Abstract
The CD40-CD40L system is a pathway which is associated with both prothrombotic and proinflammatory effects. CD40 and its ligand were first discovered on the surface of activated T cells, but its presence on B cells, antigen-presenting cells, mast cells, and finally platelets, is evident. The soluble form of CD40L (sCD40L) is derived mainly from activated platelets and contributes to the pathophysiology of atherosclerosis and atherothrombosis. Indeed, sCD40L has autocrine, paracrine, and endocrine activities, and it enhances platelet activation, aggregation, and platelet-leucocyte conjugation that may lead to atherothrombosis. It has even been suggested that sCD40L may play a pathogenic role in triggering acute coronary syndromes. Conversely, blockade of this pathway with anti-CD40L antibodies may prevent or delay the progression of atherosclerosis. Concentrations of sCD40L also predict risk of future cardiovascular disease in healthy women and clinical outcomes in patients with acute coronary syndromes. However, there are controversial and uncertain points over the application of this biomarker to clinical cardiology. In this review, we provide an overview of potential implications of CD40-CD40L signalling and sCD40L as a biomarker in patients with atherosclerotic vascular diseases.
| Abbreviations | ||
| ACS | = | acute coronary syndromes |
| AF | = | atrial fibrillation |
| CD40L | = | CD40 ligand |
| GP | = | glycoprotein |
| IL | = | interleukin |
| LDL | = | low-density lipoproteins |
| NF-κB | = | nuclear factor κB |
| sCD40L | = | soluble form of CD40 ligand |
| TNF | = | tumour necrosis factor |
| TRAF | = | TNF receptor associated factor |
Key message
The CD40-CD40L system has autocrine, paracrine, and endocrine activities, and it is actively involved in the regulation of platelet activation, aggregation, and plateletleucocyte conjugation that may lead to prothrombotic, proinflammatory, and atherogenic effects.
Introduction
CD40 (gene locus 20q12-q13.2) is a member of the tumour necrosis factor (TNF) receptor superfamily that can be activated by its ligand CD40L (Citation1,Citation2). CD40, CD40L (also known as CD154), and soluble CD40L (sCD40L) are components of a pathophysiological pathway intimately involved in inflammation and atherogenesis.
Various immune cells including B lymphocytes, T cells, monocytes, macrophages, dendritic cells, eosinophils, basophils, and mast cells, as well as smooth muscle cells, epithelial cells, and activated platelets have capability to express CD40 (Citation3–7). Its ligand, CD40L (chromosome X, gene loc us q26.3-q27.1), is a type II transmembrane protein that also belongs to the TNF superfamily (Citation8,Citation9). However, the presence of CD40L as a surface molecule was first determined on activated T lymphocytes (Citation3), and subsequent studies have established its presence on apparently all cell types that express CD40 (Citation4–7). The free and soluble form of CD40L (sCD40L) is released from the cell surface into the circulation and appears to preserve the biological activity of CD40L.
The aim of this article is to overview the current state of knowledge on the biology of the CD40-CD40L system and its role in the pathogenesis of cardiovascular disorders with focus on the involvement of this system in the processes of atherogenesis, inflammation, and thrombosis.
CD40-mediated signal transduction
Cellular CD40 expression can be induced by different proinflammatory stimuli, such as interleukin (IL)-1, IL-3, IL-4, TNF-α, and interferon-γ (Citation10,Citation11). CD40 usually appears on the cell surface within 6–12 hours following stimulation, where it remains for 24–72 h before shedding into circulation (Citation10). After ligation by CD40L, CD40 triggers transcription of proinflammatory and proatherogenic genes (Citation11).
The interaction between CD40 and CD40L initiates intracellular signalling via TNF receptor-associated factors (TRAFs) (Citation12). Subsequently, cytoplasmic kinases and effector proteins become activated. CD40 is able to bind TRAF-1, -2, -3, -5, and -6, depending on cell type and function. CD40-TRAF interaction is followed by internalization of the CD40-CD40L complex and propagation of CD40 signalling.
CD40L enhances expression of TRAF-1, -2, -3, and -6 but not TRAF-5 in endothelial cells (ECs) (Citation13). TRAF-1, -3, and -6 deficiency was found to be associated with increased CD40L-induced IL-6 and/or MCP-1 expression by endothelial cells indicating their anti-inflammatory potential. Indeed, TRAF-3 inhibits (essentially proinflammatory) CD40 signalling in the vascular wall (Citation13–15). In contrast TRAF-2 and -5 deficiency is linked to reduced cytokine expression (Citation13). However TRAF-5 deficiency is also associated with increased expression of adhesion molecules and potentiated macrophage lipid uptake and foam cell formation (Citation16).
TRAF-mediated signal transduction and modulation of response to CD40-CD40L
Impaired neointima formation and vascular remodelling has been reported in CD40 receptor-deficient mice as well as in mice with defects in CD40-TRAF-6 but not in CD40-TRAF-2, -3, and -5 (Citation17). This impairment has been linked to decreased inflammatory cell infiltration and matrix-degrading protease activity in which CD40-TRAF-6 signalling plays a key regulator role (Citation17). CD40 deficiency in an atherosclerotic mouse model was associated with a reduction in atherosclerotic burden and a stable atherosclerotic plaque phenotype (i.e. less inflammatory but more fibrotic). In the absence of CD40-TRAF-6 interaction, but not CD40-TRAF-2, -3, or -5 interactions, atherogenesis appears to be completely abrogated in mice. At atherosclerotic lesion sites, TRAF-6 triggers activation of Src/ERK1/2 and IKK/NF-κB proinflammatory pathway monocytes and macrophages (Citation18). Also, CD40-TRAF-6 signalling-deficient mice display reduced blood counts of inflammatory Ly6Chigh monocytes and impaired recruitment of proatherogenic Ly6C + monocytes to the arterial wall (Citation19).
