;126: 137–46.
- Tortella FC, Martin DA, Allot CP, Steel JA, Blackburn TP, Loveday BE, . Dextromethorphan attenuates post-ischemic hypoperfusion following incomplete global ischemia in the anesthetized rat. Brain Res. 1989;482: 179–83.
- Lerman BB, Ellenbogen KA, Kadish A, Platia E, Stein KM, Markowitz SM, . Electrophysiologic effects of a novel selective adenosine A1 agonist (CVT-510) on atrioventricular nodal conduction in humans. J Cardiovasc Pharmacol Ther. 2001;6:237–45.
- Safety Study of Tecadenoson to Treat Atrial Fibrillation. Available from: URL: http://www.clinicaltrials.gov/ct2/show/NCT00713401?term=tecadenoson&rank=1. (Accessed date: August 2010).
- Nattel S, Burstein B, Dobrev D. Atrial remodeling and atrial fibrillation: mechanisms and implications. Circ Arrhythm Electrophysiol. 2008;1:62–73.
- Verma A, Wazni OM, Marrouche NF, Martin DO, Kilicaslan F, Minor S, . Pre-existent left atrial scarring in patients undergoing pulmonary vein antrum isolation: an independent predictor of procedural failure. J Am Coll Cardiol. 2005;45:285–92.
- Wijffels MC, Kirchhof CJ, Dorland R, Allessie MA. Atrial fibrillation begets atrial fibrillation. A study in awake chronically instrumented goats. Circulation. 1995;92: 1954–68.
- Disertori M, Latini R, Barlera S, Franzosi MG, Staszewsky L, Maggioni AP, . Valsartan for prevention of recurrent atrial fibrillation. N Engl J Med. 2009;360:1606–17.
- Shroff SC, Ryu K, Martovitz NL, Hoit BD, Stambler BS. Selective aldosterone blockade suppresses atrial tachyarrhythmias in heart failure. J Cardiovasc Electrophysiol. 2006;17:534–41.
- Halonen J, Halonen P, Jarvinen O, Taskinen P, Auvinen T, Tarkka M, . Corticosteroids for the prevention of atrial fibrillation after cardiac surgery: a randomized controlled trial. JAMA. 2007;297:1562–7.
- Pellman J, Lyon RC, Sheikh F. Extracellular matrix remodeling in atrial fibrosis: mechanisms and implications in atrial fibrillation. J Mol Cell Cardiol. 2010;48:461–7.
- Griendling KK, Minieri CA, Ollerenshaw JD, Alexander RW. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ Res. 1994;74:1141–8.
- Goette A, Bukowska A, Dobrev D, Pfeiffenberger J, Morawietz H, Strugala D, . Acute atrial tachyarrhythmia induces angiotensin II type 1 receptor-mediated oxidative stress and microvascular flow abnormalities in the ventricles. Eur Heart J. 2009;30:1411–20.
- Ehrlich JR, Hohnloser SH, Nattel S. Role of angiotensin system and effects of its inhibition in atrial fibrillation: clinical and experimental evidence. Eur Heart J. 2006; 27:512–8.
- Sun Y, Ramires FJ, Weber KT. Fibrosis of atria and great vessels in response to angiotensin II or aldosterone infusion. Cardiovasc Res. 1997;35:138–47.
- Burstein B, Nattel S. Atrial structural remodeling as an antiarrhythmic target. J CardiovascPharmacol. 2008; 52:4–10.
- L'Allier PL, Ducharme A, Keller PF, Yu H, Guertin MC, Tardif JC. Angiotensin-converting enzyme inhibition in hypertensive patients is associated with a reduction in the occurrence of atrial fibrillation. J Am Coll Cardiol. 2004; 44:159–64.
- Madrid AH, Bueno MG, Rebollo JM, Marin I, Pena G, Bernal E, . Use of irbesartan to maintain sinus rhythm in patients with long-lasting persistent atrial fibrillation: a prospective and randomized study. Circulation. 2002; 106:331–6.
- Maggioni AP, Latini R, Carson PE, Singh SN, Barlera S, Glazer R, . Valsartan reduces the incidence of atrial fibrillation in patients with heart failure: results from the Valsartan Heart Failure Trial (Val-HeFT). Am Heart J. 2005;149:548–57.
