Abstract
Background. The safety of drug-eluting stents (DES) in patients on long-term warfarin treatment has been questioned due to high risk of bleeding complications during prolonged triple (aspirin, clopidogrel, and warfarin) antithrombotic therapy. Methods. We analysed the long-term outcome of 415 consecutive warfarin-treated patients who underwent DES (n = 191) or bare-metal (n = 224) stenting in six hospitals. Results. The mean duration of triple therapy was longer (4.2 ± 3.1 versus 2.1 ± 1.8 months; P < 0.001) in the DES group. The incidence of major adverse cardiovascular and cerebrovascular events was comparable in the DES and bare-metal groups (39.8% versus 42.4%; P = 0.59) during a median follow-up of 3.5 years. Similarly, major bleeding events occurred equally often in both study groups (14.7% versus 12.9%). Six patients in the DES group and seven patients in the bare-metal group suffered stent thrombosis (3.1% versus 3.1%). In the propensity score analyses of 101 matched pairs, the outcome was similar in the two groups. Conclusion. Selective use of DES with a short triple therapy seems to be safe in patients with warfarin therapy. The prognosis of this fragile patient population is quite poor, and major bleeding events are common irrespective of stent type.
Key words::
| Abbreviations | ||
| BMS | = | bare-metal stent |
| DES | = | drug-eluting stent |
| INR | = | international normalized ratio |
| MACCE | = | major adverse cardiovascular and cerebrovascular event |
| OAC | = | oral anticoagulation |
| OBRI | = | out-patient bleeding risk index |
| PCI | = | percutaneous coronary intervention |
| TIA | = | transient ischaemic attack |
Key messages
Selective use of drug-eluting stents with a short triple therapy seems to be safe in warfarin-treated patients, since late stent thromboses were rare even without long clopidogrel treatment.
Major bleeding and cardiac events are common in this fragile patient group irrespective of stent type.
Introduction
It is estimated that 5% of patients undergoing percutaneous coronary intervention (PCI) are on long-term oral anticoagulation (OAC) therapy because of underlying chronic medical conditions such as atrial fibrillation or mechanical heart valve (Citation1). PCI with drug-eluting stents (DES) has offered an effective way to reduce in-stent restenosis as compared with bare-metal stents (BMS) (Citation2,Citation3). The risk of late stent thrombosis with these devices has led to the recommendation of prolonged dual antiplatelet therapy, which in turn increases the inherent risk of bleeding, particularly in this fragile patient group (Citation4,Citation5). Premature interruption of antiplatelet therapy could induce a higher rate of DES thrombosis, and even a temporary discontinuation of OAC is associated with an increased risk of thromboembolism (Citation6–8). Current guidelines recommend that the use of DES in patients having OAC should be avoided or strictly limited to those situations where a significant benefit is expected as compared to BMS (Citation6–8). Triple therapy is recommended for the prevention of stent thrombosis, but its duration should be individualized according to the stent type and bleeding risk of the patient (Citation9,Citation10). Since there are no randomized trials assessing the best stenting strategy in patients with long-term OAC, and even the observational studies are scarce, we aimed to determine the long-term safety and efficacy of DES in patients on long-term OAC in everyday clinical practice.
Material and methods
Study design
This observational study is part of a wider protocol in progress to assess thrombotic and bleeding complications of cardiac procedures in western Finland (Citation6,Citation11–16). This substudy is based on 415 consecutive patients on chronic warfarin treatment who underwent coronary stenting in six Finnish hospitals between the years 2002 and 2006. Base-line and in-hospital data were provided by local institutional clinical registries which prospectively collect information in computerized databases. Furthermore, a non-blinded review of full medical records of the eligible patients was performed to determine the perioperative antithrombotic strategies and the incidence of in-hospital complications (Citation11).
All patients were treated according to varying local practices and followed by office visits or telephone interviews by the treating physicians with the last contact between September 2008 and February 2009. The major bleeding episodes and major adverse cardiovascular and cerebrovascular events (MACCE) were recorded. In addition, all data available from the hospital records, the institutional electronic clinical database, and the referring physicians were checked at the end of the follow-up period to record the medication at the time of MACCE and major bleeding. Hospital records and death certificates from the Central Statistical Office of Finland were used to record and classify deaths.