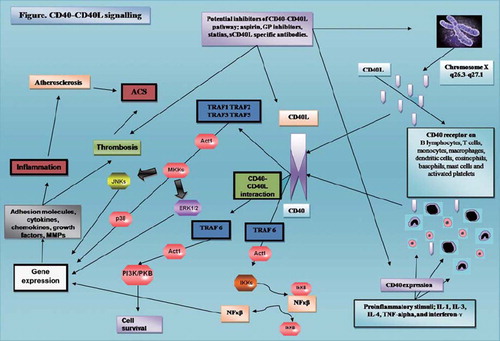
Taken together, these data indicate that the specific type of CD40-TRAF interaction represents a critical modulator of CD40 pathway proatherogenic activity. Interactions with TRAF-3 and -5 produce predominantly inhibitory effects on the CD40 signalling, whilst TRAF-6-mediated signalling promotes inflammatory responses, CD40-mediated atherosclerotic progression, and appears to play a pivotal role in neointima formation and vascular remodelling. A summary of the complex interactions within the CD40-CD40L signalling pathway is shown in .
Figure 1. Proinflammatory stimuli increasing expression of both CD40 and CD40L. Once CD40 is expressed on cell surface it interacts with its ligand sCD40L, and intracellular pathways conduct the signal. MKK, NF-κB, and PI3K/PKB (Akt) pathways induce transcription of many inflammatory and prothrombotic mediators. (Act1 = nuclear factor κB activator 1; GP = glycoprotein; IkK = inhibitory κB kinase; IL = interleukin; MKKs = mitogen-activated protein kinase complex; NF-κB = nuclear factor κB; PI3K/PKB (Akt) = phosphoinositide 3 kinases/protein kinase B; TNF-α = tumour necrosis factor α; TRAF = TNF receptor-associated factor.)
Release of soluble CD40L into circulation
Among the different cells, platelets are the major source of sCD40L in the blood (Citation20). Recent studies indicate that CD40L is cleaved from the surface of activated platelets to release sCD40L by an enzymatic reaction (Citation21). Although this release process (which leads to sCD40L shedding) has not been precisely elucidated, some evidence points towards a role of matrix metalloproteinase-2 in CD40L cleavage from the platelet surface (Citation13).
The structure of sCD40L has been a subject of debate since some researchers suggested that sCD40L might have inactive monomeric and active trimeric forms, whilst others argued that it only exists in a trimeric form (Citation21,Citation23).
The regulation of membrane-bound and soluble CD40L forms is different and depends on the activator stimulus. For example, in T cell receptor-activated cells, both forms are expressed, and CD28 co-stimulation is shown to increase their expression. The induction of sCD40L preferentially recruits a protein kinase C-dependent mechanism and potentially involvement of the ADAM-10 protease which was subsequently shown to cleave membrane CD40L to generate sCD40L (Citation24). There are limited data on the precise mechanisms that control the production of sCD40L. Although the main source of sCD40L appears to be activated platelets, it is also cleaved from T lymphocytes with a similar proteolytic mechanism of the cleavage of membrane CD40L shown for platelets (Citation25,Citation26). CD40L triggers expression of adhesion molecules and various cytokines and chemokines from different types of CD40L (Citation27). Beyond its autocrine, paracrine, and endocrine activities, the proinflammatory sCD40L enhances also platelet activation, aggregation, and platelet-leucocyte conjugation which may lead to atherothrombosis.
Soluble CD40L in thrombosis, inflammation, and atherosclerosis
Platelets are pivotal to haemostasis but also have the potential to initiate an inflammatory response of the vascular wall. Once activated, platelets promptly express CD40L and the appearance of CD40L on the platelet surface induces endothelial cells to secrete chemokines and express adhesion molecules, thereby promoting recruitment and extravasation of leucocytes at the site of injury (Citation27). CD40L can be quickly released from the platelet membrane as a soluble form, and, in fact, platelets provide the main source of sCD40L (Citation27,Citation28). Both CD40L and sCD40L have prothrombotic and proinflammatory activities, enhance platelet activation, aggregation, and platelet-leucocyte conjugation, as well as increase the release of reactive oxygen and nitrogen species from stimulated platelets (Citation29).
Although the presence of sCD40L is not essential for platelet aggregation and haemostasis, an experimental study indicated defective platelet-platelet interactions and prolonged bleeding times in CD40L knock-out mice (Citation30,Citation31). Once sCD40L is released from platelets, it triggers activation of various inflammatory and prothrombotic pathways and may serve as a molecular ‘bridge’ between hypercholesterolaemia, inflammation, and a prothrombotic state (Citation32,Citation33). For example, increased concentrations of sCD40L in hypercholesterolaemic subjects correlate with up-regulation of factor VIIa and prothrombin fragment 1+2 as well as increased platelet activation (reflected by 11-dehydro-thromboxane B2 and P-selectin expression) (Citation32). Additionally, the reduction of sCD40L by statins parallels a decrease in factor VIIa and prothrombin fragment 1+2 levels (Citation32). One recent study has even shown that activated glycoproteins (GP)Ib, IX, and V induce sCD40L release via thromboxane A2 from human platelets (Citation33). Importantly, this process was significantly up-regulated in patients with atherosclerosis (Citation33).
Accumulating evidence indicates the connective role of the CD40-CD40L system between inflammation, atherosclerosis, and thrombosis. In a mouse model, CD40L-deficient platelets failed to form platelet-leucocyte aggregates (Citation34). The injection of CD40L-positive platelets contributes to atherogenesis, whilst CD40L-deficient platelets had no effect on atherosclerotic lesion formation.
