Abstract
The eye is intricately integrated with the functions of the body. Ocular changes may precede or run concurrently with various systemic conditions and often represent important prognostic indicators of disease progression. In addition to a thorough diagnostic evaluation and treatment of underlying processes, individuals with systemic diseases and concurrent ocular changes may need comprehensive ophthalmic examination to reduce the risk of visual impairment and morbidity. In this review the authors highlight the clinically relevant ocular signs that occur parallel with systemic conditions. In particular, the study focuses on the varied clinical presentations that can lead to rapid diagnosis to improve management of eye disorders that accompany systemic diseases.
Key messages
Identification of ocular features of systemic diseases can facilitate earlier detection and treatment of many systemic diseases.
Background
The eye plays an important role in numerous physiological processes and often reflects signs of systemic disorders. In some systemic conditions, clinical signs in the eyes may offer valuable diagnostic insights into underlying disease processes. Since ophthalmic indicators often arise early on in systemic diseases, finding these changes quickly can facilitate the diagnosis and management of underlying systemic illnesses. The following article provides an overview of the clinically relevant ocular signs that accompany common cardiovascular, pulmonary, and endocrine diseases, with a particular focus on the diagnostic and prognostic features of such signs.
Cardiovascular disorders
Many ophthalmic abnormalities are associated with cardiovascular disease. Specifically, inadequate blood pressure control, arterial and venous abnormalities, and the presence of cardiovascular risk factors—including hyperlipidemia, hypertension, and smoking—often result in vision-threatening ocular manifestations.
Organs and their systems must keep up a constant, adequate blood flow to maintain their vitality. Circulating blood supplies oxygen, which is vital for the survival of tissues. It also transports molecules, leukocytes, and other cells necessary for maintaining immunity, hemostasis, and proper functioning (Citation1). Cardiovascular diseases often compromise the major vessels critical for systemic circulation as well as smaller vessels in peripheral organs including the eye. Unlike the larger blood vessels of the circulatory system which maintain low vascular resistance in healthy states, the microvascular network of the eye and its small arteries and arterioles maintain significant vascular resistance. Thus, as with other distal sites containing microvascular networks, the eye is a primary site of blood flow regulation and autoregulation (Citation2,Citation3).
Autoregulation of blood flow is critical in the body's ability to adapt to changing conditions, such as positional changes, increased metabolic demands, temperature extremes, and organ failure. To maintain adequate autoregulation, the small vessels and arterioles of the eye are under constant control of the vascular endothelial cells which release vasoactive molecules in response to physiologic changes (Citation1). Nitric oxide, which induces vasodilation of vessels, remains among the most potent vasoactive molecules released by the endothelial cells. Conversely, endothelin-1 and other vasoactive molecules induce vasoconstriction of ocular vessels in response to other physiologic conditions. In addition, circulating hormones can induce varying degrees of vasoconstriction and vasodilation.
Although vasoactive molecules may affect retinal vessels, they have little effect on retinal circulation because of the impenetrability of the blood-retinal barrier, which prevents circulating hormones in the blood from having direct access to the retinal microvascular system (Citation1,Citation3). However, circulating hormones often enter the choroid and diffuse into the optic nerve head, causing varying degrees of microvascular changes (Citation1). Any failure of autoregulation to maintain adequate blood flow ultimately results in a reduction in oxygen to peripheral tissues, leading to ischemia and tissue infarction.
Hypertension
Elevated blood pressure results in wide-spread arteriolar vasoconstriction and retinal-arteriolar narrowing which yield many pathophysiological changes within the eye. In the early stages of hypertension, arteriolar narrowing leads to opacification of arteriolar surfaces (leading to increased arteriolar light reflex or ‘copper wiring’) and sclerotic thickening of retinal arterioles and compression of underlying retinal veins, resulting in the phenomenon referred to as ‘arteriovenous nicking’ (AV nicking). As blood pressure progressively increases, the blood-retinal barrier becomes compromised. This leads to leakage of blood and lipids into ocular vessel walls resulting in ‘dot’ and ‘flame-shaped’ hemorrhages (from blood) and hard exudates (from lipid deposition) (Citation4). In very rare instances, macular edema also develops. ‘Cotton wool’ spots then arise as a result of ischemic changes within the optic nerve fiber layers. Besides hypertensive retinopathy, chronic hypertension may result in complications such as central or branch retinal vein occlusion, arterial macroaneurysms, hypertensive choroidopathy (as seen in pre-eclampsia), and hypertensive optic neuropathy (non-arteritic ischemic optic neuropathy, papilledema) (Citation4). The maintenance of target blood pressure remains the corner-stone of preventing and reducing long-term complications from these conditions.
Central retinal vein occlusion (CRVO) describes the condition in which the central retinal vein is obstructed. CRVO tends to arise with hypertension, diabetes, arteriosclerosis, and glaucoma (Citation5). Occlusion of the retinal vein typically results in accumulation of fluid and swelling within the retina, leading to abnormal retinal functioning and impaired vision (). Branch retinal vein occlusion (BRVO) occurs when one of the branches of the main vein is occluded. BRVO may arise with hypertension and may present similarly to CRVO.
Figure 1. Characteristic diffuse flame-shaped hemorrhages and edema in the posterior pole in a patient with central retinal vein occlusion.
