Abstract
Background. SCORE and FINRISK models are designed to estimate patient's risk for cardiovascular diseases (CVD). Increased circulating oxidized LDL (oxLDL) and impaired arterial elasticity, on their part, are considered as markers of subclinical atherosclerosis. Subjects with metabolic syndrome (MetS) are thought to be at high risk for CVD because of metabolic abnormalities.
Aim. To study among men with MetS whether subjects with three, four, or five MetS variables or different estimated 10-year CVD risk differ in oxLDL and arterial elasticity.
Methods. OxLDL was assessed by a capture ELISA and arterial elasticity by a radial artery tonometer among 120 men with MetS. Ten-year CVD risk was calculated for those without CVD or statin medication by FINRISK and SCORE at the actual age and at the extrapolated age of 60.
Results. High-risk subjects by FINRISK and SCORE had impaired arterial elasticity. In addition, high-risk subjects by FINRISK at the extrapolated age had elevated oxLDL levels. The number of MetS variables did not associate with arterial elasticity or oxLDL.
Conclusion. Among men with MetS, estimation of 10-year CVD risk, especially when extrapolated to age 60, seems to differentiate subjects with respect to markers of subclinical atherosclerosis.
Trial registration: ClinicalTrials.gov identifier: NCT01119404.
| Abbreviations | ||
| BMI | = | body mass index |
| C1 | = | large arterial elasticity |
| C2 | = | small arterial elasticity |
| CVD | = | cardiovascular disease |
| DBP | = | diastolic blood pressure |
| HbA1C | = | glycosylated hemoglobin |
| HDL-C | = | high-density lipoprotein cholesterol |
| LDL-C | = | low-density lipoprotein cholesterol |
| MetS | = | metabolic syndrome |
| NCEP | = | National Cholesterol Education Program |
| oxLDL | = | oxidized LDL |
| PP | = | pulse pressure |
| PWV | = | pulse wave velocity |
| SBP | = | systolic blood pressure |
Key messages
Among men with metabolic syndrome (MetS), impairment in elastic properties of arteries and increased circulating oxidized LDL were already evident among those without any symptoms but with statistically estimated high risk of cardiovascular diseases (CVD).
Among men with MetS, 10-year CVD risk estimation, especially when extrapolated to age 60, seems to find those at high CVD risk, additive to the presence of MetS only.
Introduction
Treatment of cardiovascular diseases (CVD) is well established and effective. To achieve even better results, life-style intervention and preventive medication should be targeted to those at high risk even before any clinical symptoms and events. SCORE and FINRISK risk score models have been developed to estimate a 10-year risk of CVD events and death and thus the need for primary prevention (Citation1,Citation2).
LDL oxidation in the arterial wall is a key event in atherosclerosis (Citation3). Oxidized LDL (oxLDL) together with cardiovascular risk factors damages the endothelium of the arterial wall (Citation3). Increased oxidative stress in the vascular wall causes alterations in the elastic properties and function of the arteries (Citation3). Since elevated levels of circulating oxLDL and loss of arterial elasticity associate with future cardiovascular events and mortality (Citation4–7), they are believed to serve as early markers of subclinical CVD.
Patients with metabolic syndrome (MetS) are known to be at high risk for CVD and all-cause mortality (Citation8). Traditional risk factors do not fully explain the excess risk of CVD connected to MetS (Citation9–11). However, since the definitions take account of a cluster of even mild metabolic abnormalities, the importance of MetS has been questioned (Citation12). As the number of overweight people continues to increase, the number of MetS subjects and associated diseases increase as well. A better understanding of connections between MetS and CVD would therefore be of great benefit.
We have previously reported impaired arterial elasticity and increased oxLDL among men with MetS compared to controls (Citation13). However, it is not known whether men with MetS and high estimated 10-year CVD risk but without symptoms of vascular disease have differences in oxLDL levels and vascular function. In the present study, we assessed the levels of oxLDL by a capture ELISA immunoassay, arterial elasticity by a non-invasive radial artery tonometer, and 10-year risk of CVD events and death by FINRISK and SCORE risk models among men with MetS (Citation1,Citation2). The aim was to study whether subjects with different numbers of MetS features or different estimated CVD risk have differences in these markers of subclinical atherosclerosis.