CD40L appears to promote atherogenesis in a complex way, via activation of leucocytes and increase of platelet-leucocyte and leucocyte-endothelium interactions (Citation34). Moreover, sCD40L induces tissue factor expression on macrophages and endothelial cells and down-regulates endothelial thrombomodulin expression (Citation35). In addition, sCD40L has the ability to bind the GPIIb/IIIa receptors on platelets and induce GPIIb/IIIa activation (Citation36). Vice versa, GPIIb/IIIa antagonists inhibit the release of sCD40L from activated platelets (Citation37). Of note, GPIIb/IIIa antagonists, such as abciximab, eptifibatide, or tirofiban are unable to inhibit this activation-dependent translocation of CD40L from intraplatelet compartment to the platelet surface. However, the release of sCD40L from activated platelets seems inhibited in a dose-dependent manner by abciximab, eptifibatide, and tirofiban (Citation37). Thus, GPIIb/IIIa inhibitors may obviate the proinflammatory and prothrombotic effects of sCD40L. Indeed, GPIIb/IIIa antagonists, when used at doses that optimally inhibit platelet aggregation, can reduce the inflammatory and prothrombotic state driven by sCD40L (Citation36). Nonetheless, regulation of sCD40L release from platelets is a complex process that is only partly mediated by GPIIb/IIIa but also involves actin polymerization and a matrix metalloproteinase inhibitor-sensitive pathway as mentioned above (Citation37).
The CD40-CD40L pathway and inflammation
Activation of the CD40-CD40L system promotes a chronic inflammatory state in the vascular wall, and it contributes to atherogenesis and development of its complications such as acute coronary syndromes (ACS) (Citation12,Citation38). In addition to its activity in blood, the CD40-CD40L system probably executes cell type-specific signalling in the atherosclerotic plaque. Indeed, sCD40L levels are significantly higher in samples collected from the atherosclerotic lesions compared to peripheral samples (Citation39). Increased CD40L levels in atherosclerotic sites significantly correlated with enhanced local inflammatory burden (Citation39). The contribution of the CD40-CD40L system to the atherosclerotic process is complex, and evidence indicates both early and late-phase contributions. CD40L may also promote inflammation independently from CD40 via an interaction with Mac-1 (Citation40).
Activation of the pathway may be triggered by various cardiovascular risk factors. For example, elevated levels of sCD40L have been reported in subjects with several different diseases, including diabetes, impaired glucose tolerance, metabolic syndrome, obesity, insulin resistance, and systemic hypertension (Citation41). In patients with diabetes mellitus, strong correlations between sCD40L and both IL-6 and tissue factor have been established (Citation42). Of note, an intensified medical therapy and life-style modification programme can reduce elevated plasma sCD40L levels in patients with diabetes, with and without overt cardiovascular disease (Citation42). A recent study indicated that even cigarette smoking could induce both the up-regulation of the CD40-CD40L pathway and the formation of platelet-monocyte aggregates (Citation43). Oxidized low-density lipoprotein (LDL) molecules promote the expression of CD40 and CD40L in human atheroma, whilst statins may limit the expression of both markers directly (Citation44). Accordingly, some of the anti-inflammatory pleiotropic actions of statins may be attributed to their impact on reduced CD40 signalling (Citation45).
Interactions between CD40 on antigen-presenting cells and CD40L on T lymphocytes drive the T cell expression of proatherogenic interferon-γ, TNF-α, IL-1, and IL-18 (by Th1 subset) but also potentially antiatherogenic IL-4, IL-5, and IL-10 (by Th2 subset) (Citation46). Generally, CD40L-related activities contribute local vascular inflammation and endothelial dysfunction. The CD40-CD40L system also induces expression of adhesion molecules (vascular cell adhesion molecule, intercellular adhesion molecule, and E-selectin) by the endothelium-facilitating migration of atherogenic leucocytes into the vascular wall (Citation47). However, interactions of leucocyte CD40-CD40L with innate regulatory T lymphocytes had no significant impact on atherogenesis, thus once again demonstrating the complexity of biological roles of the pathway (Citation48).
Vascular implications of the CD40-CD40L pathway
A recent study established that sCD40L promotes neointimal formation after arterial injury and may impair the function of peripheral blood angiogenic outgrowth endothelial progenitors (Citation49). In contrast, therapeutic blockade of sCD40L may accelerate endothelial regeneration and attenuate neointimal remodelling (Citation49). Plasma levels of sCD40L increase after arterial injury from percutaneous coronary interventions (PCI) (Citation50), and high sCD40L concentrations have been shown to be associated with late restenosis after PCI in which neointimal proliferation and inflammation play a pivotal role (Citation51). These observations indicate a negative impact of the pathway activation in the vascular structure function. Indeed, inhibition of the CD40-CD40L pathway reduces vascular inflammation and might potentially serve as a therapeutic target in cardiovascular disease.
In addition to providing an inflammatory background essential for the initiation of the atherosclerotic process, CD40-CD40L activity is distinctly enhanced inside the plaque, perhaps playing an important role in plaque growth and remodelling. The detrimental role of the pathway in the vascular wall is supported by the observations that CD40-CD40L signalling in macrophages induces expression and release of proinflammatory, proteolytic, and prothrombotic mediators (i.e. monocyte chemoattractant protein-1, MIP-1α, MIP-1β, RANTES, IL-1, IL-6, IL-8, interferon-γ, TNF-α, matrix metalloproteinases-1, -2, -3, -9, -11, -13, and tissue factor) (Citation52). Also, activation of CD40 signalling on vascular smooth muscle cells and endothelial cells increases the expression of inflammatory and prothrombotic mediators (Citation53).
Reflecting the systemic nature of atherosclerosis, elevated sCD40L levels have been found in patients with different localization of the disease including coronary, carotid, and peripheral vascular beds (Citation54). However, in patients with rheumatoid arthritis, despite a significant increase in sCD40L, there was no significant relation between its levels and the carotid intima-media thickness (Citation55). One may speculate that the relative impact of the pathway on the atherogenesis may be smaller in the presence of other concomitant severe inflammatory changes.