- Wachtell K, Lehto M, Gerdts E, Olsen MH, Hornestam B, Dahlof B, . Angiotensin II receptor blockade reduces new-onset atrial fibrillation and subsequent stroke compared to atenolol: the Losartan Intervention For End Point Reduction in Hypertension (LIFE) study. J Am Coll Cardiol. 2005;45:712–9.
- Camm AJ, Kirchhof P, Lip GY, Schotten U, Savelieva I, Ernst S, . Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Europace. 2010;12:1360–420.
- Patel D, Mohanty P, Di Biase L, Wang Y, Shaheen MH, Sanchez JE, . The impact of statins and renin-angiotensin-aldosterone system blockers on pulmonary vein antrum isolation outcomes in post-menopausal females. Europace. 2010;12:322–30.
- Verheule S, Sato T, Everett T 4th, Engle SK, Otten D, Rubart-von der Lohe M, . Increased vulnerability to atrial fibrillation in transgenic mice with selective atrial fibrosis caused by overexpression of TGF-beta1. Circ Res. 2004;94:1458–65.
- Burstein B, Nattel S. Atrial fibrosis: mechanisms and clinical relevance in atrial fibrillation. J Am Coll Cardiol. 2008;51:802–9.
- Van Wagoner DR. Oxidative stress and inflammation in atrial fibrillation: role in pathogenesis and potential as a therapeutic target. J Cardiovasc Pharmacol. 2008;52:306–13.
- Fauchier L, Pierre B, de Labriolle A, Grimard C, Zannad N, Babuty D. Antiarrhythmic effect of statin therapy and atrial fibrillation a meta-analysis of randomized controlled trials. J Am Coll Cardiol. 2008;51:828–35.
- Shiroshita-Takeshita A, Brundel BJ, Lavoie J, Nattel S. Prednisone prevents atrial fibrillation promotion by atrial tachycardia remodeling in dogs. Cardiovasc Res. 2006;69: 865–75.
- Shiroshita-Takeshita A, Brundel BJ, Burstein B, Leung TK, Mitamura H, Ogawa S, . Effects of simvastatin on the development of the atrial fibrillation substrate in dogs with congestive heart failure. Cardiovasc Res. 2007; 74:75–84.
- Shiroshita-Takeshita A, Schram G, Lavoie J, Nattel S. Effect of simvastatin and antioxidant vitamins on atrial fibrillation promotion by atrial-tachycardia remodeling in dogs. Circulation. 2004;110:2313–9.
- Heidt MC, Vician M, Stracke SK, Stadlbauer T, Grebe MT, Boening A, . Beneficial effects of intravenously administered N-3 fatty acids for the prevention of atrial fibrillation after coronary artery bypass surgery: a prospective randomized study. Thorac Cardiovasc Surg. 2009;57: 276–80.
- Saravanan P, Bridgewater B, West AL, O'Neill SC, Calder PC, Davidson NC. Omega-3 fatty acid supplementation does not reduce risk of atrial fibrillation after coronary artery bypass surgery: a randomized, double-blind, placebo-controlled clinical trial. Circ Arrhythm Electro physiol. 2010;3:46–53.
- Patel D, Shaheen M, Venkatraman P, Armaganijan L, Sanchez JE, Horton RP, . Omega-3 polyunsaturated fatty acid supplementation reduced atrial fibrillation recurrence after pulmonary vein antrum isolation. Indian Pacing Electrophysiol J. 2009;9:292–8.
- Pratt CM, Reiffel JA, Ellenbogen KA, Naccarelli GV, Kowey PR. Efficacy and safety of prescription omega-3-acid ethyl esters for the prevention of recurrent symptomatic atrial fibrillation: a prospective study. Am Heart J. 2009;158:163–9;e1–3.
- Macchia A, Varini S, Grancelli H, Nul D, Laffaye N, Ferrante D, . The rationale and design of the FORomegaARD Trial: A randomized, double-blind, placebo-controlled, independent study to test the efficacy of n-3 PUFA for the maintenance of normal sinus rhythm in patients with previous atrial fibrillation. Am Heart J. 2009;157:423–7.