Abstract
Introduction. Hantaviruses are important human pathogens that cause clinical diseases characterized by renal and cardiopulmonary manifestations. Their pathogenesis is currently poorly understood. We have studied the role of the complement system in the pathogenesis of Puumala (PUUV) hantavirus infection.
Material and methods. We studied the activation of complement by measuring the terminal complement complex SC5b-9 and complement component C3 and C4 levels in patients with acute PUUV infection. Several laboratory parameters and clinical findings reflecting the severity of PUUV-HFRS were evaluated with regard to complement activation. Results.
The levels of SC5b-9 were significantly increased and C3 decreased in the acute stage as compared to the levels at full recovery (P < 0.001). We found that SC5b-9 levels were higher in patients with chest X-ray abnormalities than in patients with a normal X-ray during the acute stage (P = 0.028). Furthermore, SC5b-9 and C3 levels showed significant correlation with several clinical and laboratory parameters that reflect the severity of the acute PUUV infection.
Conclusions. We showed that the complement system becomes activated via the alternative pathway in the acute stage of PUUV infection and the level of activation correlates with disease severity. The results further suggest that complement activation may contribute to the pathogenesis of acute PUUV infection.
Key words::
| Abbreviations | ||
| HCPS | = | hantavirus cardiopulmonary syndrome |
| HFRS | = | hemorrhagic fever with renal syndrome |
| MAC | = | membrane attack complex |
| NE | = | nephropathia epidemica |
| PUUV | = | Puumala virus |
Key messages
Complement activation via the alternative pathway in the acute stage of PUUV hantavirus infection correlates with disease severity.
Complement activation may contribute to the pathogenesis of acute PUUV infection.
Introduction
Puumala virus (PUUV), a member of the Hantavirus genus in the Bunyaviridae family, is a rodent-borne zoonotic virus with a segmented, negative-stranded RNA genome. PUUV is carried by the bank vole, Myodes glareolus, which is found in most of Europe (Citation1). Numerous hantaviruses are known pathogens to humans and cause two diseases: hemorrhagic fever with renal syndrome (HFRS) in Eurasia and hantavirus cardiopulmonary syndrome (HCPS) in the Americas (Citation1). Acute PUUV infection, known as nephropathia epidemica (NE), is a mild form of HFRS with approximately 0.1% mortality (Citation2).
PUUV-HFRS has an incubation period of 2–6 weeks, and the disease begins with high fever, headache, nausea, and vomiting. Signs of renal insufficiency include proteinuria, hematuria, and increased creatinine levels as well as oliguria followed by polyuria. Transient hemodialysis treatment is needed in approximately 5% of hospitalized patients (Citation3). General laboratory findings include leukocytosis, thrombocytopenia, hypoproteinemia, and increased CRP levels. The severity of the disease is associated with HLA B8, DR3, and DQ2 haplotype (Citation4).
Pulmonary involvement has been described in about one-third of PUUV-infected patients (Citation5–8), and over half of the patients have abnormal cardiac findings (Citation9). It is believed that capillary leakage due to increased capillary permeability plays a role in the pathogenesis of pulmonary changes in HFRS and HCPS, which is more frequently characterized by severe pulmonary dysfunction (Citation10,Citation11).
A previous study on complement activation in PUUV infection showed that complement activation is common and suggested that the classical pathway of complement is associated with disease severity (Citation12). However, the role of the complement system in the pathogenesis of PUUV-HFRS has not been further studied in larger patient populations. The complement system has three major pathways: the classical, alternative, and the lectin-dependent pathway. These pathways are activated differently, but they all converge on complement component C3 that has a key function in the complement system (Citation13). The end-product of the complement cascade is the cytolytic membrane attack complex (MAC), formed by sequential assembly of complement proteins C5b, C6, C7, C8, and C9 to a target cell membrane. When the complexes are formed in the absence of a target membrane in the fluid phase, C5b-9 binds to S-protein (vitronectin) or clusterin, and a non-lytic soluble SC5b-9 terminal complex is formed (Citation14).
We measured the levels of SC5b-9, C3, and C4 in 61 hospitalized patients in the acute stage of PUUV infection and at recovery to study the role of complement in the pathogenesis of PUUV-HFRS.
Material and methods
Ethics statement
Written informed consent was obtained from all patients, and the Ethics Committee of the Tampere University Hospital approved the study protocol.