CD40-CD40L pathway and cardiovascular complications
Plasma levels of sCD40L are higher in patients with unstable compared with stable coronary disease (Citation38,Citation56). Clinical studies have shown that sCD40L concentrations predict the risk of future cardiovascular disease in healthy women and clinical outcome in patients with ACS (Citation57,Citation58). Increased levels of sCD40L could therefore be considered as a new cardiovascular risk factor. Indeed, sCD40L has been shown to be involved in atherosclerotic plaque destabilization and thrombus formation during the acute phase of myocardial infarction (Citation14,Citation47,Citation52,Citation56). High sCD40L concentrations are linked to clinical outcomes in hospitalized patients with ACS (Citation58). And sCD40L predicted adverse cardiac outcomes in patients with ACS both independently of troponin-I levels and synergistically when biomarkers are used in combination (Citation50). In a recent prospective study, a 48% recurrence of vascular events in diabetic patients with stroke was predicted by increased sCD40L levels (Citation59). Accordingly it seems plausible that distinctive proinflammatory changes associated with activation of the pathway result in accelerated growth and destabilization of atherosclerotic plaques.
The effects of several medications on the CD40-CD40L pathway in cardiovascular pathology have been assessed in parallel with clinical outcomes. For example, the GPIIb/IIIa receptor antagonist, abciximab, has been shown to ameliorate increased risk of recurrent cardiovascular events in ACS patients with high sCD40L levels undergoing PCI (Citation58). Similarly, a substudy of the Myocardial Ischemia Reduction with Aggressive Cholesterol Lowering (MIRACL) trial demonstrated that atorvastatin was able to reduce prominently the risk of recurrent cardiovascular events associated with high sCD40L in patients with ACS (Citation60).
However, results of clinical trials showing a positive link between sCD40L levels and cardiovascular complications are not universal. Several studies have failed to establish a significant relationship between cardiovascular events and sCD40L levels. A poor correlation has been reported between the sCD40L and the individual components or the calculated Framingham Coronary Heart Disease Risk Scores (Citation61). A substudy of the TACTICS-TIMI-18 trial found no association of sCD40L levels with non-fatal recurrent ischaemic event risk in non-ST-elevation ACS patients treated with a platelet GPIIb/IIIa receptor antagonist (Citation62). and summarize data on the role of sCD40L in patients with ACS, stroke, and stable coronary artery disease, where some of the diverse observations could reflect the complexity of the CD40L pathway and its biological functions.
Table I. Impact of sCD40L on cardiovascular outcomes in acute coronary syndromes and stroke.
Table II. Relationship between sCD40L levels and cardiovascular outcomes in patients with stable coronary artery disease.
The relation between sCD40L and clinical outcomes has been also investigated in clinical settings other than atherosclerosis but is generally not supportive of a significant role for sCD40L in disorders such as atrial fibrillation (AF) or heart failure. Indeed, plasma sCD40L levels were only marginally increased in patients with AF (Citation63). A lack of clear correlation with relevant plasma markers (soluble P- and E-selectins, von Willebrand factor, etc.) suggested that the source of the stimulus was unlikely to be the endothelium or platelets alone (Citation63). Increased sCD40L levels have been shown to have moderate predictive value for stroke and myocardial infarction in patients with non-valvular AF (Citation64,Citation65). Preoperative platelet activation, assessed by sCD40L levels, appears to be predictive for postoperative AF (Citation66). A recent study failed to find any association between CD40L and pathophysiology of congestive heart failure as well as any predictive role of sCD40L for clinical events in this condition (Citation67).
The CD40-CD40L pathway as therapeutic target in cardiovascular medicine
The CD40-CD40L system has been investigated as a direct therapeutic target in atherothrombosis in mice and humans by infusion of anti-CD40L antibodies (Citation68,Citation69). Experimental studies have established that blockade of the CD40L pathway can inhibit atherogenesis, and mice lacking CD40L also exhibit delayed lesion progression and inflammatory cell recruitment at sites of atherogenesis (Citation70–73). Such treatment reduces the relative content of macrophages and lipids and increases the proportion of smooth muscle cells and collagen.
Unfortunately, therapeutic administration of anti-CD40L antibodies increases thromboembolic complications. Interactions between CD40 and CD40L antibodies have been suggested as a possible mechanism to explain increased thromboembolic events associated with impaired platelet function (Citation68). Although recent attempts to block the CD40 system by CD40L antibodies have not successfully provided antithrombotic effects, we believe that more precise targets in this system, for example blockage of a specific element of TRAF-mediated signal transduction, may potentially have value in treatment or prevention of atherosclerosis and thrombosis. For example, the blockage of the CD40-TRAF-6 axis is promising since experimentally it has been associated with reduced atherosclerosis (Citation19).
Determination of sCD40L in blood samples: serum or plasma?
Another controversial issue related to the potential clinical application of sCD40L as a biomarker of the cardiovascular risk stems from various methodological issues. Recent studies suggest that sample processing and temperature can profoundly affect the results of sCD40L assays. Serum clotted on ice and platelet-poor plasma are thought to minimize release of sCD40L in in-vitro conditions and probably represent preferable media to measure in-vivo levels of sCD40L (Citation74). Serum and plasma sCD40L levels appear to be similar in patients with normal platelet counts, but measurements performed with serum clotted on ice give significantly higher sCD40L values than plasma in patients with thrombocytosis (Citation74).
Levels of sCD40L measured in platelet-poor plasma did not differ significantly between stable and unstable angina patients; however, significantly lower levels of sCD40L were determined in serum (Citation75). Another study suggested that plasma samples (but not serum samples) were appropriate for sCD40L measurements (Citation76). Measurement of sCD40L, serum P-selectin levels, and light transmission platelet aggregometry were performed in healthy donors and show that citrated plasma samples yielded the most valid estimates of CD40L levels (Citation77). However, the pre-analytical conditions of these studies should be interpreted carefully because in-vitro platelet activation during sample preparation may increase sCD40L concentrations, and disease-related in-vivo activation might not contribute to the high plasma sCD40L concentrations (Citation78). The source of sCD40L that we aim to determine (e.g. platelet-derived sCD40L) and patients’ platelet count may direct the type of the sample media (i.e. serum or plasma) for its eventual quantification.