The CHADS2 score, which quantifies the annual stroke risk for patients who have non-valvular atrial fibrillation, was recorded for all patients (Citation17). Bleeding risk was evaluated retrospectively by the out-patient bleeding risk index (OBRI), which considers history of stroke, age, history of gastrointestinal bleeding, and the presence of one or more co-morbid conditions in order to stratify patients into three risk groups (Citation16,Citation18). The target international normalized ratio (INR) level in patients with atrial fibrillation was 2–3. This study complies with the Declaration of Helsinki. The study protocol was approved by the Ethics Committees of Satakunta Central Hospital and the participating hospitals.
End-point definitions
Major bleeding was defined as a decrease in the blood haemoglobin level of more than 4.0 g/dL, the need for the transfusion of two or more units of blood, the need for corrective surgery, the occurrence of an intracranial or retroperitoneal haemorrhage, or any combination of these (Citation19). Access site complications that fulfilled the criteria of major bleeding were included in the total number of major bleedings.
MACCE was defined as the occurrence of any of the following during the follow-up: all-cause death, Q-wave or non-Q-wave myocardial infarction, target vessel revascularization, stent thrombosis, or stroke. Myocardial infarction was diagnosed when a rise in the myocardial injury marker level was detected together with symptoms suggestive of acute infarction. For the diagnosis of myocardial reinfarction, a new rise > 50% above the base-line injury marker level was required (Citation11). Target vessel revascularization was defined as any reintervention driven by any lesion located in the stented vessel. Stent thromboses were classified according to the consensus of the Academic Research Consortium criteria as definite, probable, or possible (Citation20). Stroke was defined as an ischaemic cerebral infarction caused by a thrombotic occlusion of a major intracranial artery or intracerebral haemorrhage.
Statistical analysis
The normally distributed continuous variables are shown as mean ± standard deviation and were compared by unpaired t test; other continuous variables are shown as median and interquartile range and compared by Mann–Whitney U test. Dichotomous variables and outcome end-points are presented as counts and percentages and compared by chi-square test and Fisher's exact test. Two-sided P value of < 0.05 was considered statistically significant. Survival analyses were performed by Kaplan–Meier test, and differences between groups were assessed using the log-rank test.
In propensity score analyses, logistic regression with backward selection was performed to calculate the risk of these patients to be ‘assigned’ to the BMS or DES group. Variables listed in with P < 0.2 have been included into the regression model. Receiver operating characteristic (ROC) curve analysis was used to estimate the area under the curve of the model predicting the probability of being included in the DES or the BMS group. The calculated propensity score was employed for one-to-one matching as well as to adjust for other variables in estimating their impact on the postoperative outcome. Matching between study groups was done according to a difference in the propensity score < 0.005. Cox regression analysis with backward selection was used to adjust the effect of clinical variables as well as propensity score on outcome end-points. All data were analysed with the use of statistical software (SPSS v. 15.0.1, SPSS Inc., Chicago, IL, USA).
Table I. Base-line clinical and procedural characteristics of the DES and BMS study groups and their propensity score matched pairs.
Results
Base-line and procedural variables
Between December 2002 and June 2006, 191 patients were treated with DES (112 with paclitaxel-eluting stents), and 224 patients were treated with BMS. The base-line clinical and procedural characteristics of the stent groups are described in . The DES patients were younger than BMS patients, and they were less often treated for acute coronary syndromes, but the groups did not differ with respect to bleeding or thromboembolic risks (CHADS2 and OBRI scores). Atrial fibrillation was the most frequent indication for warfarin treatment (138 patients in the DES group and 159 in the BMS group) followed by transient ischaemic attacks/strokes (23 versus 27, respectively), pulmonary embolism/deep vein thrombosis (12 versus 21, respectively), mechanical heart valves (12 versus 9, respectively). There were no significant differences in lesion type, number of lesions, or other main procedural characteristics features between the study groups (). The mean hospitalization time was 2.8 days in the DES group and 3.4 days in the BMS group.
