Abstract
Background. This cross-sectional study aimed to investigate the relationship between exposure to anticholinergic and sedative medications, measured with the Drug Burden Index (DBI), and functional outcomes in community-dwelling older people living in Finland.
Methods. The study population consisted of community-dwelling older people (n = 700) enrolled in the Geriatric Multidisciplinary Strategy for the Good Care of the Elderly (GeMS) study. Outcomes included walking speed, chair stands test, grip strength, timed up and go (TUG) test, instrumental activities of daily living (IADL), and Barthel Index.
Results. Exposure to DBI drugs was identified in 37% of participants: 24% had a DBI range between >0 <1, and 13% DBI ≥1. After adjusting for confounders, exposure to DBI drugs was associated with slower walking speed (P < 0.0001), poorer performance on chair stands (P = 0.0001) and TUG (P < 0.0001), difficulties in IADL (P < 0.0001), and Barthel Index (P < 0.0001). The mean adjusted walking speed, time to complete chair stands and TUG, IADL, and Barthel scores were significantly poorer among participants with higher DBI ranges.
Conclusion. In older adults living in Finland, DBI was associated with impaired function on previously tested and new outcomes. This finding supports the use of the DBI as tool, in combination with other assessments, to identify older people at risk of functional impairment. The findings highlight the need for revision of current guidelines to improve the quality of drug use in older people.
| Abbreviations | ||
| ANCOVA | = | analyses of covariance |
| ATC | = | anatomical therapeutic chemical |
| BMI | = | body mass index |
| CI | = | confidence intervals |
| DB | = | drug burden |
| DBI | = | drug burden index |
| DR50 | = | daily dose required to achieve 50% of maximal effect |
| GDS | = | geriatric depression scale |
| GeMS | = | geriatric multidisciplinary strategy for the good care of the elderly study |
| FCI | = | functional co-morbidity index |
| IADL | = | instrumental activities of daily living |
| MMSE | = | mini-mental state examination |
| SPPB | = | short physical performance battery |
| TUG | = | timed up and go |
Key messages
This was the first study to investigate the correlation between the Drug Burden Index (DBI) and functional outcomes in community-dwelling older people living in Europe.
In older adults living in Finland, DBI drugs were widely used, and DBI exposure was associated with limitations in clinically important outcomes.
This study supports the use of the DBI, in combination with other assessments, to identify older people at risk of functional impairment.
Introduction
In older adults, polypharmacy and consensus-based lists of potentially inappropriate medications are the most commonly used measures to assess the burden of medication exposure. Polypharmacy has been associated with drug–drug interactions (Citation1), under-prescribing of clinically indicated drugs (Citation2), and functional impairment in older people (Citation3). Consensus-based lists of potentially inappropriate drugs, such as the updated Beers Criteria (Citation4), have demonstrated inconsistent ability to predict outcomes across populations of older people (Citation5). This may be due in part to the applicability of consensus-based lists being limited by differences in drug formularies, prescribing culture, and pharmaceutical regulations across countries. In addition, lists of potentially inappropriate drugs lack accuracy as stand-alone measures of prescribing quality (Citation6). There is a need for a universally accepted prescribing tool to assess the quality of drug prescribing and to define the impact of medication use on clinical outcomes in older adults (Citation7).