The controversy regarding the appropriate specimen and assay procedure for the analyses of blood sCD40L has been revisited in 20 healthy volunteers by ELISA. Serum sCD40L levels were determined to be significantly higher than plasma collected in citrate, EDTA, or heparin, without any significant differences between plasma preparation procedures. The main parameters that influence sCD40L levels appeared to be the presence of platelets in the specimen and high levels of bilirubin, haemoglobin, and triacylglycerols (Citation79). Furthermore, increasing centrifuge gravity has also a decreasing effect on sCD40L levels via platelet depletion.
Reported discrepancies in relationships between sCD40L levels and risk of cardiovascular events may reflect diverse biological roles of the CD40-CD40L pathway and differential implication of various cell types that may serve as a source of sCD40L in a specific clinical condition.
Conclusion
The CD40-CD40L system and its soluble mediator sCD40L are involved in the main inflammatory and thrombotic pathways of atherosclerotic cardiovascular diseases. The interactions between CD40L and TRAFs appear to be a major determinant for the signal transduction. The differential implication of the different TRAFs on various cell types mediates and modulates a wide range of biological effects of the CD40-CD40L pathway. Some of these CD40-TRAFs interactions are pivotal for atherogenesis, neointima formation, and vascular remodelling (e.g. TRAF-6). The system contributes in atherogenesis and thrombosis and works as a bridge between inflammation, atherosclerosis, and thrombosis. However, the clinical role of the pathway remains controversial, and even large-scale prospective studies have reported contradictory results. These discrepancies may in part be related to the methodological limitations of sCD40L assessment. Despite some promising data, our current state of the knowledge does now allow justifying markers of the CD40-CD40L pathway as reliable clinical tools for the evaluation of the risk of atherogenesis and atherothrombosis. Further studies are clearly needed to elucidate the gap between molecular mechanisms and clinical outcomes.
Declaration of interest: The work was supported by the European Society of Cardiology Atherothrombosis fellowship to Dr Burak Pamukcu. The authors declare no conflicts of interest.
References
- Noelle RJ, Ledbetter JA, Aruffo A. CD40 and its ligand, an essential ligand-receptor pair for thymus-dependent B-cell activation. Immunol Today. 1992;13:431–3.
- Ludewig B, Henn V, Schröder JM, Graf D, Kroczek RA. Induction, regulation, and function of soluble TRAP (CD40 ligand) during interaction of primary CD4+ CD45RA+ T cells with dendritic cells. Eur J Immunol. 1996;26:3137–43.
- van Kooten C, Banchereau J. CD40-CD40 ligand. J Leukoc Biol. 2000;67:2–17.
- Kroczek RA, Graf D, Brugnoni D, Giliani S, Korthauer U, Ugazio A, . Defective expression of CD40 ligand on T cells causes X-linked immunodeficiency with hyper-IgM (HIGM1). Immunol Rev. 1994;138:39–59.
- Gauchat JF, Henchoz S, Mazzei G, Aubry JP, Brunner T, Blasey H, . Induction of human IgE synthesis in B cells by mast cells and basophils. Nature. 1993;365:340–3.
- Gauchat JF, Henchoz S, Fattah D, Mazzei G, Aubry JP, Jomotte T, . CD40 ligand is functionally expressed on human eosinophils. Eur J Immunol. 1995;25:863–5.
- Hermann A, Rauch BH, Braun M, Schror K, Weber AA. Platelet CD40 ligand-subcellullar localization, regulation of expression, and inhibition by clopidogrel. Platelets. 2001;12: 74–82.
- Armitage RJ, Fanslow WC, Strockbine L, Sato TA, Clifford KN, Macduff BM, . Molecular and biological characterization of a murine ligand for CD40. Nature. 1992;357:80–2.
- Graf D, Korthauer GD, Mages HW, Senger G, Kroczek RA. Cloning of TRAP, a ligand for CD40 on human T cells. Eur J Immunol. 1992;22:3191–4.
- Schonbeck U, Libby P. The CD40/CD154 receptor/ligand dyad. Cell Mol Life Sci. 2001;58:4–43.
- Chen Y, Chen J, Xiong Y, Da Q, Xu Y, Jiang X, . Internalization of CD40 regulates its signal transduction in vascular endothelial cells. Biochem Biophys Res Commun. 2006;345:106–17.
- Schonbeck U, Libby P. CD40 signaling and plaque instability. Circ Res. 2001;89:1092–103.
- Zirlik A, Bavendiek U, Libby P, MacFarlane L, Gerdes N, Jagielska J, . TRAF-1, -2, -3, -5, and -6 are induced in atherosclerotic plaques and differentially mediate proinflammatory functions of CD40L in endothelial cells. Arterioscler Thromb Vasc Biol. 2007;27:1101–7.
- Urbich C, Mallat Z, Tedgui A, Clauss M, Zeiher AM, Dimmeler S. Upregulation of TRAF-3 by shear stress blocks CD40-mediated endothelial activation. J Clin Invest. 2001; 108:1451–8.
- Arron JR, Pewzner-Jung Y, Walsh MC, Kobayashi T, Choi Y. Regulation of the subcellular localization of tumor necrosis factor receptor associated factor (TRAF)2 by TRAF1 reveals mechanisms of TRAF2 signaling. J Exp Med. 2002;196: 923–34.
- Missiou A, Wolf D, Platzer I, Ernst S, Walter C, Rudolf P, . CD40L induces inflammation and adipogenesis in adipose cells—a potential link between metabolic and cardiovascular disease. Thromb Haemost. 2010;103:788–96.
- Donners MM, Beckers L, Lievens D, Munnix I, Heemskerk J, Janssen BJ, . The CD40-TRAF6 axis is the key regulator of the CD40/CD40L system in neointima formation and arterial remodeling. Blood. 2008;111:4596–604.
- Zapata JM. TNF-receptor-associated factors as targets for drug development. Expert Opin Ther Targets. 2003;7:411–25.
