Abstract
Background.The Modification of Diet in Renal Disease (MDRD) Study equation is the most commonly used formula for estimation of glomerular filtration rate (eGFR). Recently, the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) developed a new equation to provide a more accurate estimate of GFR among individuals with normal or mildly reduced renal function.
Aim. To compare the MDRD and CKD-EPI equations in hypertensive population treated in general practice.
Methods. The MDRD and CKD-EPI equations were applied to a cohort of 994 hypertensive subjects aged 45–70 years without cardiovascular or renal disease or previously known diabetes.
Results. The prevalence of CKD stage 3 (eGFR 30–59 mL/min per 1.73 m2) was 6.7% (95% CI 5.3–8.5) (67/994) according to the MDRD formula and 3.7% (95% CI 2.6–5.1) (37/994) according to the CKD-EPI formula. Of the 67 subjects classified as having CKD stage 3 according to the MDRD equation, 30 (44.8%) were reclassified as ‘no-CKD’ by the CKD-EPI equation. These subjects were mostly women 26/30 (87.7%).
Conclusion. Using the CKD-EPI equation leads to lower prevalence estimates for CKD than the MDRD equation in a hypertensive population treated in general practice.
| Abbreviations | ||
| ARIC | = | Atherosclerosis Research in Communities Study |
| ATR | = | angiotensin receptor |
| AusDiab | = | Australian Diabetes, Obesity and Lifestyle Study |
| CKD | = | chronic kidney disease |
| CKD-EPI | = | Chronic Kidney Disease Epidemiology Collaboration |
| eGFR | = | estimated glomerular filtration rate |
| FINDRISC | = | Finnish Diabetes Risk Score |
| GFR | = | glomerular filtration rate |
| IDMS | = | isotope dilution mass spectrometry |
| MDRD | = | Modification of Diet in Renal Disease Study |
| NHANES | = | US National Health and Nutrition Examination Surveys |
Key messages
The prevalence of moderately decreased glomerular filtration rate, defined as eGFR 30–59 mL/min/1.73 m2, was decreased from 6.7% with the MDRD formula to 3.6% when the CKD-EPI formula was applied in a cohort of hypertensive subjects without co-morbidities affecting renal function
Introduction
Chronic kidney disease (CKD) is a worldwide public health problem with a rising incidence, poor outcomes, and high costs. The estimated glomerular filtration rate (eGFR) equations, which take into account plasma creatinine, age, sex, and race, help to identify patients with CKD formerly overlooked if the renal function had been assessed by plasma creatinine alone. The four-variable Modification of Diet in Renal Disease (MDRD) Study equation (Citation1) is nowadays the most commonly used formula for eGFR. In a recent meta-analysis of general population cohorts, the risk of total mortality became significant around eGFR 60 mL/min/1.73 m2 and was two times higher around eGFR 30–45 mL/min/ 1.73 m2 compared with optimum eGFR levels of 90–104 mL/min/1.73 m2 calculated with the MDRD formula (Citation2). The MDRD formula was developed based on a database containing persons with various kidney diseases, and it has been shown to under-estimate systematically the true GFR in subjects with measured GFR ≥ 60 mL/min/1.73 m2 (Citation3–6). The Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) recently developed a new eGFR equation based on data pooled from research and clinical populations with diverse clinical characteristics in order to provide a more accurate estimate of GFR among individuals with normal or mildly reduced GFR (Citation7).
Hypertension can both initiate and worsen CKD (Citation8). According to the latest national study of the adult Finnish population, hypertension was very common with prevalence rates 52% in men and 34% in women (Citation9). Most of the hypertensive patients are treated in general practice. In this study, we applied the MDRD and CKD-EPI equations in a cohort of hypertensive subjects in general practice to compare their usefulness in quite healthy hypertensive population. We excluded patients with cardiovascular or renal disease and previously known diabetes.
Methods
Patients
The study sample of hypertensive subjects was drawn from the participants of the population survey, the Harmonica Project, which was carried out in the rural towns of Harjavalta and Kokemäki in south-western Finland from autumn 2005 to autumn 2007. A risk factor survey, a tape for the measurement of waist circumference, and a type 2 diabetes risk assessment form (Finnish Diabetes Risk Score, FINDRISC, available from www.diabetes.fi/english) (Citation10) were mailed to all inhabitants aged 45–70 years (n = 6,013). The participants were asked to mail the surveys back to the health centre if they were willing to participate in the project. Participation and all the tests included were free of charge for the participants. Participation rate was 74.0% (4,450/6,013).