Antithrombotic therapy
There were no significant differences in the periprocedural antithrombotic treatments (low-molecular-weight heparins, glycoprotein blockers, unfractionated heparin, or level of oral anticoagulation) between the groups. The antithrombotic medication at hospital discharge is described in . At discharge, 62% of patients in the DES group and 58% in the BMS group received triple therapy, and 31% in the DES group and 30% in the BMS received dual therapy where clopidogrel was combined with aspirin or warfarin. The mean duration of triple therapy was longer in the DES group.
Table II. Antithrombotic medication at discharge and duration of major treatment options.
Clinical outcomes
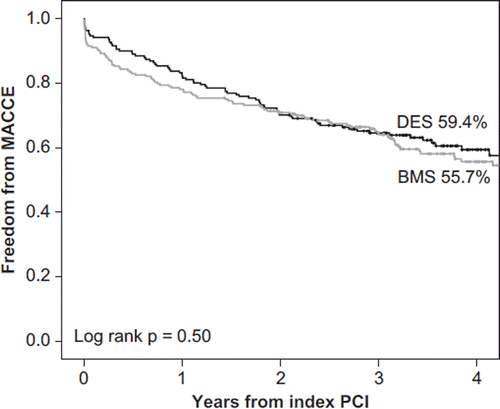
Complete follow-up was achieved in all patients (DES group: median 3.5 years; BMS group: median 3.4 years). The incidence of MACCE was comparable in the DES and the BMS groups (39.8% versus 42.4%; P = 0.59) (, ). The mean INR at the time of MACCE was similar in the DES and BMS groups (2.5 versus 2.4; P = 0.50, respectively). A total of six patients (3.1%) in the DES group and seven patients (3.1%) in the BMS group suffered stent thrombosis (). One acute and one subacute stent thrombosis were probable, but all others fulfilled the criteria of definite stent thrombosis. Only one acute and one subacute stent thrombosis occurred in stable patients. The ten cases of acute and subacute stent thromboses occurred within 17 days after stenting, and six of these patients were not receiving clopidogrel. The risk of acute or subacute (< 30 days) stent thrombosis was 15% (6/41) in patients without clopidogrel treatment and 1% (4/374) in those with clopidogrel included in the therapy (P < 0.0001 for difference). One late thrombosis of paclitaxel-eluting stent occurred at day 105 during dual therapy with clopidogrel and warfarin. Two patients with paclitaxel-eluting stents suffered very late stent thrombosis 271 days and 1,066 days after the discontinuation of clopidogrel therapy.
Table III. Outcome events in the overall series and in propensity-matched pairs according to the stent type.
Table IV. Antithrombotic therapy during stent thrombosis.
Figure 1. Kaplan–Meier estimates of freedom from MACCE according to the stent type in patients on OAC.
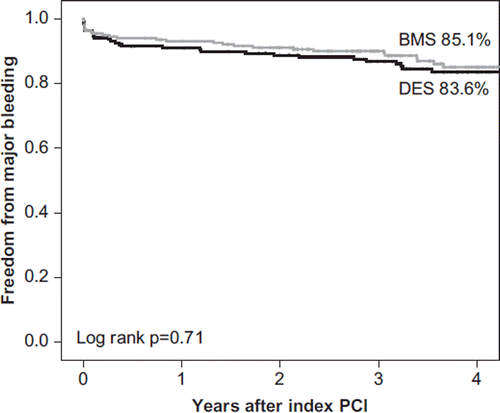
The number of patients with major bleeding episodes was comparable in DES and BMS groups (14.7% versus 12.9%; P = 0.61) (, ). Four DES and three BMS patients suffered fatal bleeding, and all these events were intracranial bleedings. Three patients suffered several (up to six) major bleeding episodes during the follow-up. A total of 14 (24.6%) major bleeding episodes occurred during index hospitalization, and 12 of them for patients with femoral access site.
Propensity score analysis
Logistic regression identified the number of stents deployed, length of stents, diameter of the stent, family history of coronary artery disease, diabetes, any previous myocardial infarction, non-ST-elevation myocardial infarction as well as ST-elevation myocardial infarction as independent ‘risk factors’ of these patients for having been included in the BMS or DES groups (Hosmer–Lemeshow's test, P = 0.32). ROC analysis showed an area under the curve of the calculated propensity score of 0.77 (95% CI 0.72–0.82; P < 0.0001), and 101 propensity score matched pairs could be obtained (). The type of stent had no significant effect in any of the outcome measures in these propensity score matched pairs when adjusted for propensity score ().