Material and methods
Study subjects were referred from consecutive patients diagnosed with MetS in a routine physical examination with laboratory tests in primary health care. Altogether 120 men were recruited, aged 30–65 and diagnosed with MetS according to National Cholesterol Education Program (NCEP) recommendations (Citation14). None of the study subjects was excluded later on. Information on the subjects’ medical history, smoking, alcohol consumption, physical activity, dietary habits, and cardiovascular diseases in the family was gathered during a standardized interview. Previously diagnosed CVD was determined based on patients’ records and their self-report during the interview. Mean alcohol intake (g/day) was calculated by multiplying the average number of alcohol portions/month by ethanol content of each taken beverage and dividing it by 30. Subjects’ weight, height, waist circumference, and blood pressure were measured according to general recommendations. Pulse pressure (PP) was calculated as systolic blood pressure (SBP, mmHg) minus diastolic blood pressure (DBP, mmHg). Body mass index (BMI) was calculated as weight (kg)/height2 (m2). Subjects filled in a questionnaire on their average times, duration, type, and intensity level (four predetermined choices) of leisure time physical exercise per week. We calculated the mean energy expenditure of daily physical exercise by multiplying the metabolic equivalent (MET) value and exercise times per week and person's weight in kilograms and mean duration of exercise in hours and finally dividing it by 7 (Citation15). The compendium of physical activities and subjects’ self-rated intensity levels of the exercise sessions were used in estimating the correct MET value (Citation16). The study was approved by the ethics committee at the Kanta-Häme Hospital District (registration number 513/2008). Study subjects were recruited within 11 months after the approval. Before any study procedures, the subjects signed an informed consent. All data were analyzed anonymously.
Study design
OxLDL and arterial elasticity indices were compared between men with three, four, or five MetS variables (n = 120). In a primary prevention setting, only men with MetS and without CVD or statin medication (n = 79) were included. Differences in oxLDL and arterial elasticity indices were assessed between subjects with different estimated 10-year CVD risk. The effect of different factors on oxLDL and arterial elasticity indices (C1 and C2) were assessed in multivariate analyses among 120 men with MetS.
Laboratory procedures
Venous blood was drawn after 12 hours of fasting. Plasma levels of oxLDL were determined as duplicates by a validated, commercial two-site immunoassay (ELISA, Mercodia, Uppsala, Sweden) (Citation17). The assay uses the same monoclonal antibody mAb-4E6 as in studies by Holvoet et al. (Citation4,Citation18). CV% of the oxLDL measurement was 7.7%. Glycosylated hemoglobin (HbA1C) was assessed by a standardized method in percent and then converted to mmol/mol (Citation19). The laboratory practices strict internal and external quality control (Labquality Oy).
Arterial elasticity
Radial artery pulse wave was recorded non-invasively by an arterial tonometry (HDI/PulseWave™ CR-2000, Hypertension Diagnostics, Inc. Eagan, MN, USA), which uses a modified Windkessel model (Citation20). Values assessed by this validated method have been reported to correlate tightly with those determined invasively (Citation20).
The measurement was conducted after at least 15 minutes of rest in a semi-sitting position. Subjects refrained from eating, smoking, drinking caffeinated drinks, and taking medication for 12 hours and drinking alcohol for two days prior to measurement. A stabilizer was used to immobilize the wrist and to stabilize the radial artery. A sensor with a manually adjustable shift was placed on the radial artery. The tonometer automatically adjusted and calibrated itself until the waveform was stable. During the measurement, the device cross-correlated the individual pulse waves. The capacitive elasticity of large arteries (C1) and the reflective elasticity of small arteries (C2) were automatically assessed by the tonometer as a mean of the five most similar pulse waves appearing during 30 seconds of measurement. The pulse waves and the data were displayed automatically on a computer screen from which the uniformity of the waveforms and possible artifacts were easily detected. The mean of four consecutive measurements was assessed to diminish the variability and possible bias caused by a single measurement. Intraindividual CV% was 9.0% for C1 and 8.8% for C2. All measurements were performed by the same experienced nurse.