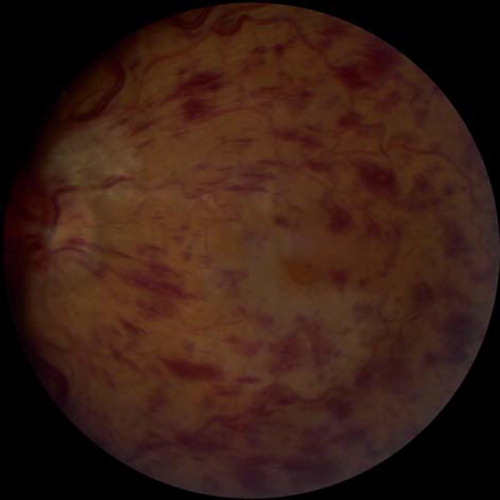
Clinical signs and symptoms. Depending upon the severity of venous obstruction, patients with CRVO can present with any of the following clinical findings: acute, unilateral painless visual loss; blurred vision; ‘floaters’ or flashes obstructing their visual field; and afferent pupil defect (Citation6). Fundoscopic examination typically reveals engorged and tortuous retinal vessels, multiple retinal hemorrhages, optic disc swelling, and retinal edema.
Management. Reducing edema and associated hemorrhage is critical for preserving retinal function in CRVO and BRVO. Intravitreal injection of corticosteroids and anti-vascular endothelial growth factor (VEGF) agents remains effective in reducing edema, prevention of neovascular glaucoma, and improving visual acuity in patients with CRVO (Citation4,Citation6). Laser photocoagulation may be effective in minimizing complications associated with neovascular glaucoma (Citation4). Many patients with CRVO experience permanent visual deficits. Thus, timely referral to an ophthalmologist may limit visual impairment.
Optic neuropathy describes the condition that arises from ischemic degeneration of the optic nerve following occlusion of the ciliary arteries that supply the nerve (Citation7). Since the optic nerve houses retinal ganglion axons, ischemic changes leading to the destruction of ganglion cells can result in irreversible visual loss. Patients with pre-existing hypertension, diabetes, and hyperlipidemia are at increased risk of developing optic neuropathy (Citation7).
Clinical signs and symptoms. Subjects experience sudden to gradual painless unilateral visual loss or blurred vision, depending on the pathology. Ophthalmoscopically, the optic nerve head may appear edematous and pale. In severe forms of the condition, ischemia of the retinal nerve fiber layer may be present and cotton wool spots detected.
Management. Corticosteroid therapy appears to improve outcomes in ischemic neuropathy not associated with mass lesions or trauma. Treatment of non-arteritic anterior ischemic optic neuropathy (NAAION) with steroids is controversial, but some studies have shown a beneficial effect (Citation7). The visual prognosis is poor in patients with optic neuropathy, therefore prompt referral to an ophthalmologist can improve outcomes.
Hypertensive retinopathy describes the microvascular retinal alterations that arise with hypertension. Persistently elevated systemic blood pressure results in thickening of ocular arterioles, necrosis of the retinal-arterial barrier, extravasation of plasma into the vessel wall, and the cascade of pathophysiological changes described above (Citation6).
Clinical signs and symptoms. Similar to hypertension, the infamous ‘silent killer’, hypertensive retinopathy typically occurs in the absence of overt clinical symptoms. Fundoscopic examination often reveals blood vessel narrowing, cotton wool spots, retinal exudates, macular edema, optic nerve swelling, and retinal hemorrhages (Citation4).
Management. Aggressive blood pressure control remains the primary management strategy in hypertensive retinopathy. With adequate blood pressure control, the clinical signs are partially reversible. In instances where vision loss occurs secondary to macular edema, intravitreal injection of corticosteroids or anti-VEGF agents can decrease edema and improve visual prognosis. Focal laser surgical treatment to the leaking blood vessels may help reduce the macular edema as well. The presence of hypertensive retinopathy appears to place individuals at increased risk for developing stroke (Citation8,Citation9), cognitive decline (Citation4), cerebral white matter lesions (Citation10), lacunar infarction (Citation11), cerebral atrophy (Citation12), and stroke mortality (Citation13,Citation14) when compared to controls without hypertensive retinopathy. Further, the presence of hypertensive retinopathy can also be a predictor of underlying or impending kidney failure (Citation15) and cardiac dysfunction (Citation16–18). Thus, aggressive blood pressure control via close management and regular follow-up represents the cardinal method of reducing the incidence of hypertensive retinopathy and its associated complications.
Macular edema
Clinical signs and symptoms. Subjects experience blurred central vision and photosensitivity.
Management. Management of macular edema (ME) typically differs according to the underlying cause. In instances where ME arises secondary to ocular trauma or ocular surgery, anti-inflammatory eye drops or ointments are the typical course of treatment. In instances of diabetes-induced ME, appropriate blood glucose control and laser photocoagulation surgery to seal leaking vessels represent effective therapeutic interventions (Citation18). In recent years, the use of intraocular injection of corticosteroids or anti-VEGF agents has grown in popularity as an effective treatment for macular edema. Any patient suspected of having ME should be referred to an ophthalmologist immediately to rule out more serious, vision-threatening conditions.
Subconjunctival hemorrhage. The increased force and velocity of circulating blood in hypertensive patients places undue stress upon vessels, leading to rupturing and leakage of blood. This is particularly true in patients with malignant hypertension and co-morbidities, which are precursors to neovascularization and retinal hemorrhages. Innocuous subconjunctival hemorrhages can occur after Valsalva maneuver, during sleep, or with minimal exertion.
Clinical signs and symptoms. Subjects experience painless, ‘bloodshot’ eyes (hyperemia).