Patients and methods
This prospective study included 61 consecutive hospitalized patients with serologically confirmed acute PUUV infection. The serological diagnosis is based on μ-capture PUUV-IgM enzyme immunoassay (Citation15). All patients, 44 males and 17 females, median age 46 years (range 22–77), were treated at the Tampere University Hospital during the period from September 2000 to January 2004.
The median number of plasma samples taken from patients during hospital care was 4 (range 1–6). The first sample (n = 61) was obtained upon admission to hospital 2–16 days (median 5) after the onset of fever. The second sample (n = 58) was obtained 4–16 (median 6), the third sample (n = 45) 6–17 (median 8), the fourth sample (n = 31) 6–14 (median 9), the fifth sample (n = 10) 7–11 (median 10), and the sixth sample (n = 2) 13–18 (median 16) days after the onset of fever. The last sample (n = 53) was taken at full recovery 18–55 days (median 38) after the onset of fever and represented the control sample in the analyses.
Laboratory parameters, determined at the Laboratory Center of the Tampere University Hospital, and clinical findings were retrieved from patient charts. All chest radiographs were studied retrospectively by a radiologist (A.P.). Complement analyses were performed at the Haartman Institute and at HUSLAB. Plasma C3 and C4 levels were measured by nephelometry (Dade Behring, Marburg, Germany) and SC5b-9 using an ELISA kit (Quidel, San Diego, CA, USA). Different sets of samples were used for SC5b-9 and C3/C4 analyses, and the samples were stored at 270°C and 220°C, respectively. A number of patient samples did not meet the quality control criteria for the measurements of C3, C4, and SC5b-9 levels defined by the manufacturer. Thus, these samples were excluded from the respective analyses.
Statistical analyses
Statistical analyses were performed using SPSS software version 18 (SPSS Inc., Chicago, IL, USA). The highest and lowest values of continuous variables measured during hospital care were designated as maximum or minimum values. For the SC5b-9 the maximum and for the C3 and C4 levels the minimum values during the acute stage, respectively, were used. The maximum level of SC5b-9 reflects the peak of complement activation, and the minimum level of C3 and C4 the degree of C3 and C4 consumption during the acute stage of PUUV infection.
We used Mann–Whitney U test to compare SC5b-9, C3, and C4 levels between different patient groups and Wilcoxon's rank sum test to compare the levels in the same individual at different time points (acute stage and full recovery). To express a relationship between the continuous variables, Spearman's rank correlation coefficients were computed. All P values are two-tailed, and statistical significance was considered at the 5% level.
Results
SC5b-9, C3, and C4 levels
We measured the plasma levels of SC5b-9, C3, and C4 during hospital care and at full recovery. The highest and lowest concentrations of SC5b-9 and C3, respectively, were found in the first or second acute-stage sample in nearly all patients (n = 50) and in the third or fourth sample in the others. The lowest concentration of C4 was found in the first or second acute-stage sample in 40 patients.
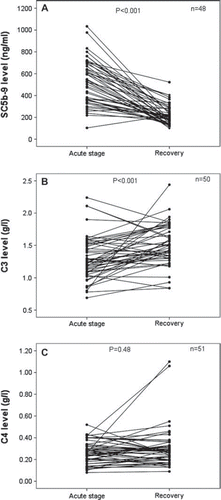
As shown in , the median maximum plasma SC5b-9 concentration in the acute stage was significantly higher than at full recovery (493 ng/mL, range 103–1034 ng/mL versus 197 ng/mL, range 100–522 ng/mL; P < 0.001). The acute-stage median minimum C3 level was decreased compared to the level measured at full recovery (1.26 g/L, range 0.65–2.24 g/L versus 1.47 g/L, range 0.84–2.44 g/L; P < 0.001) (). The acute-stage median minimum C4 level was not significantly different from the level measured at full recovery (0.26 g/L, range 0.08–0.52 g/L versus 0.27 g/L, range 0.09–1.1 g/L; P = 0.48) ().