Cardiovascular risk estimates
Since cardiovascular risk score models are designed to evaluate the need for primary prevention, only subjects without established CVD and statin medication (n = 79) were included in the analyses comparing different risk groups. Subjects with statin medication were excluded from the risk estimate analyses because they had earlier been evaluated to be at high risk for CVD events by their physician, and an effective primary prevention according to recommendations had been initiated. FINRISK and SCORE models were used to calculate the 10-year risk of cardiovascular events and death. Since younger subjects with notable risk factors have low absolute risk, we also extrapolated the risk to age 60 to assess a possible high relative risk. The extrapolated risk was assessed among those younger than 60.
The FINRISK 10-year risk of lethal and non-lethal CVD events was calculated as a sum of the risk of coronary heart disease event and the risk of stroke (Citation1). The SCORE 10-year risk of CVD death was calculated by using coefficients for populations at high CVD risk and the formula presented by the SCORE project (Citation2).
Differences in oxLDL and arterial elasticity were assessed between low-, medium-, and high-risk subjects by FINRISK and SCORE at the actual age and at the extrapolated age of 60. In FINRISK, low risk was < 5%, medium 5%–14.99%, and high ≥ 15%. In SCORE, the groups were < 3%, 3%–4.99%, and ≥ 5%, respectively.
Statistics
Statistics were analyzed with SPSS for Windows 17.0. Data are expressed as mean ± SD if not mentioned otherwise. All 120 subjects were included in the multivariate analyses. Correlations between different variables were assessed, and only the variables without a strong correlation (−0.5 < r < 0.5) with each other were included as covariates into the multivariate analyses. Covariates with evident non-linear relation to oxLDL and arterial elasticity were excluded. Linear regression model was used stepwise to assess the effect of different covariates (relevant risk factors, established CVD, and statin medication) on oxLDL and arterial elasticity. Analyses were reassured by automatic forward and backward methods as well as manually by the enter method. Smoking, diabetes, family history of CVD, statin medication, and established CVD were included as dichotomous covariates in the multivariate analyses. Smoking was dichotomized as never and current/former but also as never/former and current. The β values are presented for significant covariates included in the final models. Formulas, adjusted R2 (the level at which the model explains changes in oxLDL, C1, and C2) and P values are presented for the final models. Residual analyses were carried out for the models. All 120 men were also included in the analyses comparing subjects with different numbers of MetS variables. The results were adjusted for established CVD and statin medication. Only the subjects without established CVD and statin medication (n = 79) were included in the CVD risk estimate analyses, which are used to assess the need for primary prevention. Associations between continuous and categorical variables were assessed by t test in case of normality and by Mann–Whitney U test in case of non-normality. Associations between three-categorical and continuous variables were assessed by ANOVA in case of normality, with use of Bonferroni post-hoc analysis. When Mann–Whitney U test was used for three-categorical variables in case of non-normality, P value was corrected by multiplying it by 3. Associations between categorical variables were studied by chi-square test. P < 0.05 was considered statistically significant.
Results
Seventeen subjects had previously diagnosed CVD. Fourteen of them had been diagnosed with coronary heart disease, five with ischemic cerebrovascular disease, and one with peripheral vascular disease. Three of those with CVD were not treated with statins. Since altogether 35 patients were on statin medication, 21 patients had statin medication but not established CVD. Use of other pharmacological therapy among all study subjects (subjects without CVD and statin medication in brackets) was as follows: aspirin or other antiplatelet agent was used by 23.3% (3.8%), beta-blockers by 34.2% (19.0%), ACE inhibitors or angiotensin receptor blockers by 38.3% (31.6%), and lipid-lowering drugs by 31.7% (0%) of the subjects, respectively.
A total of 35 subjects had three, 61 subjects had four, and 24 subjects had five MetS variables. There was no difference in oxLDL, C1, or C2 between subjects with different number of MetS variables, nor did they differ in age, life-style, medical history, or laboratory measurements.