Management. Subconjunctival hemorrhages are typically self-limited; a first episode may indicate a stand-alone incident. However, in the case of recurrent episodes, the primary care physician should perform thorough history and physical examinations to rule out underlying bleeding disorders and hypertension. Patients should also be advised to avoid aspirin, warfarin, or other anti-clotting agents while symptoms persist. Patients receiving warfarin who have chronic conditions in which they cannot stop anticoagulation medications should have international normalized ratio (INR) monitored regularly to ensure therapeutic values.
Carotid artery disease
Atherosclerosis resulting in severe occlusion or stenosis of the carotid artery often results in ocular hypoperfusion. As the ophthalmic artery is the first branch of the carotid artery and the primary arterial source of the eye, a hemodynamically significant internal carotid artery occlusion can significantly affect the blood supply to the eye, leading to ischemia and improper eye function. If carotid artery disease is suspected, non-invasive diagnostic modalities—including carotid ultrasound and magnetic resonance angiograph scan (MRA)—should be considered to determine vessel wall stenosis and plaque morphology. The following ocular disorders may arise in the setting of carotid artery disease.
Amaurosis fugax, also known as transient unilateral visual loss, is the most common ocular complication of carotid artery disease. Amaurosis fugax typically arises when an atherosclerotic plaque breaks free from the carotid artery and lodges in the retinal artery causing retinal hypoperfusion and temporary vision loss (Citation19). Patients with pre-existing hypertension, coronary artery disease, hyperlipidemia, and other cardiovascular risk factors appear to be at increased risk of developing atherosclerotic lesions within the carotid.
Clinical signs and symptoms. Subjects experience sudden, painless monocular blindness or blurred vision that is partial or complete. The symptoms typically last from 5 to 30 minutes before spontaneously resolving (Citation19).
Management. Patients must undergo a comprehensive eye examination to rule out more serious conditions, such as carotid dissection, retinal ischemia, transient ischemic attack, or ocular ischemic syndrome. Patients suspected of amaurosis fugax should undergo a comprehensive cardiovascular and neurological examination, including a carotid ultrasound and MRA scan to determine the severity of carotid occlusion. In instances where carotid stenosis is 70% or greater, surgical endarterectomy, stenting, or balloon angioplasty of the stenotic region should be considered to prevent recurrent symptoms or stroke (Citation18). Given its benefits in patients at risk for stroke, daily aspirin therapy may also be warranted in eligible patients. Comprehensive laboratory evaluation, including testing of glucose and cholesterol levels, may also provide valuable insight into the patient's current and future risk for atherosclerosis.
Ocular ischemic syndrome (OIS) is most commonly encountered in elderly patients (Citation20), with the majority of reported cases occurring in those with carotid artery stenosis exceeding 90% or greater (Citation21). Serious vision-threatening complications associated with OIS include cataract formation, maculopathy, corneal decompensation, neovascularization, and neovascular glaucoma (Citation22).
Clinical signs and symptoms. Subjects experience gradual loss of vision occasionally accompanied by ocular pain. In the rare instance that OIS occurs in the setting of transient ischemic attack (TIA), patients may complain of weakness, paresthesias, or temporary aphasia.
Management. In cases of OIS arising secondary to carotid occlusion, early treatment of the affected areas is vital to preserve vision and minimize the risk of potentially fatal cerebral vascular events (Citation21). Although carotid stenting may be effective in such patients, carotid endarterectomy is commonly used to remove stenotic areas of the carotid artery, especially in cases where stenosis is less than 99% (Citation22,Citation23). Overall, among OIS patients undergoing carotid endarterectomy, approximately one-third of patients improve, one-third stay stable, and one-third worsen (Citation22). By improving arterial perfusion and ocular circulation, carotid artery stenting and endarterectomy appear to also improve ocular manifestations of OIS (Citation22). In OIS arising secondary to retinal artery occlusion, therapies focused on dislodging emboli, such as digital massage or intravenous acetazolamide, may provide relief of symptoms (Citation22).
Central retinal artery occlusion (CRAO) is a serious ophthalmologic emergency characterized by severe, sudden, painless monocular decrease in vision. Thromboembolic occlusion of the retinal artery in the setting of carotid artery disease is primarily responsible for CRAO. Despite recent advances in the understanding of CRAO, the prognosis remains poor. Patients with CRAO appear to have a 56% mortality rate over 9 years, along with a shorter life expectancy when compared to non-CRAO controls (Citation24). In addition to hypertension, hyperlipidemia, diabetes, hypercoagulopathies, and smoking appear to predispose individuals to the development of CRAO.
Clinical signs and symptoms. Subjects experience sudden, profound, and painless loss of vision.
Management. To date, no standardized therapy has proven effective in the treatment of CRAO (Citation25,Citation26). Treatments meant to dislodge retinal emboli, such as ocular massage, physical exercise, and reduction of intraocular pressure (IOP), have been suggested for the treatment of CRAO; however, none of these modalities has been proven effective according to randomized studies (Citation25).
Restoring retinal blood flow rapidly after onset of CRAO is key to reducing retinal ischemia and preserving vision. Although recent studies have recommended the use of thrombolytic therapy in the treatment of CRAO, randomized, controlled trials are needed to validate this treatment as a standard of care. Patients who are at risk for developing CRAO should be advised to avoid smoking, as cigarette use increases the incidence of retinal emboli in patients with pre-existing cardiovascular disease (Citation8). In the Blue Mountains Eye Study, patients who smoked were 2.6 times more likely to develop emboli when compared to control subjects who did not smoke (Citation27).