Figure 1. A: Paired plasma levels of SC5b-9 of PUUV-HFRS patients during the acute stage upon admission to hospital and at full recovery. The median maximum SC5b-9 concentration in the acute stage was 493 ng/mL (range 103–1034 ng/mL), and the level at full recovery was 197 ng/mL (range 100–522 ng/mL). B: Paired plasma levels of C3 of PUUV-HFRS patients during the acute stage and at full recovery. The median minimum C3 concentration in the acute stage was 1.26 g/L (range 0.65–2.24 g/L), and the level at full recovery was 1.47 g/L (range 0.84–2.44 g/L). C: Paired plasma levels of C4 of PUUV-HFRS patients during the acute stage and at full recovery. The median minimum C4 concentration in the acute stage was 0.26 g/L (range 0.08–0.52 g/L), and the level at full recovery was 0.27 g/L (range 0.09–1.1 g/L). Wilcoxon's test was used to determine the statistical significance in A, B, and C.
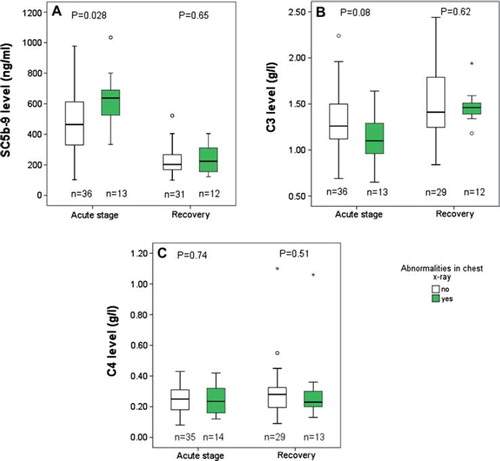
Fifty patients had chest X-ray taken upon hospitalization. Fourteen of them (28%) had abnormal findings including accumulation of pleural fluid (n = 6) and atelectasis (n = 12). The patients with chest X-ray abnormalities during the acute stage had significantly higher levels of SC5b-9 than patients with normal X-ray (P = 0.028), but the levels were no longer different at the time of recovery (). The patients with pathological findings in chest X-ray in the acute stage tended to have lower minimum C3 levels than patients with normal X-ray, but the difference was not statistically significant (P = 0.08) (). No differences were observed in C4 levels ().
Figure 2. A: Box plots of plasma SC5b-9 levels of patients with and without chest X-ray abnormalities in the acute stage of PUUV-HFRS and at full recovery. Box plot illustrates the median value (center horizontal line), interquartile range (the lower and upper quartiles), and the highest and lowest values (whiskers) that are not outliers. The outliers are presented as circles. The median maximum SC5b-9 levels in patients with and without chest X-ray abnormalities in the acute stage were 637 ng/mL (range 334–1034 ng/mL) and 464 ng/mL (range 103–977 ng/mL) and at full recovery 222 ng/mL (range 123–365) and 203 ng/mL (100–522 ng/mL), respectively. B: Box plots of plasma C3 levels of patients with and without chest X-ray abnormalities in the acute stage of PUUV-HFRS patients and at full recovery. Box plot illustrates the median value (center horizontal line), interquartile range (the lower and upper quartiles), and the highest and lowest values (whiskers) that are not outliers. The outliers are presented as circles (minor outlier) or asterisks (major outlier). The median minimum C3 levels in patients with and without chest X-ray abnormalities in the acute stage were 1.1 g/L (range 0.65–1.64) and 1.26 g/L (range 0.7–2.24) and at full recovery 1.46 g/L (range 1.2–1.94) and 1.41 (range 0.84–2-44), respectively. C: Box plots of plasma C4 levels of patients with and without chest X-ray abnormalities in the acute stage of PUUV-HFRS and at full recovery. Box plot illustrates the median value (center horizontal line), interquartile range (the lower and upper quartiles), and the highest and lowest values (whiskers) that are not outliers. The outliers are presented as circles. The median minimum C4 levels in patients with and without chest X-ray abnormalities in the acute stage were 0.24 g/L (range 0.12–0.42 g/L) and 0.25 g/L (range 0.08–0.43 g/L) and at full recovery 0.23 g/L (range 0.13–1.06 g/L) and 0.28 g/L (range 0.09–1.10), respectively. Mann–Whitney test was used to determine statistical significance in A, B, and C.