The use of anticholinergic and sedative drugs has been linked with adverse events in older adults. Exposure to anticholinergic drugs increases the risk of falls (Citation8), functional impairment (Citation9), and cognitive dysfunction (Citation10). Similarly, sedative drugs have been associated with falls (Citation11) and impairments of physical (Citation12) and cognitive function (Citation13) in older people. The Drug Burden Index (DBI) is a tool that calculates individuals’ exposure to anticholinergic and sedative medications utilizing dose-response and cumulative effect parameters (Citation14). The DBI has been developed to predict functional impairment in older adults. The association of DBI with functional outcomes has been tested in older people living in the community (Citation14–16) and intermediate residential aged care facilities (Citation17). In cross-sectional studies of community-dwelling older adults living in the US (Citation14,Citation16) and Australia (Citation15), higher DBI scores were associated with physical and cognitive functional impairment. For instance, in older people living in self-care retirement villages in Australia, every one unit increase in DBI score was associated with a decrease in Short Physical Performance Battery (SPPB) score by 1.3 (Citation18), which is considered a clinically meaningful change (Citation19). In older Australian people residing in intermediate residential aged care facilities, DBI was not associated with poorer function (Citation17). In a longitudinal study, cumulative DBI load at year 1 predicted functional decline over a 5-year period (Citation20). However, the association between DBI and function in populations of older people living outside the US and Australia with different access to health care and pharmaceuticals and different pharmacogenomics remains unknown. The objective of this cross-sectional study was to investigate the relationship between DBI and functional outcomes in community-dwelling older people living in Finland.
Patients and methods
Study population
This study utilized base-line data from the Geriatric Multidisciplinary Strategy for the Good Care of the Elderly (GeMS) study (Citation21–23). This was a randomized comparative study conducted in the city of Kuopio, eastern Finland, from 2004 to 2007. The objective of the GeMS study was to evaluate a model for geriatric assessment, care, and rehabilitation. The study sample (n = 1,000) was randomly selected from all persons listed in the population register aged ≥75 years (born before 1 November 1928). Of 1,000 randomly selected persons, 781 provided written informed consent to participate (162 persons refused participation, 2 persons had moved residence, and 55 persons died before the scheduled base-line examination). For the purpose of this study, participants who lived in institutional care facilities (n = 81) were excluded because they were likely to have higher anticholinergic and sedative drug use and different predictors of functional disability compared to community-dwelling participants (Citation24,Citation25). A total of 700 participants were included in the analyses. The study was approved by the Research Ethics Committee of the Northern Savo Hospital District, Kuopio, Finland.
Data collection
All participants were invited to visit the out-patient study clinic. Those who attended the clinic were interviewed by a trained nurse. Participants who were unable to attend the clinic were interviewed in their own home. Data on socio-demographic factors (age, sex, and education level), medication use, medical conditions, and functional outcomes were collected.
Medication assessment
Use of prescription and non-prescription medications was self-reported by participants during the interviews. To reduce the possibility of recall error, participants were asked to bring their prescription forms and medication packages to the interviews. Each participant was asked to indicate what medications they used on a regular or as-needed basis over the previous two weeks. The nurse interviewers had access to each participant's medical records from the municipal health centre, home nursing service, local hospitals, and Kuopio University Hospital at the time of the interviews. This meant that the nurses were able to verify each participant's self-reported list of medications by using the medical records as a prompt. The lists of regular and as-needed medications prepared by the nurses were based on each participant's actual patterns of use rather than patterns of prescribed or intended use. Data on the medication name, frequency, and administration pattern (regular or as-needed) were recorded for each participant. Medication use was considered regular if the drug was being taken daily or at regular intervals (once a week or once a month). Medication use was considered as-needed if there was an irregular pattern of use. Medications were categorized according to Anatomical Therapeutic Chemical (ATC) classification system recommended by the World Health Organization (Citation26). Use of regularly scheduled anticholinergic and sedative medications was quantified using the DBI. Total drug burden (DB) was calculated by adding the burden from medications with anticholinergic (BAC) and sedative properties (BS) (Citation14).
DBI for each participant was calculated as the sum of exposure to each medication with clinically significant anticholinergic or sedative effects using Equation 2 below (Citation14,Citation15),
where D represents the daily dose taken by the subject and δ the minimum recommended daily dose registered by the Finnish registered medication product information (Citation27). The DBI represents a hyperbolic function ranging in value from 0 to 1 for each medication depending on the dose. For example, if an individual is exposed to three anticholinergic and/or sedative medications at the minimum recommended daily dose, their DBI will be 1.5. Originally the DBI equation included the dose required to achieve 50% of the maximum of anticholinergic and/or sedative effect (DR50) in place of δ in the denominator (Citation14). However, because DR50 of anticholinergic or sedative effects is not readily identifiable, the minimum recommended daily dose listed in the Finnish registered medication product information was used as an estimate of the DR50. For the purpose of the analyses, medications with both anticholinergic and sedative effects were classified as anticholinergic only. Complementary medications, topical medications, and medications used on an as-needed basis were excluded from the DBI calculations.