Pulmonary disorders
Sleep apnea
Obstructive sleep apnea/hypopnea syndrome (OSAHS) is characterized by nocturnal airway obstruction resulting in cessation of breathing (apnea), loud snoring, and multiple arousals from sleep. Patients with OSAHS are at increased risk of ocular complications including primary open-angle glaucoma (POAG), non-arteritic anterior ischemic optic neuropathy (NAAION), idiopathic intracranial hypertension (IIH), and floppy eyelid syndrome (Citation28). Early screening and adequate treatment of underlying OSAHS can reduce long-term ocular complications associated with OSAHS.
Primary open-angle glaucoma. Glaucoma describes the group of eye diseases characterized by visual impairment due to progressive damage to the optic nerve (Citation29). The optic nerve, which is primarily responsible for transmittal of visual data to the brain, is incapable of regeneration. Thus, vision loss in glaucoma is irreversible because it arises secondary to optic nerve damage. Over 90% of reported cases of glaucoma in the US are of the primary open-angle glaucoma (POAG) subset, which is usually correlated with increased intraocular pressure (IOP) (Citation30). POAG's asymptomatic course has made early detection a challenge to clinicians, and it is described as a ‘silent thief of sight’, robbing an individual of vision without warning. Although it is the second most common cause of blindness in the United States, glaucoma remains the leading cause of blindness among African-Americans (Citation31). Further, approximately 5.9%–12.9% of individuals with OSAHS also have POAG (Citation32,Citation33).
Clinical signs and symptoms. Subjects are typically asymptomatic with gradual loss of the peripheral vision culminating in ‘tunnel’ vision in later stages.
Management. Given the irreversible nature of vision loss in POAG, the condition remains incurable. However, early recognition and treatment can help prevent further visual loss. In addition to screening, at-risk individuals such as African-Americans, those with family history of glaucoma, and those over 65 years of age should undergo annual comprehensive ophthalmic examinations. These exams typically include a thorough evaluation of the optic nerve, monitoring of IOP, and a corneal assessment (Citation34). In addition to regularly scheduled ophthalmic examinations, individuals with diagnosed POAG also benefit from IOP-lowering eye drops, including prostaglandin analogs, alpha-adrenergic agonists, beta-adrenergic antagonists, and carbonic anhydrase inhibitors.
Non-arteritic anterior ischemic optic neuropathy (NAAION) is the disorder in which hypoperfusion of the posterior ciliary artery results in optic nerve ischemia and irreversible blindness. NAAION is a subset of ischemic optic neuropathy disorders and can arise with the onset of cardiac or pulmonary compromise. The elderly, patients with cardiovascular risk factors, those with decreased central retinal artery pressure, and those predisposed to nocturnal hypotension appear to be at greatest risk of developing NAAION (Citation35,Citation36). Although a recent study suggested that as many 70% of patients with OSAHS may have NAAION, larger, long-term cohort studies are required to validate this association (Citation28,Citation37).
Clinical signs and symptoms. NAAION characteristically results in sudden loss of vision in the involved eye, with the lower hemifield of vision most commonly effected (Citation28).
Management. Numerous therapies have been promoted for the treatment of NAAION, including oral corticosteroids, aspirin, and intravitreal injection of triamcinolone acetonide or bevacizumab (Citation36). Addressing underlying cardiopulmonary conditions, thus reducing risk factors, remains a central component to reducing the complications of NAAION (Citation35).
Idiopathic intracranial hypertension (IIH) (also known as benign intracranial hypertension or pseudotumor cerebri) is a disorder where intracranial pressure (ICP) is increased in the absence of an acute cerebral process. The diagnosis of IIH hinges upon normal neuro-imaging and cerebrospinal fluid composition in the setting of increased ‘opening pressure’ during lumbar puncture (Citation28). Women and obese individuals appear to be at greatest risk for developing IIH (Citation38).
Clinical signs and symptoms. Patients with IIH often exhibit swelling of the optic disc or papilledema though a physical exam. Typically, patients complain of daily headaches, nausea, transient loss of vision, blurred vision, double vision (e.g. diplopia), and tinnitus (Citation28,Citation38).
Management. Since IIH is a diagnosis of exclusion, a thorough history and physical examination as well as neuro-imaging are needed to rule out other causes of increased ICP. Such causes may include intracranial mass, infections (e.g. encephalitis, meningitis), aneurysm, or head injury (Citation39). After diagnosis, the two main goals are to relieve headaches and to prevent visual loss through the use of acetazolamide or thiazides, weight loss, regular perimetry, and even neurosurgery.
Floppy eyelid syndrome (FES), or lax eye syndrome, is a rare disorder where the upper eyelids become highly elastic and easily distortable. Specifically, the ‘floppiness’ of the eyelids exposes the ocular surface to external threats and may lead to chronic irritation of the ocular surface and other structures (Citation28,Citation40). Patients with OSAHS appear to be at increased risk for FES and vice versa (Citation41,Citation42).
Clinical signs and symptoms. Individuals with FES often seek medical attention for ocular irritation that responds poorly to medical treatments. Characteristic findings in FES arise from exposure of the ocular surface to external stimuli and may include dry eyes, foreign body sensation, ocular pruritus, blurred vision, ocular discharge, ptosis, ectropion, dystopia, corneal ulcer, and unilateral conjunctivitis (Citation28,Citation40).
Management. Supportive measures such as nocturnal eye shields and daily lubricant drops can significantly improve symptoms associated with FES. Severe forms of the condition may require surgical correction in the form of eyelid tightening or removal of redundant eyelid tissues along with strict blood pressure control (Citation43,Citation44).
Endocrine disorders
Common endocrine disorders including diabetes mellitus, hypothyroidism, and hyperthyroidism may present with potentially serious ocular manifestations. The following highlights the most common ophthalmic manifestations associated with endocrine disorders.