Correlation of SC5b-9, C3, and C4 levels with disease severity
Clinical and laboratory findings of patients are shown in . Acute-stage levels of SC5b-9 and C3 correlated significantly with a number of variables that reflect the clinical severity of PUUV infection (). As for maximum levels of SC5b-9, the strongest correlations were found with the treatment time at hospital, the highest blood leukocyte count, and the change in weight during hospital care (r = 0.46, r = 0.43, and r = 0.42, respectively; P < 0.001 for all). The variables that showed the strongest correlation with minimum C3 levels were the highest levels of interleukin 6 (IL-6), the lowest blood hematocrit level, and lowest serum sodium level (r = 20.62, r = 0.52, and r = 0.44, respectively; P < 0.001 for all). The highest blood leukocyte count also correlated inversely with C3 levels (r = 20.3; P = 0.020). Acute-stage levels of C4 did not show significant correlation with any of the clinical or laboratory findings ().
Table I. Clinical and laboratory findings in 61 patients with acute PUUV-HFRS.
Table II. Spearman rank correlations of minimum acute-stage level of C3 and C4 and maximum acute-stage level of SC5b-9 with clinical and laboratory findings in PUUV-HFRS patients.
Discussion
Our study shows that the alternative pathway of the complement system becomes activated in the acute stage of PUUV infection, observed as elevated levels of SC5b-9 and decreased levels of C3 (). The levels of C4 were not significantly altered between the acute stage and full recovery. The actual peak of complement activation, however, had possibly occurred before the patients were hospitalized. Furthermore, we showed that SC5b-9 levels were significantly higher in patients with chest X-ray abnormalities than in patients with a normal X-ray during the acute stage of PUUV-HFRS (). We also found that consumption of C3 was higher among patients with X-ray abnormalities, although this was not statistically significant (), possibly due to the small sample size. In general, we consider the level of SC5b-9 a better measure of complement activation, since C3 levels are influenced not only by consumption but also by increased synthesis related to the acute-phase response. This likely explains why some individuals had higher C3 levels in the acute stage of PUUV-HFRS than at the recovery ().
Previous studies have suggested that the pathogenesis of lung disease in HCPS and HFRS is attributable to increased capillary permeability in lungs (Citation11). Puumala and Sin Nombre hantaviruses can infect endothelial cells in vitro without causing any visible changes in cell morphology or necrosis in the infected endothelium in vivo (Citation10,Citation16). Therefore, it is believed that immunological mechanisms contribute to changes disturbing the function of the endothelium and thereby lead to capillary leakage. It has been suggested that cytotoxic CD8+ T cells would trigger capillary leakage (Citation17) and that cytokines may contribute to the increased capillary permeability (Citation5,Citation18).
Studies in the rat have shown that the soluble form of C5b-9 (SC5b-9) can increase the endothelial permeability by ligating αvβ3-integrin of lung endothelium, increasing the hydraulic conductivity in a dose-dependent manner (Citation19,Citation20). SC5b-9 can also promote the permeability in human endothelial cells through the release of bradykinin and platelet-activating factor (Citation21), and SC5b-9 has been shown to promote pulmonary edema in the adult respiratory distress syndrome (ARDS) (Citation22). Based on our findings, it is possible that complement activation during the acute stage of PUUV-HFRS may contribute to the pathogenesis of vascular leakage in the lungs. Although pleural fluid indicating leakage was not visible in all patients with pathological findings in the chest X-ray, the presence of fluid cannot be excluded since small amounts of fluid accumulation is not detectable in posteroanterior radiographs (Citation23). Our data cannot, however, prove a direct causal link between complement activation and vascular leakage, since no direct experimental evidence is provided. Additional immunopathological mechanisms could be involved.
The role of the complement system in the pathogenesis of other viral diseases has recently been studied. Increased permeability of the endothelium without morphological damage to the cells is a key feature of dengue hemorrhagic fever (DHF) and dengue shock syndrome (DSS) (Citation24), and recent studies showed that patients with DHF had significantly higher SC5b-9 and lower C3 levels in plasma than did patients with less severe dengue fever during the acute stage of the disease (Citation25,Citation26). The authors observed the highest levels of SC5b-9 in the pleural fluids and plasma of DSS patients (Citation25). The SC5b-9 levels found in our study were, however, clearly lower than in DSS patients, which is in line with the milder course of PUUV infection compared to DSS. However, our levels were similar to those seen in DHF patients. A recent study also suggested that complement activation contributes to a more severe clinical outcome of H1N1 pandemic influenza (Citation27).