- Lutgens E, Lievens D, Beckers L, Wijnands E, Soehnlein O, Zernecke A, . Deficient CD40-TRAF6 signaling in leukocytes prevents atherosclerosis by skewing the immune response toward an antiinflammatory profile. J Exp Med. 2010;207:391–404.
- Wenzel F, Baertl A, Zimmermann N, Hohlfeld T, Giers G, Oldenburg J, . Different behaviour of soluble CD40L concentrations can be reflected by variations of preanalytical conditions. Clin Hemorheol Microcirc. 2008;39:417–22.
- Henn V, Steinbach S, Büchner K, Presek P, Kroczek RA. The inflammatory action of CD40 ligand (CD154) expressed on activated human platelets is temporally limited by coexpressed CD40. Blood. 2001;98:1047–54.
- Reinboldt S, Wenzel F, Rauch BH, Hohlfeld T, Grandoch M, Fischer JW, . Preliminary evidence for a matrix metalloproteinase-2 (MMP-2)-dependent shedding of soluble CD40 ligand (sCD40L) from activated platelets. Platelets. 2009;20: 441–4.
- Inwald DP, McDowall A, Peters MJ, Callard RE, Klein NJ. CD40 is constitutively expressed on platelets and provides a novel mechanism for platelet activation. Circ Res. 2003;92: 1041–8.
- Matthies KM, Newman JL, Hodzic A, Wingett DG. Differential regulation of soluble and membrane CD40L proteins in T cells. Cell Immunol. 2006;241:47–58.
- Hsu YM, Lucci J, Su L, Ehrenfels B, Garber E, Thomas D. Heteromultimeric complexes of CD40 ligand are present on the cell surface of human T lymphocytes. J Biol Chem. 1997; 272:911–5.
- Hirohata S. Human Th1 responses driven by IL-12 are associated with enhanced expression of CD40 ligand. Clin Exp Immunol. 1999;115:78–85.
- Henn V, Slupsky JR, Grafe M, Anagnostopoulos I, Forster R, Muller-Berghaus G, . CD40 ligand on activated platelets triggers an inflammatory reaction of endothelial cells. Nature. 1998;391:591–4.
- Foy TM, Aruffo A, Bajorath J, Buhlmann JE, Noelle RJ. Immune regulation by CD40 and its ligand GP39. Annu Rev Immunol. 1996;14:591–617.
- Chakrabarti S, Varghese S, Vitseva O, Tanriverdi K, Freedman JE. CD40 ligand influences platelet release of reactive oxygen intermediates. Arterioscler Thromb Vasc Biol. 2005;25:2428–34.
- Prasad KS, Andre P, He M, Bao M, Manganello J, Phillips DR. Soluble CD40 ligand induces beta3 integrin tyrosine phosphorylation and triggers platelet activation by outside-in signaling. Proc Natl Acad Sci U S A. 2003;100:12367–71.
- Crow AR, Leytin V, Starkey AF, Rand ML, Lazarus AH. CD154 (CD40 ligand)-deficient mice exhibit prolonged bleeding time and decreased shear-induced platelet aggregates. J Thromb Haemost. 2003;1:850–2.
- Cipollone F, Mezzetti A, Porreca E, Di Febbo C, Nutini M, Fazia M, . Association between enhanced soluble CD40L and prothrombotic state in hypercholesterolemia. Effects of statin therapy. Circulation. 2002;106:399–402.
- Enomoto Y, Adachi S, Matsushima-Nishiwaki R, Doi T, Niwa M, Akamatsu S, . Thromboxane A(2) promotes soluble CD40 ligand release from human platelets. Atherosclerosis. 2010;209:415–21.
- Lievens D, Zernecke A, Seijkens T, Soehnlein O, Beckers L, Munnix IC, . Platelet CD40L mediates thrombotic and inflammatory processes in atherosclerosis. Blood. 2010;116: 4317–27.
- Aukrust P, Damas JK, Solum NO. Soluble CD40 ligand and platelets: self-perpetuating pathogenic loop in thrombosis and inflammation? J Am Coll Cardiol. 2004;43:2326–8.
- Nannizzi-Alaimo L, Alves VL, Phillips DR. Inhibitory effects of glycoprotein IIb/IIIa antagonists and aspirin on the release of soluble CD40 ligand during platelet stimulation. Circulation. 2003;107:1123–8.
- Furman MI, Krueger LA, Linden MD, Barnard MR, Frelinger III AL, Michelson AD. Release of soluble CD40L from platelets is regulated by glycoprotein IIb/IIIa and actin polymerization. J Am Coll Cardiol. 2004;43:2319–25.
- Garlichs CD, Eskafi S, Raaz D, Schmidt A, Ludwig J, Herrmann M, . Patients with acute coronary syndromes express enhanced CD40 ligand/CD154 on platelets. Heart. 2001;86:649–55.
- Aggarwal A, Schneider DJ, Terrien EF, Sobel, BE, Dauerman HL. Increased coronary arterial release of interleukin-1 receptor antagonist and soluble CD40 ligand indicative of inflammation associated with culprit coronary plaques. Am J Cardiol. 2004;93:6–9.
- Zirlik A, Maier C, Gerdes N, MacFarlane L, Soosairajah J, Bavendiek U, . CD40 ligand mediates inflammation independently of CD40 by interaction with Mac-1. Circulation. 2007;115:1571–80.
- Penno G, Pucci L, Dell’Omo G, Lucchesi D, Miccoli R, Del Prato S, . Soluble CD40 ligand levels in essential hypertensive men: evidence of a possible role of insulin resistance. Am J Hypertens. 2009;22:1007–13.
- Lim HS, Blann AD, Lip GYH. Soluble CD40 ligand, soluble P-selectin, interleukin-6, and tissue factor in diabetes mellitus. Relationships to cardiovascular disease and risk factor intervention. Circulation. 2004;109:2524–8.
- Harding SA, Sarma J, Josephs DH, Cruden NL, Din JN, Twomey PJ, . Upregulation of the CD40/CD40 ligand dyad and platelet-monocyte aggregation in cigarette smokers. Circulation. 2004;109:1926–9.