Respondents with a latest measured blood pressure of at least 140/90 mmHg, or those using antihypertensive medication, having history of gestational hypertension or diabetes, having family history of premature cardiovascular disease, or those with FINDRISC of at least 12 points in Harjavalta, or at least 15 points in Kokemäki, were invited for laboratory tests and further examinations (n = 2,752). A FINDRISC of ≥9 has a sensitivity of 0.81 and a specificity of 0.76 to predict new cases of drug-treated type 2 diabetes (Citation10).
The diagnosis of hypertension was made during further examinations performed by a trained nurse as described in the measurements section. A total of 1,130 hypertensive participants were identified. For the analyses described here, we excluded participants with known diabetes or diagnosed cardiovascular or renal disease (polycystic kidney disease, chronic nephropathies, one single kidney), yielding an analytic cohort of 994.
Measurements
GFR was estimated from plasma creatinine values using the four-variable MDRD Study equation (Citation1) and the CKD-EPI equation for white men and women (Citation7). Plasma creatinine assays were performed at one central laboratory using a method (enzymatic method; Olympus® AU640, Japan) which is calibrated to be traceable to isotope dilution mass spectrometry (IDMS).
Plasma glucose levels, creatinine levels, and lipid profiles were determined in blood samples which were obtained after at least 12 hours of fasting. Oral glucose tolerance test was performed by measuring fasting plasma glucose and 2-hour plasma glucose from capillary blood after ingestion of a glucose load of 75 g anhydrous glucose dissolved in water. Glucose homeostasis was classified according to the World Health Organization 1999 criteria updated in 2006 (Citation11). On the basis of 2-hour post-load plasma glucose, individuals were classified into categories of newly diagnosed diabetes, impaired glucose tolerance, and normal glucose tolerance if their 2-hour plasma glucose concentrations were ≥12.2, 8.9–12.1, and <8.9 mmol/L, respectively. Impaired fasting glucose was diagnosed if the fasting plasma glucose was ≥6.1 mmol/L and the 2-hour plasma glucose was <8.9 mmol/L.
Blood pressure was measured by a trained nurse with a calibrated mercury sphygmomanometer with subjects in a sitting posture, after resting at least 5 minutes. In each subject the mean of the two readings taken at intervals of at least 2 minutes was used. If the mean systolic blood pressure was ≥140 mmHg or the mean diastolic blood pressure ≥90 mmHg, subjects were taught to use an automatic validated blood pressure monitor (Omron® M4-1, the Netherlands) which was lent for home blood pressure monitoring. In the subjects whose arm circumference was >32 cm, a larger cuff was used. The subjects were instructed to take duplicate blood pressure measurements in the seated position after 5 minutes of rest in the morning and evening for 1 week. The recorded measurements except those from the first day were used to calculate the mean home blood pressure.
Hypertension was diagnosed if any of the following conditions were met: 1) the patient was already taking antihypertensive medication; 2) the mean systolic blood pressure taken by the nurse was ≥140 mmHg or the mean diastolic blood pressure was ≥90 mmHg, and the mean of home blood pressure monitoring was ≥135 mmHg systolic or ≥85 mmHg diastolic.
Height and weight were measured by the nurse. Body mass index was calculated as weight (kg) divided by the square of height (m²).
Informed consent
The study protocol and consent forms were reviewed and approved by the ethics committee of Satakunta hospital district. All participants provided written informed consent for the project and subsequent medical research.
Statistical analysis
Data are presented as means with standard deviations or as counts with percentages. The most important results are given with the 95% confidence intervals. Statistical comparison between groups in measures with binary distribution was made by chi-square or Fisher's exact test, when appropriate. The independent samples t test was used for continuous variables. Positive agreement between the MDRD and CKD-EPI formulas was calculated as Chamberlain's per cent positive agreement, defined as a proportion in which positive agreement is divided by all positive findings (eGFR < 60 mL/min/1.73 m2) made by either CKD-EPI or MDRD equation; 95% confidence intervals were calculated by using the jack-knife equation (Citation12). Concordance of the two formulas was evaluated by using Bland and Altman methods. Statistical analysis was made with the statistical software Stata 11.1 (StataCorp., College Station, TX, USA).