We showed in our study that complement system activation, observed as elevated levels of SC5b-9 and/or lower levels of C3, in the acute stage of PUUV-HFRS correlated significantly with several laboratory and clinical findings that reflect the disease severity such as leukocytosis and thrombocytopenia (). One previous study showed that low serum protein level, a good marker of capillary leakage, was strongly associated with abnormal chest X-ray findings (Citation7). However, we did not have protein levels available in this study. The length of hospital treatment, probably the most objective measure to define severity, showed significant correlation with both high SC5b-9 and low C3 concentrations.
We observed an inverse correlation between the C3 and IL-6 levels in the acute stage of PUUV infection (). Significant increase in plasma IL-6 levels has been reported in patients with acute PUUV infection (Citation28), and IL-6 secretion has been found to stimulate C3 synthesis (Citation29). In our study, the low C3 levels associated with high IL-6 levels probably reflect the level of tissue injury and consumption of C3 in the acute stage of the disease. An increase in C3 levels due to IL-6 secretion was probably not observed at the time samples were taken because it usually follows a 2–5 days delay.
Our hypothesis is supported by our recent findings concerning two fatal cases of PUUV-HFRS (unpublished data). The first patient with severe pulmonary edema had high SC5b-9 and low C3 levels during the acute stage of PUUV infection as compared to the levels found in this study, and the immunohistochemical staining showed extensive accumulation of C5b-9 and C3 in lung tissue. Heavy deposition of C5b-9 and C3 complexes was also detected in hypophysis, characterized by hemorrhages, and in the liver. As for the second patient, plasma levels of complement proteins were not obtained but immunohistochemical staining showed similar accumulation, although to a slightly lower extent, of C5b-9 and C3 in lungs and hypophysis.
We measured the level of SC5b-9 that reflects overall activation of the complement system in the specimen. SC5b-9 is the soluble equivalent of the membrane-associated MAC complex. Therefore, the result indirectly suggests that formation of membrane attack complexes, known to trigger cellular reactions and production of inflammatory cytokines (Citation30), also contributes to the pathology of PUUV-HFRS. In addition to the terminal complement complex, elevated plasma levels of C5a, known to play a role in the pathogenesis of ARDS (Citation31), may also contribute to pulmonary dysfunction in PUUV HFRS as C5a attracts and aggregates leukocytes that can result in capillary obstruction and leakage (Citation31). Correlation between complement activation and blood leukocyte count was observed in our study.
The sample size of this study was relatively small. Thus, it has an impact on the statistical power and addresses the need for further studies. However, for a prospective panel of hantavirus patient samples studied comprehensively with similar methodology, the number of cases is considerable. The time elapsed from the onset of fever to the time point when the first acute-stage samples were taken varied between individual patients. This time frame was not, however, significantly different between the patients with chest X-ray abnormalities and other patients (data not shown). Thus, analyses on SC5b-9 and C3 levels were presumably not biased. There were no significant differences in the age and sex distribution or in the frequency of previous illnesses between the patients with chest X-ray abnormalities and other patients.
The only previous study on complement activation in PUUV infection (Citation12) indicated that complement activation via the classical pathway is associated with the disease severity. Our study, with a considerably larger sample size, confirmed that complement becomes activated in the acute stage of PUUV and further extends the findings by demonstrating that SC5b-9 and C3 levels, indicating the alternative pathway activation, correlated with disease severity. Although our data showed that C4 levels were not significantly altered between the acute stage and recovery, it cannot be excluded that classical pathway activation also occurs as it is possible that consumption is compensated by increased synthesis. However, our data indicated that the activation of the classical pathway is not so strong or long-standing that it would be observable in changes in total C4 levels.
Our study did not address the question of what actually triggers the complement activation in PUUV-HFRS and why some individuals have stronger responses than others. A previous study showed that individuals with the HLA B8, DR3, and DQ2 haplotype are likely to have severe PUUV-HFRS (Citation4), and a more recent study established that the presence and the severity of abnormal chest radiography findings are apparently associated with this haplotype (Citation6). This haplotype regularly carries a deletion of the C4A gene that encodes the C4A protein, one of the two isotypes of complement component C4. C4 is an essential component of the complement system, and deficiencies of C4A are associated with defective clearance of immune aggregates and persistence of viral infections (Citation32). Abnormal complement activation may thus be associated with this particular HLA haplotype, and mechanistically it may relate to the deficiency of C4A. The C4A deficiency may lead to impaired classical pathway activation and an inability to process immune aggregates, e.g. antigen–antibody complexes, which in turn would lead to further inflammation and complement activation via the alternative pathway. However, the total plasma levels of C4 measured in this study do not indicate the level of C4A proteins specifically. Individuals with elevated complement activation may also have impaired regulation of the alternative complement pathway. A recent study showed that alternative complement pathway deregulation correlates with the severity of DHF, and this may be linked to complement factor H dysfunction (Citation26).