Diabetes mellitus
Diabetes mellitus (DM) is a severe, chronic, condition affecting both pediatric and adult populations. In patients with DM, the sustained hyperglycemia in small vessels leads to microvascular complications in the eyes and other organs. As many as 37% of diabetics will exhibit microvascular complications (Citation45). Approximately 200 million people worldwide are affected by DM, with the estimates projecting as many as 300 million diabetics by the year 2025 (Citation46). Diabetics appear to be at increased risk for developing a number of ocular conditions including cataracts, diabetic retinopathy, diabetic macular edema, tractional retinal detachment, and cranial nerve palsies. Strict glycemic control reflected in consistently appropriate glycosylated hemoglobin (HbA1c) levels remains critical to preventing these and other microvascular complications in diabetics. Given the many ocular morbidities associated with diabetes, the Preferred Practice Pattern Guidelines of the American Academy of Ophthalmology (AAO) recommend regular consultation with ophthalmologists for any diabetic patients who fall into the following categories (Citation47):
1) Individuals with type I DM aged 10–30 years: The AAO recommends an initial dilated and comprehensive eye examination within 5 years of DM onset, with annual comprehensive ophthalmic examinations thereafter.
2) Type II DM patients: Patients are encouraged to schedule a dilated and comprehensive ophthalmic examination at the time of diagnosis, with annual eye examinations thereafter.
3) Diabetic females: Women with DM should be informed about the risk of developing or exacerbating diabetic retinopathy (DR) during pregnancy. Pregnant women with DM are encouraged to have a comprehensive eye examination in the first trimester, with regular follow-up exams thereafter.
Diabetic retinopathy (DR) remains the most common ocular manifestation of diabetic microvascular disease. It is a condition in which hyperglycemia may lead to a malfunction in the blood-retinal barrier (mainly in the peripheral ischemia), resulting in increased vessel permeability. This chain of events may promote neovascularization. The increased retinal vascular permeability that occurs from chronic hyperglycemia contributes to leakage from blood vessels and swelling of the macula, which is the region within the central retina responsible for visual acuity. This process describes diabetic macular edema (DME), a vision-threatening phenomenon that typically accompanies DR.
The poor glycemic control and the length of time the patient has had diabetes appear to be the most important risk factors for developing DR.
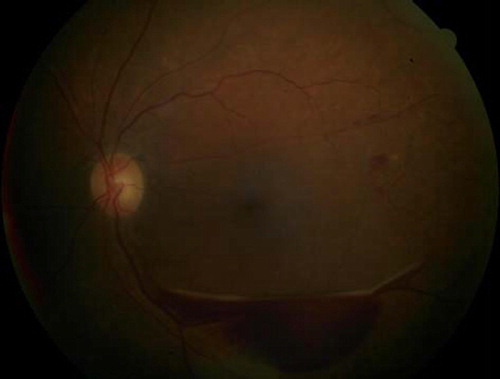
Clinical signs and symptoms. DR is characteristically asymptomatic except in severe stages when macular edema or neovascularization results in impaired vision. Diabetic macular edema is the most common cause of vision loss in DR (, ). Findings depend on the stage of the disease and include hemorrhages, exudates, and cotton wool spots. Tractional retinal detachment, rubeosis irides, and vitreous hemorrhage are signs of advanced disease. Neovascularization either of the disc (NVD) or of the retina (NVE) are signs of proliferative DR (, ).
Figure 2. Fundus photograph demonstrating accumulation of lipid exudates in a patient with clinically significant diabetic macular edema.
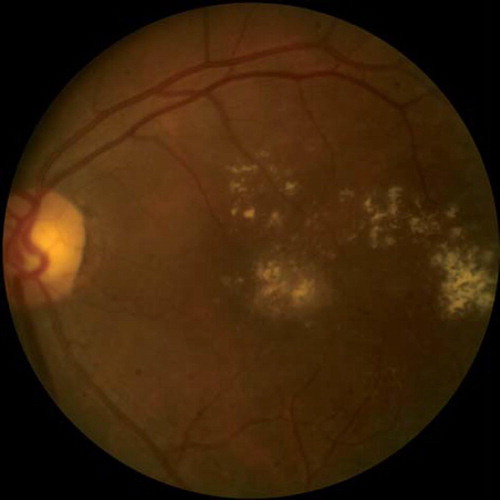
Figure 3. Fundus photograph demonstrating accumulation of lipid exudates in a patient with clinically significant diabetic macular edema.
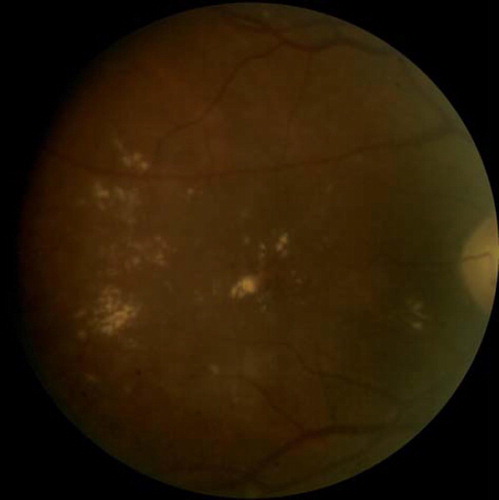
Figure 4. Tractional retinal detachment secondary to proliferative diabetic retinopathy. Note presence of neovascularization of the disc as well as elsewhere.