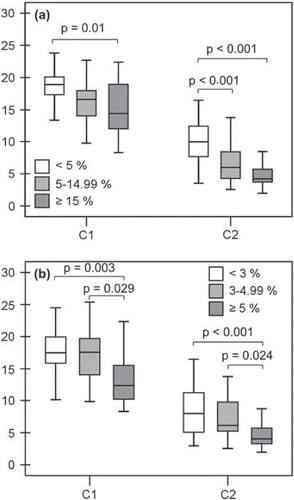
High-risk (≥15%) subjects according to the FINRISK model at the actual age had lower C1 and C2 compared to low-risk (<5%) subjects (). Medium-risk (5%–14.99%) subjects also had lower C2 than low risk subjects. High-risk (≥5%) subjects according to SCORE at the actual age had impaired C1 and C2 compared to medium-risk (3%–4.99%) and low-risk (<3%) subjects (). In oxLDL levels, there was no difference between the risk groups by either FINRISK or SCORE at the actual age.
Figure 1. Large (C1) and small (C2) arterial elasticity among low-, medium-, and high-risk subjects according to FINRISK (A) and SCORE (B) models at the actual age. A: C1 was 18.5 ± 3.2 mL/mmHg × 10 among low-risk (<5%; n = 26), 16.4±4.1 mL/ mmHg × 10 among medium-risk (5%–14.99%; n = 36), and 14.8±4.5 mL/mmHg × 10 among high-risk (≥ 15%; n =17) subjects according to FINRISK at the actual age. C2 was 10.1 ±3.6 mL/mmHg × 100, 6.7±3.0 mL/mmHg × 100, and 4.9±2.0 mL/mmHg × 100, respectively. B: C1 was 17.6±3.6 mL/mmHg × 10 among low-risk (<3%; n = 44), 17.2±4.1 mL/ mmHg × 10 among medium-risk (3%–4.99%; n = 20), and 13.6±4.4 mL/mmHg × 10 among high-risk (>5%; n = 15) subjects according to SCORE at the actual age. C2 was 8.4 ± 3.7 mL/mmHg × 100, 7.3 ±3.4 mL/mmHg × 100, and 4.6 ± 2.1 mL/ mmHg × 100, respectively.
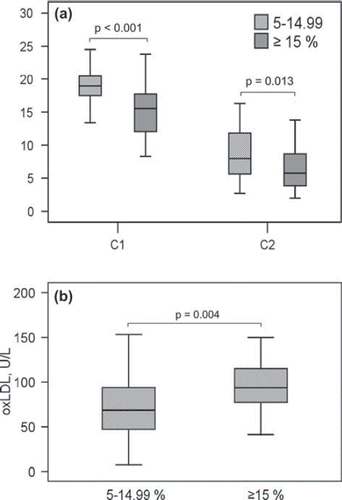
According to the FINRISK model at the projected age of 60, all subjects belonged to medium-risk (5%–14.99%) or high-risk (≥15%) groups. Clinical characteristics of these subjects are presented in . Eight (18.1%) of high-risk and one (2.9%) of medium-risk subjects were on metformin medication (P = 0.03). The groups did not differ in the number of any other medication. High-risk subjects had impaired C1 and C2 as well as elevated levels of oxLDL compared to medium-risk subjects (). Besides the differences in oxLDL, C1 and C2, the risk groups differed significantly only in risk factors included in the FINRISK model. The differences in C1 and oxLDL remained significant also after adjustment for age, smoking, prediabetes/diabetes, LDL cholesterol, and previously diagnosed hypertension, P = 0.001 and P = 0.022, respectively. The difference in C2 did not remain significant after adjustment for age or diabetes. After adjustment for SBP and previously diagnosed hypertension, the difference remained significant in C1 (P = 0.045) but not in C2. The high-risk group was further on divided into high (15%–19.99%) and very high (≥20%) risk, but this did not yield any additional benefit.