Role of the funding source
This study was supported by the State Provincial Office of Western Finland and by the Central Satakunta Health Federation of Municipalities.
Results
We evaluated 994 hypertensive subjects aged 45–70 years (mean age 59 ± 7 years, 54.1% women) without established cardiovascular or renal disease or previously known diabetes. The crude prevalence of CKD stage 3 (eGFR 30–59 mL/min/1.73 m2) was 6.7% (95% CI 5.3–8.5) (67/994) according to the MDRD formula and 3.6% (95% CI 2.6–5.1) (36/994) according to the CKD-EPI formula. Positive agreement was 55% (95% CI 43%–67%).
In women, the prevalence of CKD stage 3 was 10.0% (54/538) and 5.0% (27/538) estimated by the MDRD and CKD-EPI equations, respectively. In men, the corresponding figures were 2.9% (13/456) and 2.0% (9/456).
displays the Bland–Altman plots of the difference in eGFR, as assessed by the MDRD and the CKD-EPI formulas in each patient, against their mean value. The difference between these formulas turns negative with rising eGFR values until 90–100 mL/min/1.73 m2 and grows positive thereafter at higher eGFR values.
Figure 1. Bland–Altman plots of difference versus average of the MDRD and the CKD-EPI formulas in women and men. Solid lines show mean differences and dotted lines 95% limits of agreement. (eGFR = estimated glomerular filtration rate; CKD-EPI = Chronic Kidney Disease Epidemiology Collaboration; MDRD = Modification of Diet in Renal Disease).
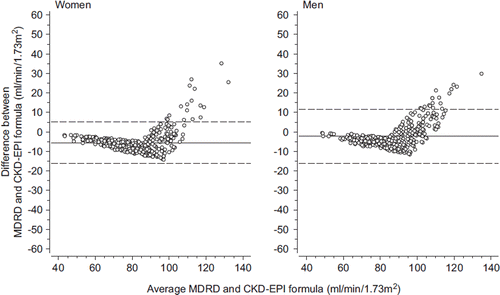
The mean value of eGFR was 81.2 mL/min/ 1.73 m2 (SD 16.4) with the MDRD formula and 85.2 mL/min/1.73 m2 (SD 13.5) with the CKD-EPI formula (P < 0.001). In women, the mean eGFR by the MDRD formula was 77.3 mL/min/1.73 m2 (SD 15.3) and 82.8 mL/min/1.73 m2 (SD 13.9) by the CKD-EPI formula (P < 0.001). In men, the mean eGFR was 85.8 mL/min/1.73 m2 (SD 16.3) and 88.1 mL/min/1.73 m2 (SD 12.4) according to the MDRD and CKD-EPI formulas, respectively (P < 0.001). None of the study subjects had eGFR < 30 mL/min per 1.73 m2.
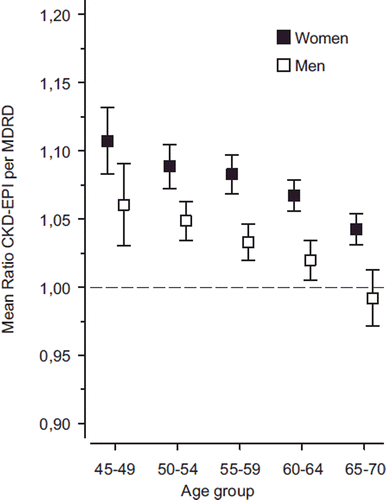
Mean values of eGFR according to gender and age-groups calculated by the CKD-EPI and MDRD equations are shown in . In older age-groups, the difference between the two formulas seems to diminish. However, shows that eGFR values generated by the CKD-EPI formula are on average significantly higher than those by the MDRD formula (mean ratio > 1) in both genders and in all age-groups except in males aged 65–70 years. In women, the mean ratio between the equations was 1.07 (95% CI 1.06–1.08) and in men 1.03 (95% CI 1.02–1.03).