- Schonbeck U, Gerdes N, Varo N, Reynolds RS, Horton DB, Bavendiek U, . Oxidized low-density lipoprotein augments and 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors limit CD40 and CD40L expression in human vascular cells. Circulation. 2002;106:2888–93.
- Andre P, Prasad KS, Denis CV, He M, Papalia JM, Hynes RO, . CD40L stabilizes arterial thrombi by a B3 integrindependent mechanism. Nat Med. 2002;8:247–52.
- Robertson AK, Hansson GK. T cells in atherogenesis: for better or for worse? Arterioscler Thromb Vasc Biol. 2006;26: 2421–32.
- Wagner AH, Guldenzoph B, Lienenluke B Hecker M. CD154/CD40-mediated expression of CD154 in endothelial cells: consequences for endothelial cell-monocyte interaction. Arterioscler Thromb Vasc Biol. 2004;24:715–20.
- Smook ML, Heeringa P, Damoiseaux JG, Daemen MJ, de Winther MP, Gijbels MJ, . Leukocyte CD40L deficiency affects the CD25(+) CD4 T cell population but does not affect atherosclerosis. Atherosclerosis. 2005;183: 275–82.
- Hristov M, Gümbel D, Lutgens E, Zernecke A, Weber C. Soluble CD40 ligand impairs the function of peripheral blood angiogenic outgrowth cells and increases neointimal formation after arterial injury. Circulation. 2010;121: 315–24.
- Varo N, de Lemos JA, Libby P, Morrow DA, Murphy SA, Nuzzo R, . Soluble CD40L: risk prediction after acute coronary syndromes. Circulation. 2003;108:1049–52.
- Cipollone F, Ferri C, Desideri G, Paloscia L, Materazzo G, Mascellanti M, . Preprocedural level of soluble CD40L is predictive of enhanced inflammatory response and restenosis after coronary angioplasty. Circulation. 2003;108: 2776–82.
- Mach F, Schonbeck U, Bonnefoy JY, Pober JS, Libby P. Activation of monocyte/macrophage functions related to acute atheroma complication by ligation of CD40: induction of collagenase, stromelysin, and tissue factor. Circulation. 1997;96:396–9.
- Bavendiek U, Libby P, Kilbride M, Reynolds R, Mackman N, Schonbeck U. Induction of tissue factor expression in human endothelial cells by CD40 ligand is mediated via activator protein 1, nuclear factor kappa B, and Egr-1. J Biol Chem. 2002;277:25032–9.
- Blann AD, Tan KT, Tayebjee MH, Davagnanam I, Moss M, Lip GY. Soluble CD40L in peripheral artery disease. Relationship with disease severity, platelet markers and the effects of angioplasty. Thromb Haemost. 2005;93:578–83.
- Pamuk GE, Vural O, Turgut B, Demir M, Pamuk ON, Cakir N. Increased platelet activation markers in rheumatoid arthritis: are they related with subclinical atherosclerosis? Platelets. 2008;19:146–54.
- Aukrust P, Muller F, Ueland T, Berget T, Aaser E, Brunsvig A, . Enhanced levels of soluble and membrane-bound CD40 ligand in patients with unstable angina. Possible reflection of T lymphocyte and platelet involvement in the pathogenesis of acute coronary syndromes. Circulation. 1999;100:614–20.
- Schonbeck U, Varo N, Libby P, Buring J, Ridker PM. Soluble CD40L and cardiovascular risk in women. Circulation. 2001;104:2266–8.
- Heeschen C, Dimmeler S, Hamm CW, van den Brand MJ, Boersma E, Zeiher AM, . Soluble CD40 ligand in acute coronary syndromes. N Engl J Med. 2003;348:1104–11.
- Davi G, Tuttolomondo A, Santilli F, Basili S, Ferrante E, Di Raimondo D, . CD40 ligand and MCP-1 as predictors of cardiovascular events in diabetic patients with stroke. J Atheroscler Thromb. 2009;16:707–13.
- Kinlay S, Schwartz GG, Olsson AG, Rifai N, Sasiela WJ, Szarek M, . Effect of atorvastatin on risk of recurrent cardiovascular events after an acute coronary syndrome associated with high soluble CD40 ligand in the Myocardial Ischemia Reduction with Aggressive Cholesterol Lowering (MIRACL) Study. Circulation. 2004;110:386–91.
- Verma S, Wang CH, Li SH, Lonnb E, Charbonneau F, Title LM, . The relationship between soluble CD40 ligand levels and Framingham coronary heart disease risk score in healthy volunteers. Atherosclerosis. 2005;182:361–5.
- Morrow DA, Sabatine MS, Brennan ML, de Lemos JA, Murphy SA, Ruff CT, . Concurrent evaluation of novel cardiac biomarkers in acute coronary syndrome: myeloperoxidase and soluble CD40 ligand and the risk of recurrent ischaemic events in TACTICS-TIMI 18. Eur Heart J. 2008;29:1096–102.
- Blann AD, Choudhury A, Freestone B, Patel J, Lip GY. Soluble CD40 ligand and atrial fibrillation: relationship to platelet activation, and endothelial damage/dysfunction. Int J Cardiol. 2008;127:135–7.
- Ferro D, Loffredo L, Polimeni L, Fimognari F, Villari P, Pignatelli P, . Soluble CD40 ligand predicts ischemic stroke and myocardial infarction in patients with nonvalvular atrial fibrillation. Arterioscler Thromb Vasc Biol. 2007;27: 2763–8.
- Choudhury A, Chung I, Panja N, Patel J, Lip GYH. Soluble CD40 ligand, platelet surface CD40 ligand, and total platelet CD40 ligand in atrial fibrillation. Relationship to soluble p-selectin, stroke risk factors, and risk factor intervention. Chest. 2008;134:574–81.
- Antoniades C, Van-Assche T, Shirodaria C, Diesch J, Antonopoulos AS, Lee J, . Preoperative sCD40L levels predict risk of atrial fibrillation after off-pump coronary artery bypass graft surgery. Circulation. 2009;120: S170–S6.