Outcome measures
Physical performance was assessed using a standard performance battery. The battery comprised three tests: 1) 10-metre walking speed (metre/second), 2) chair stands, i.e. time (seconds) needed to complete five chair stands, and 3) grip strength (kilograms) measured using a Saehan dynamometer (Saehan Corporation, South Korea), and recorded as the grip strength of the dominant hand (higher of the two strengths). These tests were selected because physical performance is an important marker of functional independence in older adults. The walking speed is a component of frailty syndrome (Citation28) that predicts disability in older adults (Citation29). Grip strength is an important marker of muscle function (Citation30) and is associated with frailty (Citation28), disability, falls, and mortality in older adults (Citation31). For 10-metre walking speed higher scores indicated faster speed. For chair stands lower scores indicated better performance, and for grip strength higher scores indicated better performance. A clinical test of basic functional mobility, timed up and go test (TUG) (seconds), was also administered, with higher score indicating worse performance (Citation32). The TUG test assessed each participant's speed performing several functional manoeuvres which included standing up, walking 3 m, turning, coming back, and sitting down. The TUG clinical test identifies older adults at risk of falls (Citation33). Functional status was assessed using the eight-item self-reported Lawton and Brody Instrumental Activities of Daily Living (IADL) Scale (Citation34), and the ten-item Barthel Index (Citation35), with higher scores indicating better performance on both tests.
Covariates
Rather than adjusting for single diseases we computed a co-morbidity score using a modified Functional Co-morbidity Index (FCI) (Citation36). The FCI is a validated scale that predicts physical function in older people (Citation36). The FCI takes into account the medical conditions associated with function, with a higher score indicating greater co-morbidity. In this study, data on the following medical conditions, either from self-reports or obtained from medical records were available and included in the FCI: arthritis (rheumatoid arthritis and other connective tissue diseases), osteoporosis, diabetes mellitus (type I and II), chronic asthma or chronic obstructive pulmonary disease, angina (coronary artery disease), congestive heart failure or heart disease, myocardial infarction, stroke, depressive symptoms, visual impairment, hearing impairment, Parkinson's disease or multiple sclerosis, and obesity (body mass index (BMI) >30). Patient diagnoses obtained from the Finnish National Prescription and Special Reimbursement Registers maintained by the Social Insurance Institution of Finland (SII) were used to screen for the presence of arthritis (rheumatoid arthritis and other connective tissue diseases), chronic asthma or chronic obstructive pulmonary disease, Parkinson's disease, or multiple sclerosis (Citation37). The presence of depressive symptoms was assessed using the 15-item Geriatric Depression Scale (GDS), with scores ≥5 considered indicative of depressive symptoms (Citation38). Cognitive function was assessed using the Mini-Mental State Exam (MMSE) (Citation39), with scores <25 considered indicative of cognitive impairment (Citation40). Respondents’ self-rated health was determined using a five-point scale (good, very good, moderate, poor and very poor).