Figure 2. Mean values of estimated glomerular filtration rates with 95% confidence intervals calculated by the CKD-EPI and MDRD equations by gender and age-group. (CKD-EPI = Chronic Kidney Disease Epidemiology Collaboration; MDRD = Modification of Diet in Renal Disease; eGFR = estimated glomerular filtration rate).
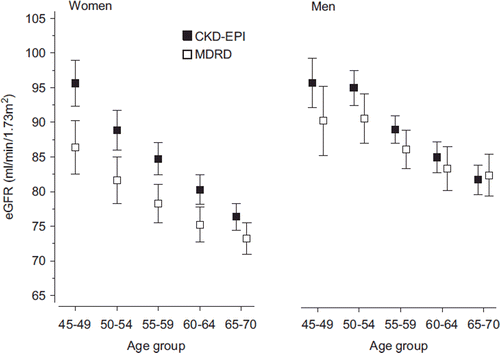
Figure 3. The mean ratio with 95% confidence intervals of eGFR values calculated by the CKD-EPI and MDRD equations by gender and age-group. (eGFR = estimated glomerular filtration rate; CKD-EPI = Chronic Kidney Disease Epidemiology Collaboration; MDRD = Modification of Diet in Renal Disease).
Of the 67 subjects classified as having CKD stage 3 according to the MDRD equation, 31 (46.3%) were reclassified as ‘no-CKD’ when the CKD-EPI equation was applied. The CKD-EPI formula did not find any subjects suffering from renal insufficiency among no-CKD defined by the MDRD formula.
shows the characteristics of the subjects with no-CKD, CKD stage 3 according to both formulas (‘CKD defined’), and subjects reclassified to no-CKD according to the CKD-EPI equation. The reclassified subjects were mostly women 27/30 (90.0%) and slightly younger than the subjects having CKD according to both formulas. There was no difference between the groups in cardiovascular risk factors or treatment with antihypertensive medication or daily use of non-steroidal anti-inflammatory drugs.
Table I. Characteristics of the subjects with no-CKD, subjects reclassified to no-CKD according to the CKD-EPI equation, and CKD according to both formulas (CKD defined).
Discussion
Our study shows that among middle-aged hypertensive subjects without cardiovascular or renal disease or previously known diabetes, the prevalence of CKD stage 3 is almost 2-fold higher when calculated by the MDRD formula than by the CKD-EPI formula. This result is mainly driven by the fact that the prevalence of CKD stage 3 in women was decreased from 10.0% based on the MDRD equation to 5.0% using the CKD-EPI equation.
The MDRD Study equation was developed by studying 1,628 subjects (mean age 51 years) who had non-diabetic CKD, with mean GFR of 40 mL/min/ 1.73 m2 (Citation13). The CKD-EPI equation was derived from studies including 5,504 subjects (mean age 47 years) with or without CKD who had a wide range of GFRs (mean GFR 67 mL/min/1.73 m2) measured by external filtration markers (Citation8).
Although our study subjects were older (mean age 59 years), they were quite healthy compared to the patients of the MDRD Study. Thus, the characteristics of the subjects treated in primary care resemble more the patients from whom the CKD-EPI equation was derived.
The CKD-EPI and MDRD Study equations have recently been compared in three large population-based cohorts. In the US National Health and Nutrition Examination Surveys (NHANES 1999–2006), the mean eGFR was 93.2 mL/min/1.73 m2 using the CKD-EPI formula versus 86.3 mL/min/1.73 m2 using the MDRD formula, and the CKD prevalence in the US adult population aged ≥ 20 years was 11.6% versus 13.1%, respectively (Citation8). The Australian Diabetes, Obesity and Lifestyle (AusDiab) Study reported a CKD prevalence of 11.5% using the CKD-EPI equation and 13.4% with the MDRD equation in the general Australian adult population (Citation14). The Atherosclerosis Research in Communities (ARIC) Study showed that the prevalence of CKD stage 3 (eGFR 30–59 mL/min/1.73 m2) was decreased from 2.5% with the MDRD formula to 1.4% when the CKD-EPI formula was applied in a cohort of individuals aged 45–64 years (Citation15). In all these studies, as well as in our study, the prevalence of CKD was lower, especially in women, but remained high in older subjects. Importantly, participants of the AusDiab and ARIC studies who were reclassified upward from eGFR 30–59 mL/min/ 1.73 m2 based on the MDRD formula to eGFR 60–89 mL/min/1.73 m2 using the CKD-EPI formula had lower risk of all-cause mortality, major cardiovascular events, and end-stage renal disease compared with those who were not reclassified (Citation14,Citation15).