- Chung I, Choudhury A, Patel J, Lip GY. Soluble CD40L, platelet surface CD40L and total platelet CD40L in congestive heart failure: relationship to platelet volume, mass and granularity. J Intern Med. 2008;263:313–21.
- Langer F, Ingersoll SB, Amirkhosravi A, Meyer T, Siddiqui FA, Ahmad S, . The role of CD40 in CD40L- and antibody-mediated platelet activation. Thromb Haemost. 2005;93: 1137–46.
- Kawai T, Andrews D, Colvin RB, Sachs DH, Cosimi AB. Thromboembolic complications after treatment with monoclonal antibody against CD40 ligand. Nat Med. 2000; 6:114.
- Mach F, Schönbeck U, Sukhova GK, Atkinson E, Libby P. Reduction of atherosclerosis in mice by inhibition of CD40 signalling. Nature. 1998;394:200–3.
- Lutgens E, Gorelik L, Daemen MJ, de Muinck ED, Grewal IS, Koteliansky VE, . Requirement for CD154 in the progression of atherosclerosis. Nat Med. 1999;5:1313–6.
- Schönbeck U, Sukhova G, Shimizu K, Mach F, Libby P. Inhibition of CD40 signaling limits evolution of established atherosclerosis in mice. Proc Natl Acad Sci U S A. 2000; 7458:458–63.
- Lutgens E, Cleutjens KB, Heeneman S, Koteliansky VE, Burkly LC, Daemen MJ. Both early and delayed anti-CD40L antibody treatment induces a stable plaque phenotype. Proc Natl Acad Sci U S A. 2000;97:7464–9.
- Ahn ER, Lander G, Jy W, Bidot CJ, Jimenez JJ, Horstman LL, . Differences of soluble CD40L in sera and plasma: Implications on CD40L assay as a marker of thrombotic risk. Thromb Res. 2004;114:143–8.
- Mason PJ, Chakrabarti S, Albers AA, Rex S, Vitseva O, Varghese S, . Plasma, serum and platelet expression of CD40 ligand in adults with cardiovascular disease. Am J Cardiol. 2005;96:1365–9.
- Weber M, Rabenau B, Stanisch M, Nef HM, Möllmann H, Elsässer A, . Influence of sample type on soluble CD40 ligand assessment in patients with acute coronary syndromes. Thromb Res. 2007;120:811–4.
- Riondino S, Martini F, La Farina F, Spila A, Guadagni F, Ferroni P. Increased plasma levels of soluble CD40 ligand correlate with platelet activation markers and underline the need for standardized pre-analytical conditions. Clin Biochem. 2010;43:666–70.
- Ivandic BT, Spanuth E, Haase D, Lestin HG, Katus HA. Increased plasma concentrations of soluble cd40 ligand in acute coronary syndrome depend on in vitro platelet activation. Clin Chem. 2007;53:1231–4.
- Varo N, Nuzzo R, Natal C, Libby P, Schonbeck U. Influence of pre-analytical and analytical factors on soluble CD40L measurements. Clin Sci (Lond). 2006;111:341–7.
- Wykrzykowska JJ, Warnholtz A, de Jaeger P, Curzen N, Oldroyd KG, Collet JP, . Effect of clopidogrel discontinuation at 1 year after drug eluting stent placement on soluble CD40L, P-selectin and C-reactive protein levels: DECADES (Discontinuation Effect of Clopidogrel After Drug Eluting Stent): a multicenter, open-label study. J Thromb Thrombolysis. 2009;28:410–7.
- Olenchock BA, Wiviott SD, Murphy SA, Cannon CP, Rifai N, Braunwald E, . Lack of association between soluble CD40L and risk in a large cohort of patients with acute coronary syndrome in OPUS TIMI-16. J Thromb Thrombolysis. 2008;26:79–84.
- Novo S, Basili S, Tantillo R, Falco A, Davì V, Novo G, . Soluble CD40L and cardiovascular risk in asymptomatic low-grade carotid stenosis. Stroke. 2005;36:673–5.
- Malarstig A, Lindahl B, Wallentin L, Siegbahn A. Soluble CD40L levels are regulated by the -3459 A > G polymorphism and predict myocardial infarction and the efficacy of antithrombotic treatment in non-ST elevation acute coronary syndrome. Arterioscler Thromb Vasc Biol. 2006;26:1667–73.
- Manenti ER, Bodanese LC, Camey SA, Polanczyk CA. Prognostic value of serum biomarkers in association with TIMI risk score for acute coronary syndromes. Clin Cardiol. 2006;29:405–10.
- Apple FS, Pearce LA, Chung A, Ler R, Murakami MM. Multiple biomarker use for detection of adverse events in patients presenting with symptoms suggestive of acute coronary syndrome. Clin Chem. 2007;53:874–81.
- Obradovic SD, Antovic JP, Antonijevic NM, Ratkovic NG, Vojvodic DV, Subota VS, . Elevations in soluble CD40 ligand in patients with high platelet aggregability undergoing percutaneous coronary intervention. Blood Coagul Fibrinolysis. 2009;20:283–9.
- Rondina MT, Lappé JM, Carlquist JF, Muhlestein JB, Kolek MJ, Horne BD, . Soluble CD40 ligand as a predictor of coronary artery disease and long-term clinical outcomes in stable patients undergoing coronary angiography. Cardiology. 2008;109:196–201.
- Tousoulis D, Antoniades C, Nikolopoulou A, Koniari K, Vasiliadou C, Marinou K, . Interaction between cytokines and sCD40L in patients with stable and unstable coronary syndromes. Eur J Clin Invest. 2007;37:623–8.
- Tanne D, Haim M, Goldbourt U, Boyko V, Reshef T, Adler Y, . CD40 ligand and risk of ischemic stroke or coronary events in patients with chronic coronary heart disease. Int J Cardiol. 2006;107:322–6.