Statistical analyses
The characteristics of the sample were summarized using means, medians, standard deviations (SDs), and proportions. The Shapiro–Wilk W test was performed to test the normality of continuous variables, and the Levene test for homogeneity was used to test the equality of variance. In this sample, DBI was tested as a dichotomous (DBI = 0 or DBI >0) and categorical ordinal variable (0, >0 <1, ≥1). The chair stands test and TUG-dependent variables were log10-transformed to increase the normality of their distribution. In these cases, the values presented are the back-transformed. The correlation analysis (Spearman rho coefficient) between the FCI and self-reported health status was performed, with r = 0.34 ruling out the possibility of substantial correlation between the scales (Citation41). Unadjusted and adjusted analyses of covariance (ANCOVA) were performed to compare functional outcomes between participants not exposed to DBI drugs (DBI = 0) with those exposed to DBI drugs (DBI > 0). ANCOVA were used to determine whether there were differences in the adjusted means of 10-m walking speed, chair stands test, grip strength, TUG, IADL, and Barthel Index scores across participants with different DBI subgroups (0, >0 <1, ≥1). Models were adjusted for confounders considered to be clinically relevant including age, sex, education (≤6 or >6 years), FCI (continuous score), MMSE <25 (yes, no), and self-reported health status (good, very good, moderate, poor, and very poor). Data were analysed using SAS software (version 9.2, SAS Institute, Cary, NC, USA). All tests were two-tailed, and P < 0.05 was set as statistically significant.
Results
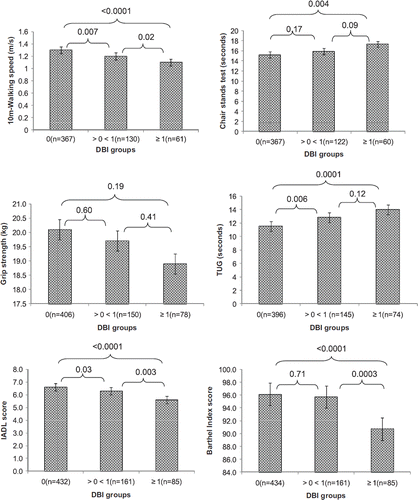
The base-line characteristics of the study sample are described in . The mean age (± SD) of the population was 81.3 ± 4.6 years old, and 69% were female. A total of 257 (37%) participants were exposed to DBI contributing drugs. Of these, 24% had a DBI range between >0 <1, and 13% DBI of ≥1. The mean DBI in the GeMS study was 0.33 ± 0.55. The presence of cognitive decline (MMSE <25) was identified in 25% of the participants. The mean co-morbidity score was 2.5 ± 1.7. The exposure to DBI-contributing drugs is presented in . In this sample, zopiclone (8%), temazepam (5%), and tamsulosin (4%) were the most prevalent DBI drugs reported.
Table I. Base-line study characteristics (n = 700).
Table II. The most common DBI medications used in the GeMS population.
summarizes unadjusted and adjusted models comparing each of the functional outcomes between participants with DBI of zero and participants with DBI greater than zero. On unadjusted models, exposure to DBI drugs was associated with slower 10-m walking speed (P < 0.0001), longer time to complete chair stands test (P < 0.0001), weaker grip strength (P = 0.0003), longer time to complete TUG (P < 0.0001), and lower IADL (P < 0.0001) and Barthel Index scores (P < 0.0001). After adjusting for age, sex, education, comorbidites, self-reported status and cognitive impariment, exposure to DBI drugs was associated with slower 10-m walking speed (P < 0.0001), poorer performance on chair stands test (P = 0.0001), poorer performance on TUG (P < 0.0001), and lower IADL (P < 0.0001) and Barthel Index scores (P = 0.0001), but not with weaker grip strength (P = 0.07).
Table III. Unadjusted and adjusted analysis of covariance for association of DBI with outcomes.
The associations between the functional outcome measures and increasing DBI exposure are presented in . The mean adjusted 10-m walking speed, time taken to complete chair stands, time taken to complete TUG, and IADL and Barthel scores were significantly poorer among participants with DBI ≥1 compared with participants not exposed to DBI drugs and participants with DBI range >0 <1. The mean adjusted grip strength was not significantly different across DBI groups.
Figure 1. Adjusted means of functional outcome measures according to the DBI exposure. 10-m walking speed: higher scores indicated faster speed; chair stands test and TUG: lower score indicated better performance; grip strength, IADL, and Barthel Index: higher score indicating better performance. Models adjusted for age, sex, education, comorbidites, self-reported status and cognitive impariment. The y-axis does not consistently start at 0. Error bars represent standard error. DBI = drug burden index; IADL = instrumental activities of daily living; TUG = timed up and go.