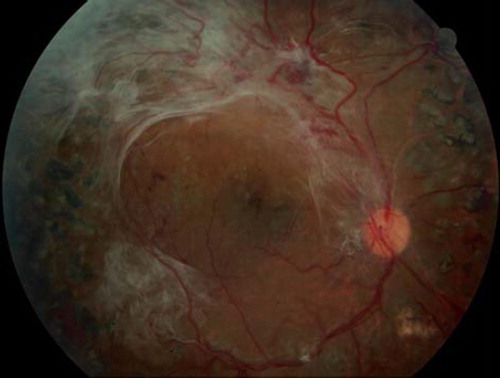
Figure 5. Well circumscribed sub-hyaloid hemorrhage in a patient with proliferative diabetic retinopathy.
Management. Annual comprehensive eye examinations are critical for diabetics, particularly because signs and symptoms of DR are clinically recognizable early in the course of the disease (Citation46). Because poor glycemic control accelerates the progression of DR, comprehensive diabetes management combined with regularly scheduled eye examinations can prevent vision loss. Retinal laser photocoagulation is the standard management of more advanced DR and DME, and is effective at preventing further visual loss. Thus, patients with DR should be followed regularly to evaluate disease severity and to provide intervention before vision loss occurs.
Cataracts. A cataract is the painless, progressive opacification of the lens of the eye. In most cases cataracts develop in both eyes. The accumulation of sorbitol in the crystalline lens of the eye predisposes diabetics to developing cataracts. In fact, diabetics are at approximately 60% increased risk for developing cataracts over the course of their lives when compared to the general population.
Clinical signs and symptoms. Common symptoms of cataracts include ‘cloudy’ or blurred vision, difficulty seeing in dim light (or in the evening), and light sensitivity (e.g. photophobia).
Management. Although improved lighting and stronger lenses may diminish the impact of cataracts early in their course, as the opacification progresses, cataract extraction and intraocular lens implantation is oftentimes necessary. Cataract surgery is highly successful; over 90% of patients report improved visual function, with 20/40 visual acuity or better after surgery (Citation48).
Cranial nerve palsies and the ‘diabetic pupil’. Sustained hyperglycemia can compromise vascular supply to extraocular muscles, resulting in cranial nerve palsies and other neuro-ophthalmic sequelae (Citation48). These microvascular insults may lead to palsies of cranial nerves (CN) III, IV, and VI, and cause ptosis, deficits in abduction, reduced motility, and other symptoms. Although the vasculopathic palsies that accompany DM generally spare the pupil, patients often develop the ‘diabetic pupil’, which is characterized by a poorly constricting tonic pupil. This occurs secondarily to microvascular damage to the parasympathetic nerves in the ciliary ganglion. The impaired pupil in individuals with the ‘diabetic pupil’ may result in compromised accommodation leading to poor visual acuity. As with DR, the length of time the patient has had diabetes corresponds with the likelihood of developing cranial nerve palsies (Citation48).
Clinical signs and symptoms. Depending on where the insult occurs, patients may exhibit diplopia (CN III, IV, or VI palsy), impaired ipsilateral abduction (CN VI palsy), impaired ipsilateral adduction (CN III palsy), or ptosis (CN III palsy).
Management. Although most vasculopathic palsies in DM resolve within 4–6 weeks (Citation48), a thorough neuro-ophthalmic evaluation is encouraged to rule out more serious causes of palsies, including intracranial masses or potentially serious neuro-ophthalmic conditions.
Thyroid eye disease
Thyroid eye disease (TED), or Graves’ ophthalmopathy, refers to the constellation of orbital and periorbital findings that accompany dysthyroid states. Although it is generally associated with hyperthyroidism, TED can happen with euthyroidism or even hypothyroidism. Diagnosis requires radiographic evidence (by orbital CT scan or ultrasound) of extraocular muscle enlargement, serologic evidence of dysthyroid state, and gross orbital signs (Citation48). The most common ocular signs encountered in TED include eyelid retraction, proptosis, strabismus, periorbital edema, extraocular muscle hypertrophy (), and extraocular fat hypertrophy (Citation48,Citation49) ( and ). If TED continues to worsen, it can lead to compression of the optic nerve and permanent vision loss.
Figure 6. Bilaterally enlargement of extraocular muscles, especially in the inferior and medial rectus muscles, in a patient with thyroid eye disease.
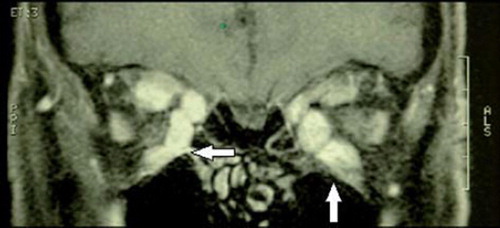
Figure 7. A patient with thyroid eye disease exhibiting prominent exophthalmos that is greater in the right eye, with significant restriction of right eye movement.
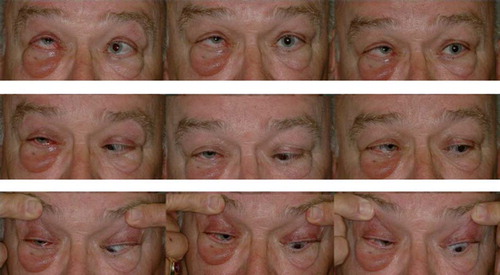
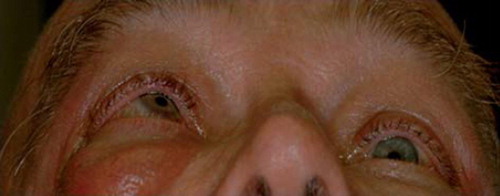
Figure 8. A patient with thyroid eye disease exhibiting prominent exophthalmos that is greater in the right eye with significant restriction of right eye movement.