Table I. Demographics of the subjects with medium (5%–14.99%) or high (≥ 15%) 10-year cardiovascular risk according to the FINRISK model at the projected age of 60.
Figure 2. Large (C1) and small (C2) arterial elasticity (A) and oxidized LDL (oxLDL) (B) among medium- and high-risk subjects according to the FINRISK model at the projected age of 60. C1 was 18.9 ±3.3 mL/mmHg × 10 among medium-risk (5%–14.99%; n = 35) and 15.1 ± 4.0 mL/mmHg × 10 among high-risk (≥ 15%; n = 44) subjects. C2 was 8.6 ±3.6 mL/mmHg × 100 and 6.5 ± 3.4 mL/mmHg × 100, respectively. OxLDL was 70.7 ± 38.1 U/L among medium-risk and 94.8 ± 32.1 U/L among high-risk subjects.
With an alternative risk score division of FINRISK at the actual age (low < 5%, medium 5%–9.99%, and high ≥ 10%), there was still a significant difference between low- and high-risk subjects in C1 (P = 0.004) as well as between low and medium risk in C2 (P = 0.001). The same division in FINRISK at the projected age of 60 could not be analyzed because there were not enough subjects with risk <10%.
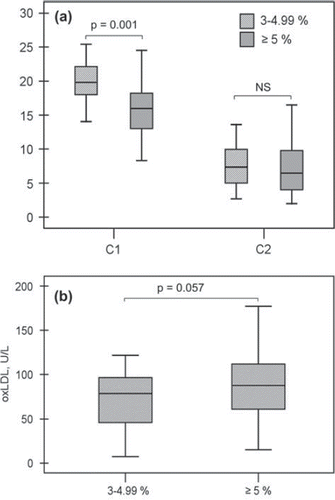
Also by the SCORE model at the projected age of 60, all subjects belonged to medium- (3%–4.99%) or high-risk (≥5%) groups. High-risk subjects had impaired C1 compared to subjects with medium risk (). The difference remained significant even after adjustment for age (P = 0.001). High-risk subjects also had higher levels of oxLDL, but it did not quite reach statistical significance (). There was no difference in C2 between the groups ().
Figure 3. Large (C1) and small (C2) arterial elasticity (A) and oxidized LDL (oxLDL) (B) among medium- and high-risk subjects according to the SCORE model at the projected age of 60. C1 was 20.0±3.0 mL/mmHg×10 among medium-risk (3%–4.99%; n = 19) and 15.7 ±3.9 mL/mmHg × 10 among high-risk (>5%; n = 60) subjects. C2 was 7.6±3.4 mL/mmHg × 100 and 7.3±3.7 mL/mmHg × 100, respectively. OxLDL was 70.1 ± 35.8 U/L among medium-risk and 88.8 ± 36.0 U/L among high-risk subjects.
Covariates selected to the multivariate analyses are presented in and the covariates included in the final models in . LDL-C and triglycerides were positively and physical exercise negatively associated with oxLDL. The formula for oxLDL (U/L) was 22.1 + 3.54 × triglycerides (mmol/L) + 17.20 × LDL-C (mmol/L) – 0.04 × physical exercise (kcal/day), P < 0.001. Diabetes and PP were negatively and waist circumference positively associated with C1. The formula for C1 (mL/mmHg × 10) was 23.7 – 0.33 × PP (mmHg) – 1.18 × diabetes + 0.11 × waist circumference (cm), P < 0.001. Age and PP were negatively associated with C2. The formula for C2 (mL/mmHg × 100) was 22.2 – 0.08 × PP (mmHg) – 0.22 × age (years), P < 0.001. When SBP or DBP were selected separately to the models instead of PP, they both were negatively associated with C1, β = −0.19, P < 0.001 and β = −0.22, P < 0.001, respectively. SBP (β = −0.05, P = 0.006) was negatively associated with C2. Dichotomizing smoking to never and current/former, or never/former and current, did not reveal a significant association to oxLDL or arterial elasticity.
Table II. Cardiovascular risk factors included in the multivariate analyses of circulating levels of oxidized LDL and arterial elasticity (n = 120).