Discussion
This was the first study to investigate the correlation between the DBI and functional outcomes in community-dwelling older people living in Europe. In this population of older adults living in Finland, DBI drugs were widely used, and DBI exposure was significantly and independently associated with a wide range of limitations in function. This was the first study to evaluate the association of DBI with the clinical composite measure of mobility, the TUG. The established association is consistent with other studies that have investigated the impact of DBI exposure on outcomes in older adults living in Australia and the US (Citation14,Citation15,Citation20).
A recent systematic review highlighted the importance of objective measures of physical performance such as the walking speed and the chair stands test as predictors of mortality in older community-dwelling people (Citation42). In the present study, DBI exposure was independently associated with a decrease in walking speed of 0.13 m/s. This is considered as a substantial clinically meaningful change (Citation19). In addition, DBI was associated with limitations on the chair stands test and functional mobility as measured with the TUG. Poorer performance on the chair stands test has been linked with adverse health outcomes including lower extremity limitations, hospitalization, and death (Citation43). The mean adjusted time taken to complete TUG amongst participants with DBI ≥1 was 14.0 seconds. This is clinically important as older adults who take longer than 14.0 seconds to complete the TUG have a higher risk for falls (Citation33). DBI exposure was also associated with disability in performing basic and complex tasks of daily living. In this population, exposure to DBI drugs was associated with difficulties in performing three ADL tasks. Older adults with disabilities in three or more ADL tasks are 3.3 times more likely to enter a nursing home over a 2–6 year period (Citation44). In this study, DBI exposure was not associated with weaker grip strength, which is not consistent with previous studies in community-dwelling older people (Citation15,Citation16,Citation20).
In this cross-sectional study, the use of DBI drugs was reported by 37% of the participants. This exposure is higher than that in community-dwelling people living in Australia (Citation15) and the US (Citation14), but lower than that observed among older Australians residing in low-level care facilities (Citation45) and intermediate level care facilities (Citation17). This could be in part explained by the differences in medication access and use across different settings and countries. Finland has higher rates of psychotropic drug use compared to other European countries (Citation46,Citation47), the US (Citation48), and Australia (Citation49). Psychotropic drugs frequently possess anticholinergic and sedative properties. The most frequently used DBI drug classes in Finland were benzodiazepines, compared to antihistamines and opioid analgesics in US (Citation16), and antidepressants in Australia (Citation15). Despite the differences in population characteristics, medication exposures, and functional measures across studies conducted in US and Australia (Citation14–16,Citation20), and this study, DBI was consistently associated with functional impairment in community-dwelling older people. This study further highlights the need for revision of current guidelines to improve the quality of drug use which may reduce the incidence of adverse outcomes in older adults.
There are several strengths to our study. The generalizability of our findings is enhanced by the high participation rate (78%) in the GeMS study. Objective clinically meaningful measures of physical function were administered in this study (Citation42). Participants were interviewed about their medication use and were asked to bring all prescription and over-the-counter medications. The self-reported medication use was verified against medical records obtained from health centres, home nursing services, and hospitals. This represents an advantage because pharmacy administrative data may not include drugs that are not reimbursed and do not take into account medication non-adherence (Citation23). Another strength of our study is that anticholinergic and sedative medication use was calculated using a method that incorporates the principles of dose-response and cumulative effect (Citation14). There were no missing data on daily doses or frequency for anticholinergic or sedative medications.
This study had several limitations as well. The cross-sectional study design limits causality implications. However, the reduction in walking speed was clinically sizeable (Citation19), suggesting that DBI-contributing medications may have an effect on physical function. Slow walking speed is considered as the single best indicator of functional impairment (Citation50). Participants may have had sound clinical reasons for using drugs with anticholinergic and sedative properties. We have attempted to address possible confounding by adjusting for important co-morbidities using the modified FCI that takes into account medical conditions that have been shown to predict physical function in older people. However, we cannot discount the possibility of residual confounding by other medical conditions and confounding by indication. Also, analyses were limited to those participants for whom functional outcomes data were available. Exclusion of participants with missing data may have compromised the reliability and validity of the outcome data, and it may have resulted in the misclassification bias of findings regarding the association between DBI and outcomes.