When the reporting of eGFR using the MDRD Study equation was initially introduced in the United Kingdom, the number of referrals from primary care to nephrologists rose 2.7-fold (Citation16). This reflects the increased identification of patients not previously suspected of having CKD based on simple creatinine measurement but also the fact that the majority of CKD patients are treated in primary care. Using the CKD-EPI equation leads to lower prevalence estimates for CKD, which possibly limits the number of referrals to nephrology clinics.
Our study is, presumably, the first one to compare the MDRD and CKD-EPI equations in hypertensive subjects without previously diagnosed co-morbidities. In our patient population, there were no cases with severely decreased renal function (GFR < 30 mL/min/1.73 m2), and the prevalence of moderately decreased renal function (GFR 30–59 mL/min/1.73 m2) was reduced from 10.0% to 5.0% in women and from 2.9% to 2.0% in men when the CKD-EPI equation was applied instead of the MDRD equation. The higher prevalence of moderately decreased renal function in women is in concordance with other large-scale studies in hypertensive participants (Citation17,Citation18). The GFR is equal to the sum of the filtration rates in all functioning nephrons and can be regarded as a rough measure of the number of them. In autopsy studies women had fewer glomeruli than men (Citation19,Citation20), and the number of nephrons was reduced in white patients with primary hypertension (Citation21). Renal excretory capacity, as represented by GFR, deteriorates with age beginning in the third or fourth decade of life, and by the sixth decade, GFR commonly declines by 1–2 mL/min/1.73 m2 per year (Citation22). This age-related loss of renal function is proportional to blood pressure level, and the rate of GFR deterioration can accelerate to 4–8 mL/min/1.73 m2 per year if high systolic blood pressure remains uncontrolled (Citation22)—possibly more in women.
All formulas for calculating eGFR depend strongly on the accuracy of serum creatinine measurement. In our study, all plasma creatinine assays were performed in one laboratory with one method calibrated to be traceable to isotope dilution mass spectrometry (IDMS), the gold standard. Improved accuracy of eGFR is obtainable by using IDMS correction especially in the early stages of chronic kidney disease (Citation23).
Some limitations of the present analysis should be mentioned. First, albuminuria or haematuria was not measured, and thus only eGFR levels <60 mL/min/1.73 m2 can be reported as CKD. Second, because our results are derived from only one visit and a single creatinine measurement, it is not possible to evaluate the chronicity of renal insufficiency in the study population. However, Weiner et al. have recently shown that also a single measurement of eGFR to classify CKD in a community population appears to have prognostic value (Citation24).
In conclusion, the prevalence of CKD stage 3 was decreased from 6.7% with the MDRD formula to 3.6% when the CKD-EPI formula was applied in a cohort of hypertensive subjects without co-morbidities affecting renal function. The prevalence of CKD was lower especially in women.
Declaration of interest: The authors have no conflict of interest to declare.
References
- Levey AS, Coresh J, Greene T, Marsh J, Stevens LA, Kusek J, . Expressing the MDRD study equation for estimating GFR with IDMS traceable (gold standard) serum creatinine values. J Am Soc Nephrol. 2005;16:69A.
- ; Chronic Kidney Disease Prognosis ConsortiumMatsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, de Jong PE, . Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375:2073–81.
- Stevens LA, Coresh J, Feldman HI, Greene T, Lash JP, Nelson RG, . Evaluation of the Modification of Diet in Renal Disease Study equation in a large diverse population. J Am Soc Nephrol. 2007;18:2749–57.
- Rule AD, Larson TS, Bergstralh EJ, Slezak JM, Jacobsen SJ, Cosio FG. Using serum creatinine to estimate glomerular filtration rate: accuracy in good health and in chronic kidney disease. Ann Intern Med. 2004;141:929–37.
- Rule AD, Gussak HM, Pond GR, Bergstralh EJ, Stegall MD, Cosio G, . Measured and estimated GFR in healthy potential kidney donors. Am J Kidney Dis. 2004;43:112–9.