Discussion
This is the largest and most comprehensive evaluation of the long-term outcome in warfarin-treated patients after coronary stenting. The main finding of our study was that selective use of DES in this population was not associated with excess of major bleeding or thrombotic complications as compared to BMS. Our findings also suggest that late (> 3 months) stent thromboses are rare even without long clopidogrel treatment, and so a short triple therapy seems to be sufficient to prevent stent thrombosis and might help to reduce the incidence of bleeding complications in this fragile patient group.
There are 16 earlier studies describing outcomes in OAC patients undergoing coronary stenting and providing information on the stent use (Citation10). Most of these are small retrospective single-centre series, and no randomized trials are available. The use of DES ranged in these reports from 2% to 100%, and only two of these trials presented data on comparison of DES versus BMS. Rogacka et al. (Citation21) showed no significant difference between DES and BMS with respect to major bleeding and thrombotic complications, whereas Ruiz-Nodar et al. (Citation22) concluded that the routine use of DES in this patient population does not seem to be justified on the basis of adverse outcome in the DES-treated patients.
Bleeding complications are the most frequent non-ischaemic complications of PCI, especially in the treatment of acute coronary syndromes. Most of the bleeding events occur early during the hospital phase, and, e.g. in the large CRUSADE registry, the incidence of in-hospital major bleeding events was as high as 9.4% (Citation23). The incidence of bleeding events seems to be even higher when these patients are on long-term OAC and need concomitant potent antiplatelet agents due to PCI (Citation7). Triple therapy is the most frequently recommended drug regimen in this scenario. The downside of the combination is the high incidence of bleeding complications, since the incidence of major bleedings increases to a range of 3.2–6.6-fold compared with dual antiplatelet therapy (Citation10). Duration of triple therapy seems to be critical, since in a pooled analysis the rate of major bleeding increased from 4.6% to 10.3% when the treatment period increased from 1 month to 6–12 months or more (Citation7).
Our results confirm earlier studies showing a high rate of major bleeding complications after PCI in patients with long-term OAC (Citation6,Citation11,Citation22,Citation24). The use of DES did not, however, increase the rate of major bleeding episodes relative to BMS use. This was not unexpected, since only 62% of patients in the DES group and 58% of patients in the BMS group received triple therapy at discharge and the duration of triple therapy was shorter than currently recommended reflecting the contemporary treatment policies. Our findings diverge from a study by Ruiz-Nodar et al. (Citation22) where the use of DES was associated with a higher rate of major bleedings with no difference in thrombotic complications in patients with atrial fibrillation. Differences in base-line characteristics (e.g. only half of the patients were on warfarin before stenting) and longer duration of triple therapy (exact data not available) in the DES-treated patients may contribute to this difference, since also Sarafoff et al. (Citation25) recently showed that the use of DES was safe in patients receiving either 12 weeks of triple therapy or dual antiplatelet therapy. In that trial, the choice of triple therapy with a low target INR of 2.0–2.5 was made on the basis of an individual assessment of thromboembolic risk.
The use of DES has significantly reduced the problem of restenosis inherent to BMS. In the present study, DES patients received significantly longer and smaller stents than BMS patients, apparently reflecting the higher risk of restenosis in the DES-treated lesions. The actual rate of clinical restenosis was not increased in the DES group, suggesting that the stenting policy was effective in the prevention of restenosis.
Longer delay in arterial healing may extend the risk of stent thrombosis far beyond 30 days after the DES implantation. Consequently, there has been concern for late stent thrombosis after DES implantation, and early discontinuation of antiplatelet therapy has been the most important predisposing factor for stent thrombosis. Accordingly, recent recommendations have suggested that, for patients with a strong indication for concomitant OAC, triple therapy should be considered for 1 year following DES implantation, especially after acute coronary syndromes (Citation7,Citation9,Citation26,Citation27). The risk of stent thrombosis after stopping short triple therapy was, however, low in the present study, and the two cases of stent thrombosis occurred so late that the recommended 12 months' treatment would not have prevented these very late events. Accordingly, our present findings suggest that a shorter triple therapy may be considered also after DES implantation, especially when the clinical risks and consequences of stent thrombosis are considered reasonable or the individual bleeding risk is high (Citation10,Citation16). On the other hand, our results confirm that clopidogrel treatment is highly beneficial in the early phase after stenting, since a 15-fold increase in early stent thrombosis rate was observed in the patients left without clopidogrel.