There are limitations to calculating exposure to anticholinergic and sedative medications using the DBI pharmacological model. The minimum recommended daily dose was used as an estimate of DR50 of anticholinergic and sedative drugs. The minimum daily dose may vary with pharmacokinetic and pharmacodynamic properties of different anticholinergic and sedative medications and between populations of older people in different settings and countries. The DBI measures exposure to regular rather than as-needed anticholinergic and sedative medications. This means true exposure to anticholinergic and sedative medications may have been under-estimated in our study. While the findings of this study are consistent with those in two other continents, they may not be applicable to other countries as differences in prescribing culture, access, and anticholinergic and sedative drug use are likely to vary across countries. The study population was restricted to community-dwelling older people. Whether these findings are applicable to older people living in institutional settings, who are more likely to have multiple co-morbidities and decreased functional status, warrants a separate investigation. Also, the study results may primarily reflect the association between DBI and function in females since the number of male participants was small.
In conclusion, DBI was associated with poorer performance on clinically important outcomes in older community-dwelling adults living in Finland. This finding supports the inclusion of the DBI tool with other assessments to predict functional impairment in older people. The DBI, pharmacologically based tool can be used to derive clinically relevant information for the assessment of risk and benefit when reviewing an older patient's medications. The DBI tool may be used to identify high-risk prescribing in older people. The clinical feasibility of the DBI tool to guide prescribing needs further evaluation, and intervention studies may need to be tailored to individual populations and health care systems. Further longitudinal studies are needed to determine whether DBI is associated with functional decline over time.
Acknowledgements
The authors gratefully acknowledge the funding support from the Geoff and Elaine Penney Ageing Research Unit, and the Scientific Staff Council, Royal North Shore Hospital, Sydney, Australia. The authors also thank the Social Insurance Institution of Finland and City of Kuopio for supporting the Geriatric Multidisciplinary Strategy for the Good Care of the Elderly (GeMS) Study. No other sources of funding were used to assist in the preparation of the manuscript.
Declaration of interest: Dr Hilmer holds an international patent for the Drug Burden Index with Drs Abernethy and Mager.
References
- Haider SI, Johnell K, Thorslund M, Fastbom J. Trends in polypharmacy and potential drug-drug interactions across educational groups in elderly patients in Sweden for the period 1992–2002. Int J Clin Pharmacol Ther. 2007;45: 643–53.
- Kuijpers MA, van Marum RJ, Egberts AC, Jansen PA. Relationship between polypharmacy and underprescribing. Br J Clin Pharmacol. 2008;65:130–3.
- Hilmer SN, Gnjidic D. The effects of polypharmacy in older adults. Clin Pharmacol Ther. 2009;85:86–8.
- Fick DM, Cooper JW, Wade WE, Waller JL, Maclean JR, Beers MH. Updating the Beers criteria for potentially inappropriate medication use in older adults: results of a US consensus panel of experts. Arch Intern Med. 2003;163: 2716–24.
- Jano E, Aparasu RR. Healthcare outcomes associated with beers’ criteria: a systematic review. Ann Pharmacother. 2007;41:438–47.
- Steinman MA, Rosenthal GE, Landefeld CS, Bertenthal D, Kaboli PJ. Agreement between drugs-to-avoid criteria and expert assessments of problematic prescribing. Arch Intern Med. 2009;169:1326–32.
- O'Mahony D, Gallagher PF. Inappropriate prescribing in the older population: need for new criteria. Age Ageing. 2008; 37:138–41.
- Aizenberg D, Sigler M, Weizman A, Barak Y. Anticholinergic burden and the risk of falls among elderly psychiatric inpatients: a 4-year case-control study. Int Psychogeriatr. 2002; 14:307–10.