- Stevens LA, Coresh J, Greene T, Levey AS. Assessing kidney function—measured and estimated glomerular filtration rate. N Engl J Med. 2006;354:2473–83.
- Levey AS, Stevens LA, Schmid CH, Zhang Y, Castro AF, Feldman HI, . A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–12.
- Levey AS, Coresh J, Balk E, Kausz AT, Levin A, Steffes MW, . National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med. 2003;139:137–47.
- Kastarinen M, Antikainen R, Peltonen M, Laatikainen T, Barengo NC, Jula A, . Prevalence, awareness and treatment of hypertension in Finland during 1982–2007. J Hypertens. 2009;27:1552–9.
- Lindström J, Tuomilehto J. The diabetes risk score: a practical tool to predict type 2 diabetes risk. Diabetes Care. 2003; 26:725–31.
- World Health Organization: Definition and diagnosis of diabetes mellitus and intermediate hyperglycemia. Report of a WHO/IDF consultation. Geneva: World Health Organization; 2006.
- Chamberlain J, Ginks S, Rogers P, Nathan BE, Price JL, Burn I. Validity of clinical examination in mammography as screening test for breast cancer. Lancet. 1975;2:1026–30.
- Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–70.
- White SL, Polkinghome KR, Atkins RC, Chadban SJ. Comparison of the prevalence and mortality risk of CKD in Australia using the CKD Epidemiology Collaboration (CKD-EPI) and Modification of Diet in Renal Disease (MDRD) Study GFR estimating equations: The AusDiab (Australian Diabetes, Obesity and Lifestyle) Study. Am J Kidney Dis. 2010;55:660–70.
- Matsushita K, Selvin E, Bash LD, Astor BC, Coresh J. Risk implications of the new CKD Epidemiology Collaboration (CKD-EPI) equation compared with the MDRD Study equation for estimated GFR: The Atherosclerosis Risk in Communities (ARIC) Study. Am J Kidney Dis. 2010;55: 648–59.
- Richards N, Harris K, Whitfield M, O'Donoghue D, Lewis R, Mansell M, . The impact of population-based identification of chronic kidney disease using estimated glomerular filtration rate (eGFR) reporting. Nephrol Dial Transplant. 2008;23:556–61.
- Redon J, Morales-Olivas F, Galgo A, Brito MA, Mediavilla J, Marín R, . Urinary albumin excretion and glomerular filtration rate across the spectrum of glucose abnormalities in essential hypertension. J Am Soc Nephrol. 2006;17: S236–45.
- Redón J, Cea-Calvo L, Lozano JV, Fernández-Pérez C, Navarro J, Bonet A, .; the ERIC-HTA 2003 Study. Kidney function and cardiovascular disease in the hypertensive population: the ERIC-HTA study. J Hypertens. 2006;24: 663–9.
- Nyengaard JR, Bendtsen TF. Glomerular number and size in relation to age, kidney, weight, and body surface in normal man. Anat Rec. 1992;232:194–201.
- Hoy WE, Douglas-Denton RN, Hughson MD, Cass A, Johnson K, Bertram JF. A stereological study of glomerular number and volume: Preliminary findings in a multiracial study of kidneys at autopsy. Kidney Int. 2003;63:531–7.
- Keller G, Zimmer G, Mall G, Ritz E, Amann K. Nephron number in patients with primary hypertension. N Engl J Med. 2003;348:101–8.
- Bakris GL, Williams M, Dworkin L, Elliott WJ, Epstein M, Toto R, . Preserving renal function in adults with hypertension and diabetes: a consensus approach. National Kidney Foundation Hypertension and Diabetes Executive Committees Working Group. Am J Kidney Dis. 2000;36: 646–61.
- Quinn MP, Rainey A, Cairns KJ, Marshall AH, Savage G, Kee F, . The practical implications of using standardized estimation equations in calculating the prevalence of chronic kidney disease. Nephrol Dial Transplant. 2008;23:542–8.
- Weiner DE, Krassilnikova M, Tighiouart H, Salem DN, Levey AS, Sarnak MJ. CKD classification based on estimated GFR over three years and subsequent cardiac and mortality outcomes: a cohort study. BMC Nephrol. 2009;10:26.