Taken together, our data indicate that complement activation via the alternative pathway in the acute stage of PUUV hantavirus infection correlates with disease severity and may contribute to the pathology of PUUV-HFRS. The complement system may thus be considered a potential therapeutic target to suppress severe hantavirus disease.
Acknowledgements
The skillful technical assistance of Ms Katriina Yli-Nikkilä, Ms Mirja Ikonen, Ms Marita Siren, and Tytti Manni is greatly appreciated.
The study was financially supported by the Medical Research Fund of Tampere University Hospital, the Finnish Kidney Foundation, and the European Commission Project ‘Diagnosis and control of rodent-borne viral zoonoses in Europe’ (QLK2-CT-2002-01358), the Academy of Finland, Sigrid Jusélius Foundation, HUS/EVO grants TYH 7214 and 2008235, and the Helsinki Biomedical Graduate School.
Declaration of interest: The authors report no conflicts of interest.
References
- Vaheri A, Mills JN, Spiropoulou CF, Hjelle B. Hantaviruses. Palmer SR, Lord Soulsby, Torgerson PR, Brown DWG, Oxford Textbook of Zoonoses—Biology, Clinical Practice and Public Health Control. 2nd Oxford: Oxford University Press; 2011:307–322.
- Makary P, Kanerva M, Ollgren J, Virtanen MJ, Vapalahti O, Lyytikainen O. Disease burden of Puumala virus infections, 1995–2008. Epidemiol Infect. 2010: 138:1484–92.
- Vapalahti O, Mustonen J, Lundkvist A, Henttonen H, Plyusnin A, Vaheri A. Hantavirus infections in Europe. Lancet Infect Dis. 2003;310:653–61.
- Mustonen J, Partanen J, Kanerva M, Pietila K, Vapalahti O, Pasternack A, . Genetic susceptibility to severe course of nephropathia epidemica caused by Puumala hantavirus. Kidney Int. 1996;49:217–21.
- Kanerva M, Paakkala A, Mustonen J, Paakkala T, Lahtela J, Pasternack A. Pulmonary involvement in nephropathia epidemica: radiological findings and their clinical correlations. Clin Nephrol. 1996;46:369–78.
- Paakkala A, Makela S, Hurme M, Partanen J, Huhtala H, Mustonen J. Association of chest radiography findings with host-related genetic factors in patients with nephropathia epidemica. Scand J Infect Dis. 2008;40:254–8.
- Paakkala A, Mustonen J. Radiological findings and their clinical correlations in nephropathia epidemica. Acta Radiol. 2007; 48:345–50.
- Clement J, Colson P, McKenna P. Hantavirus pulmonary syndrome in New England and Europe. N Engl J Med. 1994;331:545–6; author reply 547–8.
- Makela S, Kokkonen L, Ala-Houhala I, Groundstroem K, Harmoinen A, Huhtala H, . More than half of the patients with acute Puumala hantavirus infection have abnormal cardiac findings. Scand J Infect Dis. 2009;41:57–62.
- Zaki SR, Greer PW, Coffield LM, Goldsmith CS, Nolte KB, Foucar K, . Hantavirus pulmonary syndrome. Pathogenesis of an emerging infectious disease. Am J Pathol. 1995; 146:552–79.
- Kanerva M, Mustonen J, Vaheri A. Pathogenesis of puumala and other hantavirus infections. Rev Med Virol. 1998; 82:67–86.
- Paakkala A, Mustonen J, Viander M, Huhtala H, Pasternack A. Complement activation in nephropathia epidemica caused by Puumala hantavirus. Clin Nephrol. 2000; 536:424–31.
- Walport MJ. Complement. First of two parts. N Engl J Med. 2001;344:1058–66.
- Podack ER, Muller-Eberhard HJ. Isolation of human S-protein, an inhibitor of the membrane attack complex of complement. J Biol Chem. 1979;254:9808–14.