Our study is limited by typical retrospective study design including individual risk-based decision-making in the treatment choices. We attempted to overcome this bias using propensity score analysis. The outcome assessment was not blinded, and the small sample size might cause some bias in records and limits subgroup analyses. Data on medication in clinical databases were not complete in all patients during the follow-up, especially when a patient underwent repetitive medication changes. Information on minor bleeding complications was incomplete and could not be reported, which might under-estimate the bleeding complications observed in clinical practice.
In the light of current findings, selective use of DES in patients on long-term OAC leads to an acceptable outcome. Our findings also suggest that a shorter duration of triple therapy may be an option in this patient group, but randomized data on the optimal duration of triple therapy are needed. The selection of stent should, however, be based on individual evaluation, balancing of bleeding and thromboembolic risks of the patient, and thrombotic and restenotic risks of the treated coronary lesion.
Declaration of interest: The authors have no conflict of interest to declare. This study was supported by grants from the Finnish Foundation for Cardiovascular Research, Helsinki, Finland and South-Western Finland Hospital District Research Fund (Turku, Finland).
References
- Helft G, Gilard M, Le Feuvre C, Zaman AG. Drug insight: antithrombotic therapy after percutaneous coronary intervention in patients with an indication for anticoagulation. Nat Clin Pract Cardiovasc Med. 2006;3:673–80.
- Babapulle MN, Joseph L, Belisle P, Brophy JM, Eisenberg MJ. A hierarchical bayesian meta-analysis of randomized clinical trials of drug-eluting stents. Lancet. 2004;364: 583–91.
- Stone GW, Moses JW, Ellis SG, Schofer J, Dawkins KD, Morice MC, . Safety and efficacy of sirolimus- and paclitaxel-eluting coronary stents. N Engl J Med. 2007;356: 998–1008.
- Bavry AA, Kumbhani DJ, Helton TJ, Bhatt DL. What is the risk of stent thrombosis associated with the use of paclitaxel-eluting stents for percutaneous coronary intervention? J Am Coll Cardiol. 2005;45:941–6.
- Iakovou I, Schmidt T, Bonizzoni E, Ge L, Sangiorgi GM, Stankovic G, . Incidence, predictors, and outcome of thrombosis after successful implantation of drug-eluting stents. JAMA. 2005;293:2126–30.
- Karjalainen PP, Porela P, Ylitalo A, Vikman S, Nyman K, Vaittinen MA, . Safety and efficacy of combined antiplatelet-warfarin therapy after coronary stenting. Eur Heart J. 2007;28:726–32.
- Rubboli A, Halperin JL, Airaksinen KE, Buerke M, Eeckhout E, Freedman SB, . Antithrombotic therapy in patients treated with oral anticoagulation undergoing coronary artery stenting. An expert consensus document with focus on atrial fibrillation. Ann Med. 2008;40:428–36.
- Gilard M, Blanchard D, Helft G, Carrier D, Eltchaninoff H, Belle L, . Antiplatelet therapy in patients with anticoagulants undergoing percutaneous coronary stenting (from STENTIng and oral antiCOagulants [STENTICO]). Am J Cardiol. 2009;104:338–42.
- Lip GY, Huber K, Andreotti F, Arnesen H, Airaksinen KJ, Cuisset T, . Management of antithrombotic therapy in atrial fibrillation patients presenting with acute coronary syndrome and/or undergoing percutaneous coronary intervention/ stenting. Thromb Haemost. 2010;103:13–28.
- Airaksinen KEJ, Ylitalo A, Karjalainen PP. Drug-eluting stents in patients on long-term oral anticoagulation therapy: a mission impossible? Interv Cardiol. 2010;2:127–35.
- Karjalainen PP, Vikman S, Niemela M, Porela P, Ylitalo A, Vaittinen MA, . Safety of percutaneous coronary intervention during uninterrupted oral anticoagulant treatment. Eur Heart J. 2008;29:1001–10.