Management. The primary management strategy in patients with TED is regular serologic and clinical follow-up for the underlying thyroid dysfunction. It is important to test not only thyroid-related hormones but also anti-thyroglobulin antibodies.
Additionally, since the hypertrophied ocular structures in TED can compress and damage the optic nerve, all patients with TED should be followed by an ophthalmologist. Although corticosteroids and external beam radiotherapy can alleviate symptoms associated with mild to moderate orbital edema, orbital decompression surgery is the immediate, permanent solution to acute optic neuropathy in patients with TED (Citation49). Since cigarette smoking is an important risk factor for progression of TED, patients should be regularly counseled on the importance of smoking cessation. Finally, aggressive treatment with lubricant eye drops is encouraged in patients with TED to minimize damage to the ocular surface secondary to exposure.
Conclusion
The ocular manifestations of several major systemic conditions have been described with their key diagnostic and prognostic features. As illustrated in the examples above, communication between the primary care physician and the ophthalmologist is key to identifying these manifestations to provide appropriate, timely treatment. The second article in this series will explore additional ocular conditions related to common systemic diseases.
Declaration of interest: The authors report no financial or any other conflict of interest related to the contents of the material within this article. Funding: none.
References
- Flammer J, Mozaffarieh M. Autoregulation, a balancing act between supply and demand. Can J Ophthalmol. 2008;43: 317–21.
- Flammer J, Orgül S, Costa VP, Orzalesi N, Krieglstein GK, Serra LM, . The impact of ocular blood flow in glaucoma. Prog Retin Eye Res. 2002;21:359–93.
- Gherghel D, Hosking SL, Orgül S. Autonomic nervous system, circadian rhythms, and primary open-angle glaucoma. Surv Ophthalmol. 2004;49:491–508.
- Wong TY, Mitchell P. The eye in hypertension. Lancet. 2007;369:425–35.
- Karia N. Retinal vein occlusion: pathophysiology and treatment options. Clin Ophthalmol. 2010;4:809–16.
- Bressler NM, Schachat AP. Management of macular edema from retinal vein occlusions: you can never have too many choices. Ophthalmology. 2010;117:1061–3.
- Atkins EJ, Bruce BB, Newman NJ, Biousse V. Treatment of nonarteritic anterior ischemic optic neuropathy. Surv Ophthalmol. 2010;55:47–63.
- Klein R, Klein BE, Moss SE, Meuer SM. Retinal emboli and cardiovascular disease: the Beaver Dam Eye Study. Arch Ophthalmol. 2003;121:1446–51.
- Yatsuya H, Folsom AR, Wong TY, Klein R, Klein BE, Sharrett AR; ARIC Study Investigators. Retinal microvascular abnormalities and risk of lacunar stroke: Atherosclerosis Risk in Communities Study. Stroke. 2010;41:1349–55.
- Wong TY, Klein R, Sharrett AR, Couper DJ, Klein BE, Liao DP, . Cerebral white matter lesions, retinopathy, and incident clinical stroke. JAMA. 2002;288:67–74.
- Cheung N, Mosley T, Islam A, Kawasaki R, Sharrett AR, Klein R, . Retinal microvascular abnormalities and subclinical magnetic resonance imaging brain infarct: a prospective study. Brain. 2010;133:1987–93.
- Kawasaki R, Cheung N, Mosley T, Islam AF, Sharrett AR, Klein R, . Retinal microvascular signs and 10-year risk of cerebral atrophy: The Atherosclerosis Risk in Communities (ARIC) Study. Stroke. 2010;41:1826–8.
- Wong TY, Klein R, Nieto FJ, Klein BE, Sharrett AR, Meuer SM, . Retinal microvascular abnormalities and 10-year cardiovascular mortality: a population-based case-control study. Ophthalmology. 2003;110:933–40.
- Mitchell P, Wang JJ, Wong TY, Smith W, Klein R, Leeder SR. Retinal microvascular signs and risk of stroke and stroke mortality. Neurology. 2005;65:1005–9.
- Wong TY, Coresh J, Klein R, Muntner P, Couper DJ, Sharrett AR, . Retinal microvascular abnormalities and renal dysfunction: the atherosclerosis risk in communities study. J Am Soc Nephrol. 2004;15:2469–76.
- Saitoh M, Matsuo K, Nomoto S, Kondoh T, Yanagawa T, Katoh Y, . Relationship between left ventricular hypertrophy and renal and retinal damage in untreated patients with essential hypertension. Intern Med. 1998;37:576–80.
- Blumenkranz MS, Haller JA, Kuppermann BD, Williams GA, Ip M, Davis M, . Correlation of visual acuity and macular thickness measured by optical coherence tomography in patients with persistent macular edema. Retina. 2010;30:1090–4.
- Karim R, Tang B. Use of antivascular endothelial growth factor for diabetic macular edema. Clin Ophthalmol. 2010;4:493–517.
- Cohen R, Padilla J, Light D, Diller R. Carotid artery occlusive disease and ocular manifestations: Importance of identifying patients at risk. Optometry. 2010;81:359–63.
- Takaki Y, Nagata M, Shinoda K, Tatewaki S, Yamada K, Matsumoto CS, . Severe acute ocular ischemia associated with spontaneous internal carotid artery dissection. Int Ophthalmol. 2008;28:447–9.
- Kawaguchi S, Sakaki T, Iwahashi H, Fujimoto K, Iida J, Mishima H, . Effect of carotid artery stenting on ocular circulation and chronic ocular ischemic syndrome. Cerebrovasc Dis. 2006;22:402–8.