- Vapalahti O, Lundkvist A, Kallio-Kokko H, Paukku K, Julkunen I, Lankinen H, . Antigenic properties and diagnostic potential of puumala virus nucleocapsid protein expressed in insect cells. J Clin Microbiol. 1996;341:119–25.
- Temonen M, Vapalahti O, Holthofer H, Brummer-Korvenkontio M, Vaheri A, Lankinen H. Susceptibility of human cells to Puumala virus infection. J Gen Virol. 1993; 74(Pt 3):515–8.
- Terajima M, Hayasaka D, Maeda K, Ennis FA. Immunopathogenesis of hantavirus pulmonary syndrome and hemorrhagic fever with renal syndrome: Do CD8+ T cells trigger capillary leakage in viral hemorrhagic fevers? Immunol Lett. 2007;113:117–20.
- Linderholm M, Ahlm C, Settergren B, Waage A, Tarnvik A. Elevated plasma levels of tumor necrosis factor (TNF)-alpha, soluble TNF receptors, interleukin (IL)-6, and IL-10 in patients with hemorrhagic fever with renal syndrome. J Infect Dis. 1996;173:38–43.
- Tsukada H, Ying X, Fu C, Ishikawa S, McKeown-Longo P, Albelda S, . Ligation of endothelial alpha v beta 3 integrin increases capillary hydraulic conductivity of rat lung. Circ Res. 1995;77:651–9.
- Ishikawa S, Tsukada H, Bhattacharya J. Soluble complex of complement increases hydraulic conductivity in single microvessels of rat lung. J Clin Invest. 1993;91:103–9.
- Bossi F, Fischetti F, Pellis V, Bulla R, Ferrero E, Mollnes TE, . Platelet-activating factor and kinin-dependent vascular leakage as a novel functional activity of the soluble terminal complement complex. J Immunol. 2004; 173:6921–7.
- Langlois PF, Gawryl MS. Accentuated formation of the terminal C5b-9 complement complex in patient plasma precedes development of the adult respiratory distress syndrome. Am Rev Respir Dis. 1988;138:368–75.
- Colins JD, Burwell D, Furmanski S, Lorber P, Steckel RJ. Minimal detectable pleural effusions. A roentgen pathology model. Radiology. 1972;105:51–3.
- Basu A, Chaturvedi UC. Vascular endothelium: the battlefield of dengue viruses. FEMS Immunol Med Microbiol. 2008;53:287–99.
- Avirutnan P, Punyadee N, Noisakran S, Komoltri C, Thiemmeca S, Auethavornanan K, . Vascular leakage in severe dengue virus infections: a potential role for the nonstructural viral protein NS1 and complement. J Infect Dis. 2006;193:1078–88.
- Nascimento EJ, Silva AM, Cordeiro MT, Brito CA, Gil LH, Braga-Neto U, . Alternative complement pathway deregulation is correlated with dengue severity. PLoS One. 2009; 48:e6782.
- Monsalvo AC, Batalle JP, Lopez MF, Krause JC, Klemenc J, Hernandez JZ, . Severe pandemic 2009 H1N1 influenza disease due to pathogenic immune complexes. Nat Med. 2011;17:195–9.
- Makela S, Mustonen J, Ala-Houhala I, Hurme M, Koivisto AM, Vaheri A, . Urinary excretion of interleukin-6 correlates with proteinuria in acute Puumala hantavirus-induced nephritis. Am J Kidney Dis. 2004;43:809–16.
- Katz Y, Revel M, Strunk RC. Interleukin 6 stimulates synthesis of complement proteins factor B and C3 in human skin fibroblasts. Eur J Immunol. 1989;19:983–8.
- Morgan BP. Regulation of the complement membrane attack pathway. Crit Rev Immunol. 1999;19:173–98.
- Weigelt JA, Chenoweth DE, Borman KR, Norcross JF. Complement and the severity of pulmonary failure. J Trauma. 1988;28:1013–9.
- Blanchong CA, Zhou B, Rupert KL, Chung EK, Jones KN, Sotos JF, . Deficiencies of human complement component C4A and C4B and heterozygosity in length variants of RP-C4-CYP21-TNX (RCCX) modules in caucasians. The load of RCCX genetic diversity on major histocompatibility complex-associated disease. J Exp Med. 2000;191: 2183–96.