- Landi F, Russo A, Liperoti R, Cesari M, Barillaro C, Pahor M, . Anticholinergic drugs and physical function among frail elderly population. Clin Pharmacol Ther. 2007;81: 235–41.
- Carriere I, Fourrier-Reglat A, Dartigues JF, Rouaud O, Pasquier F, Ritchie K, . Drugs with anticholinergic properties, cognitive decline, and dementia in an elderly general population: the 3-city study. Arch Intern Med. 2009;169:1317–24.
- Tinetti ME, Speechley M, Ginter SF. Risk factors for falls among elderly persons living in the community. N Engl J Med. 1988;319:1701–7.
- Gray SL, LaCroix AZ, Hanlon JT, Penninx BW, Blough DK, Leveille SG, . Benzodiazepine use and physical disability in community-dwelling older adults. J Am Geriatr Soc. 2006;54:224–30.
- Hanlon JT, Horner RD, Schmader KE, Fillenbaum GG, Lewis IK, Wall WE Jr, . Benzodiazepine use and cognitive function among community-dwelling elderly. Clin Pharmacol Ther. 1998;64:684–92.
- Hilmer SN, Mager DE, Simonsick EM, Cao Y, Ling SM, Windham BG, . A drug burden index to define the functional burden of medications in older people. Arch Intern Med. 2007;167:781–7.
- Gnjidic D, Cumming RG, Le Couteur DG, Handelsman DJ, Naganathan V, Abernethy DR, . Drug Burden Index and physical function in older Australian men. Br J Clin Pharmacol. 2009;68:97–105.
- Cao YJ, Mager DE, Simonsick EM, Hilmer SN, Ling SM, Windham BG, . Physical and cognitive performance and burden of anticholinergics, sedatives, and ACE inhibitors in older women. Clin Pharmacol Ther. 2008;83: 422–9.
- Wilson NM, Hilmer SN, March LM, Cameron ID, Lord SR, Seibel MJ, . Associations between drug burden index and physical function in older people in residential aged care facilities. Age Ageing. 2010;39:503–7.
- Gnjidic D, Le Couteur DG, Abernethy DR, Hilmer SN. Drug Burden Index and Beers criteria: Impact on functional outcomes in older people living in self-care retirement villages. J Clin Pharm. 2011 Feb 2 (Epub ahead of print).
- Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc. 2006;54:743–9.
- Hilmer SN, Mager DE, Simonsick EM, Ling SM, Windham BG, Harris TB, . Drug burden index score and functional decline in older people. Am J Med. 2009; 122:1142–9.e1–2.
- Pokela N, Bell JS, Lihavainen K, Sulkava R, Hartikainen S. Analgesic use among community-dwelling people aged 75 years and older: A population-based interview study. Am J Geriatr Pharmacother. 2010;8:233–44.
- Lampela P, Hartikainen S, Sulkava R, Huupponen R. Adverse drug effects in elderly people—a disparity between clinical examination and adverse effects self-reported by the patient. Eur J Clin Pharmacol. 2007;63:509–15.
- Rikala M, Hartikainen S, Sulkava R, Korhonen MJ. Validity of the Finnish Prescription Register for measuring psychotropic drug exposures among elderly Finns: a population-based intervention study. Drugs Aging. 2010; 27:337–49.
- Rigler SK, Perera S, Jachna C, Shireman TI, Eng M. Comparison of the association between disease burden and inappropriate medication use across three cohorts of older adults. Am J Geriatr Pharmacother. 2004;2:239–47.
- Van Rensbergen G, Nawrot T. Medical conditions of nursing home admissions. BMC Geriatr. 2010;10:46.
- WHO Collaborating Centre for Drug Statistics Methodology. The Anatomical Therapeutic Chemical Classification System. Norwegian Institute of Public Health. Available at: http://www.whocc.no/atcddd/ (accessed 14 April 2010).
- Pharmaca Fennica 2010. Available at: http://www.laaketie tokeskus.fi/page.php?page_id=493 (accessed 28 October 2010).
- Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, . Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56: M146–56.
- Guralnik JM, Ferrucci L, Pieper CF, Leveille SG, Markides KS, Ostir GV, . Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci. 2000;55:M221–31.
- Roubenoff R. Sarcopenia: a major modifiable cause of frailty in the elderly. J Nutr Health Aging. 2000;4:140–2.
- Bohannon RW. Hand-grip dynamometry predicts future outcomes in aging adults. J Geriatr Phys Ther. 2008;31: 3–10.
- Podsiadlo D, Richardson S. The timed ‘Up & Go’: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39:142–8.
- Shumway-Cook A, Brauer S, Woollacott M. Predicting the probability for falls in community-dwelling older adults using the Timed Up & Go Test. Phys Ther. 2000;80: 896–903.
- Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179–86.
- van der Putten JJ, Hobart JC, Freeman JA, Thompson AJ. Measuring change in disability after inpatient rehabilitation: comparison of the responsiveness of the Barthel index and the Functional Independence Measure. J Neurol Neurosurg Psychiatry. 1999;66:480–4.
- Groll DL, To T, Bombardier C, Wright JG. The development of a comorbidity index with physical function as the outcome. J Clin Epidemiol. 2005;58:595–602.
- Furu K, Wettermark B, Andersen M, Martikainen JE, Almarsdottir AB, Sorensen HT. The Nordic countries as a cohort for pharmacoepidemiological research. Basic Clin Pharmacol Toxicol. 2010;106:86–94.
- Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, . Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982; 17:37–49.
- Folstein MF, Folstein SE, McHugh PR. ‘Mini-mental state’. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98.
- Crum RM, Anthony JC, Bassett SS, Folstein MF. Population-based norms for the Mini-Mental State Examination by age and educational level. JAMA. 1993;269:2386–91.
- Hartl K, Janni W, Kastner R, Sommer H, Strobl B, Rack B, . Impact of medical and demographic factors on long-term quality of life and body image of breast cancer patients. Ann Oncol. 2003;14:1064–71.
- Cooper R, Kuh D, Hardy R. Objectively measured physical capability levels and mortality: systematic review and meta-analysis. BMJ. 2010;341:c4467.
- Cesari M, Kritchevsky SB, Newman AB, Simonsick EM, Harris TB, Penninx BW, . Added value of physical performance measures in predicting adverse health-related events: results from the Health, Aging And Body Composition Study. J Am Geriatr Soc. 2009;57:251–9.
- Gaugler JE, Duval S, Anderson KA, Kane RL. Predicting nursing home admission in the US: a meta-analysis. BMC Geriatr. 2007;7:13.
- Gnjidic D, Le Couteur DG, Abernethy DR, Hilmer SN. A pilot randomized clinical trial utilizing the drug burden index to reduce exposure to anticholinergic and sedative medications in older people. Ann Pharmacother. 2010;44: 1725–32.
- Alanen HM, Finne-Soveri H, Fialova D, Topinkova E, Jonsson PV, Soerbye LW, . Use of antipsychotic medications in older home-care patients. Report from nine European countries. Aging Clin Exp Res. 2008;20:260–5.
- Fialova D, Topinkova E, Gambassi G, Finne-Soveri H, Jonsson PV, Carpenter I, . Potentially inappropriate medication use among elderly home care patients in Europe. JAMA. 2005;293:1348–58.
- Curtis LH, Ostbye T, Sendersky V, Hutchison S, Dans PE, Wright A, . Inappropriate prescribing for elderly Americans in a large outpatient population. Arch Intern Med. 2004;164:1621–5.
- Draper B, Brodaty H, Low LF, Saab D, Lie D, Richards V, . Use of psychotropics in Sydney nursing homes: associations with depression, psychosis, and behavioral disturbances. Int Psychogeriatr. 2001;13:107–20.
- Gill TM. Assessment of function and disability in longitudinal studies. J Am Geriatr Soc. 2010;58 Suppl 2: S308–12.