- Hazin R, Daoud YJ, Khan F. Ocular ischemic syndrome: recent trends in medical management. Curr Opin Ophthalmol. 2009;20:430–3.
- Mazlumzadeh M, Hunder GG, Easley KA, Calamia KT, Matteson EL, Griffing WL, . Treatment of giant cell arteritis using induction therapy with high-dose glucocorticoids: a double-blind, placebo-controlled, randomized prospective clinical trial. Arthritis Rheum. 2006;54:3310–8.
- Wang JJ, Cugati S, Knudtson MD, Rochtchina E, Klein R, Klein BE, . Retinal arteriolar emboli and long-term mortality: pooled data analysis from two older populations. Stroke. 2006;37:1833–6.
- Hazin R, Dixon JA, Bhatti MT. Thrombolytic therapy in central retinal artery occlusion: cutting edge therapy, standard of care therapy, or impractical therapy? Curr Opin Ophthalmol. 2009;20:210–8.
- Biousse V. Thrombolysis for acute central retinal artery occlusion: is it time? Am J Ophthalmol. 2008;146: 631–4.
- Mitchell P, Wang JJ, Li W, Leeder SR, Smith W. Prevalence of asymptomatic retinal emboli. Stroke. 1997;28:63–6.
- Hazin R, Abuzetun JY, Khan MF, Bhatti MT. Ocular health and sleep apnea: A comprehensive overview. Neuroophthalmology. 2008;32:127–36.
- Pasquale LR, Kang JH. Lifestyle, nutrition, and glaucoma. J Glaucoma. 2009;18:423–8.
- Primary Open-Angle Glaucoma; Limited Revision (Preferred Practice Pattern). San Francisco: American Academy of Ophthalmology; 2003.
- Racette L, Liebmann JM, Girkin CA, Zangwill LM, Jain S, Becerra LM, . African Descent and Glaucoma Evaluation Study (ADAGES): III. Ancestry differences in visual function in healthy eyes. Arch Ophthalmol. 2010;128: 551–9.
- Sergi M, Salerno DE, Rizzi M, Blini M, Andreoli A, Messenio D, . Prevalence of normal tension glaucoma in obstructive sleep apnea syndrome patients. J Glaucoma. 2007;16:42–6.
- Sharma T, Salmon JF. Ten-year outcomes in newly diagnosed glaucoma patients: mortality and visual function. Br J Ophthalmol. 2007;91:1282–4.
- Kowing D, Messer D, Slagle S, Wasik A; V-POAG Study Group. Programs to optimize adherence in glaucoma. Optometry. 2010;81:339–50.
- Hayreh SS, Zimmerman MB. Nonarteritic anterior ischemic optic neuropathy: refractive error and its relationship to cup/disc ratio. Ophthalmology. 2008;115:2275–81.
- Hayreh SS, Podhajsky PA, Zimmerman B. Ipsilateral recurrence of nonarteritic anterior ischemic optic neuropathy. Am J Ophthalmol. 2001;132:734–42.
- Behbehani R, Mathews MK, Sergott RC, Savino PJ. Nonarteritic anterior ischemic optic neuropathy in patients with sleep apnea while being treated with continuous positive airway pressure. Am J Ophthalmol. 2005;139:518–21.
- Fraser JA, Bruce BB, Rucker J, Fraser LA, Atkins EJ, Newman NJ, . Risk factors for idiopathic intracranial hypertension in men: a case-control study. J Neurol Sci. 2010;290:86–9.
- Wall M. Idiopathic intracranial hypertension. Neurol Clin. 2010;28:593–617.
- Ezra DG, Beaconsfield M, Sira M, Bunce C, Wormald R, Collin R. The associations of floppy eyelid syndrome: a case control study. Ophthalmology. 2010;117:831–8.
- McNab AA. Floppy eyelid syndrome and obstructive sleep apnea. Ophthal Plast Reconstr Surg. 1997;13:98–114.
- Karger RA, White WA, Park WC, Rosales AG, McLaren JW, Olson EJ, . Prevalence of floppy eyelid syndrome in obstructive sleep apnea-hypopnea syndrome. Ophthalmology. 2006;113:1669–74.
- Ezra DG, Beaconsfield M, Sira M, Bunce C, Shah-Desai S, Verity DH, . Long-term outcomes of surgical approaches to the treatment of floppy eyelid syndrome. Ophthalmology. 2010;117:839–46.
- Mills DM, Meyer DR, Harrison AR. Floppy eyelid syndrome: Quantifying the effect of horizontal tightening on upper eyelid position. Ophthalmology. 2007;114: 1932–6.
- Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA, . Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000; 321:405–12.
- Younis N, Broadbent DM, Vora JP, Harding SP. Incidence of sight-threatening retinopathy in patients with type 2 diabetes in the Liverpool Diabetic Eye Study: a cohort study. Lancet. 2003; 361:195–200.
- American Academy of Ophthalmology Retina Panel. Preferred Practice Pattern Guidelines. Diabetic Retinopathy. San Francisco, CA: American Academy of Ophthalmology; 2008. Available at: http://www.aao.org/ppp. Accessed 21 January 2011.
- Hendrick AM, Kahook MY, Daoud YJ, Hazin R. Ophthalmic manifestations of endocrine disorders: approaches and medical management. Curr Opin Ophthalmol. 2009;20:495–503.
- Phillips ME, Marzban MM, Kathuria SS. Treatment of thyroid eye disease. Curr Treat Options Neurol. 2010;12:64–9.