- Korkeila P, Ylitalo A, Koistinen J, Airaksinen KEJ. Progression of venous pathology after pacemaker and cardioverter-defibrillator implantation: A prospective serial venographic study. Ann Med. 2009;41:216–23.
- Annala A-P, Karjalainen PP, Porela P, Nyman K, Ylitalo A, Airaksinen KEJ. Safety of diagnostic coronary angiography during uninterrupted therapeutic warfarin treatment. Am J Cardiol. 2008;102:386–90.
- Korkeila P, Mustonen P, Koistinen J, Nyman K, Ylitalo A, Karjalainen P, . Clinical and laboratory risk factors of thrombotic complications after pacemaker implantation: a prospective study. Europace. 2010;12:817–24.
- Lahtela H, Karjalainen PP, Niemelä M, Vikman S, Kervinen K, Ylitalo A, . Are glycoprotein inhibitors safe during percutaneous coronary intervention in patients on chronic warfarin treatment? Thromb Haemost. 2009;102:1227–33.
- Airaksinen KE, Suurmunne H, Porela P, Niemelä M, Vikman S, Puurunen M, . Usefulness of outpatient bleeding risk index to predict bleeding complications in patients with long-term oral anticoagulation undergoing coronary stenting. Am J Cardiol. 2010;106:175–9.
- Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke. Results from the National Registry of Atrial Fibrillation. JAMA. 2001;285:2864–70.
- Beyth R, Quinn L, Landefeld C. Prospective evaluation of an index for predicting the risk of major bleeding in outpatients treated with warfarin. Am J Med. 1998;105:91–9.
- Inhibition of the platelet glycoprotein IIb/IIIa receptor with tirofiban in unstable angina and non-Q-wave myocardial infarction. Platelet Receptor Inhibition in Ischemic Syndrome Management in Patients Limited by Unstable Signs and Symptoms (PRISM-PLUS) Study Investigators. N Engl J Med. 1998;338:1488–97.
- Cutlip DE, Windecker S, Mehran R, Boam A, Cohen DJ, van Es GA, . Clinical end points in coronary stent trials. A case for standardized definitions. Circulation. 2007;115: 2344–51.
- Rogacka R, Chieffo A, Michev I, Airoldi F, Latib A, Cosgrave J, . Dual antiplatelet therapy after percutaneous coronary intervention with stent implantation in patients taking chronic oral antiocoagulation. J Am Coll Cardiol Interv. 2008;1:56–61.
- Ruiz-Nodar JM, Marín F, Sánchez-Payá J, Hurtado JA, Valencia-Martín J, Manzano-Fernándes S, . Efficacy and safety of drug-eluting stent use in patients with atrial fibrillation. Eur Heart J. 2009;30:932–9.
- Subherwal S, Bach RG, Chen AY, Gage BF, Rao SV, Newby LK, . Baseline risk of major bleeding in non-ST-segment-elevation myocardial infarction: the CRUSADE (Can Rapid risk stratification of Unstable angina patients Suppress ADverse outcomes with Early implementation of the ACC/AHA Guidelines) Bleeding Score. Circulation. 2009; 119:1873–82.
- Rossini R, Musumeci G, Lettieri C, Molfese M, Mihalcsik L, Mantovani P, . Long-term outcomes in patients undergoing coronary stenting on dual oral antiplatelet treatment requiring oral anticoagulant therapy. Am J Cardiol. 2008;102: 1618–23.
- Sarafoff N, Ndrepepa G, Mehilli J, Dörrler K, Schulz S, Lijima R, . Aspirin and clopidogrel with or without phenprocoumon after drug eluting coronary stent placement in patients on chronic oral anticoagulation. J Intern Med. 2008;264:472–80.
- Becker RC, Meade TW, Berger PB, Ezekowitz M, O'Connor CM, Vorchheimer DA, . The primary and secondary prevention of coronary artery disease. Chest. 2008;133: 776S–814S.
- Holmes DR Jr, Kereiakes DJ, Kleiman NS, Moliterno DJ, Patti G, Grines CL. Combining antiplatelet and anticoagulant therapies. J Am Coll Cardiol. 2009;54:95–109.