Table III. Final models of the multivariate analyses of oxidized LDL (oxLDL) as well as large (C1) and small (C2) arterial elasticity. The β values of significant covariates as well as adjusted R2 and P values of the models are presented.
Discussion
In the present study among men with MetS, subjects with high estimated 10-year CVD risk by the FINRISK and SCORE models had impaired arterial elasticity compared to those with lower risk. In addition, subjects with high risk by the FINRISK model at the projected age of 60 had elevated levels of oxLDL compared to subjects with medium risk. It is intriguing that impairment in elastic properties of arteries and increased circulating oxLDL were already evident among those without any symptoms but with statistically estimated high risk of CVD. Since increased oxLDL and impaired arterial elasticity associate with future CVD events (Citation4–7), our findings imply that CVD risk estimates are able to detect MetS subjects at high CVD risk, additive to the presence of MetS only.
To our knowledge, this is the first report on impaired arterial elasticity and increased oxLDL among men with MetS and high calculated CVD risk. Our findings support previous studies reporting an association between arterial stiffness, assessed by pulse wave velocity (PWV), and Framingham risk score (Citation21). Framingham risk score has also been reported to associate with increased oxLDL levels (Citation22,Citation23). Impaired large arterial elasticity and increased oxLDL also seem to associate with MetS (Citation13,Citation24,Citation25). Since all our study subjects had MetS, the presence and magnitude of CVD risk factors additional to those of MetS criteria contributed to the deterioration in the elasticity of arteries and increase in oxLDL levels.
The importance of MetS has been questioned (Citation12). However, the value of diabetes in predicting future CVD events is unquestionable (Citation12,Citation26,Citation27). In the present study, there were significantly more diabetics among those at high estimated 10-year CVD risk according to FINRISK at the projected age of 60 compared to those at medium risk (). Thus, the presence of diabetes may explain part of the difference in oxLDL and arterial elasticity between the groups. However, even after adjustment for the presence of prediabetes and diabetes, the difference in C1 and oxLDL between the groups remained significant. Thus, besides the prevention and treatment of diabetes, an aggressive management of other risk factors among MetS subjects should be taken into account.
FINRISK at the projected age of 60 was superior to FINRISK at the actual age in differentiating MetS subjects with increased oxLDL and impaired arterial elasticity. This supports the suggestion of the European Society of Cardiology to extrapolate risk to age 60 to reveal those young subjects at high relative risk (Citation28). In FINRISK at the projected age of 60, differences were seen between risk scores 5%–14.99% and ≥15%. As the small arterial elasticity was impaired already among subjects with ≥5% risk in FINRISK at the actual age, a lower threshold might be considered if actual age is used in FINRISK estimate. We also found significant differences in arterial elasticity between risk scores <4.99% and ≥5% in SCORE both at the actual and at the projected age of 60. It supports the use of the ≥5% threshold in SCORE for more aggressive primary prevention.
The FINRISK model has been constructed from a series of prospective studies among Finns, and it takes account of more cardiovascular risk factors than SCORE (Citation29). This may explain why FINRISK differentiated MetS subjects with impaired arterial elasticity and increased oxLDL better than SCORE in the present study. Although we studied only Finnish MetS subjects, the use of the FINRISK model has not been limited to a Finnish population (Citation30).
The number of MetS variables had no effect on oxLDL levels or arterial elasticity. Earlier, Koskinen et al. found no difference in carotid intima media thickness (IMT) progression between subjects with three and four-to-five MetS components (Citation11). This is in agreement with our study as IMT is also considered a marker of subclinical atherosclerosis. However, Lapointe et al. (Citation31) reported higher oxLDL among subjects with five compared to those with only three MetS components. In another study, impaired arterial flow-mediated function associated with increasing number of MetS variables (Citation32). The variation of findings may be caused by different study populations or methods used.
In the multivariate analyses of the present study, increased pulse pressure (PP) and the presence of diabetes associated with impaired C1, and increased PP and age with impaired C2. An elevated PP is known to reflect the increased stiffness of large arteries (Citation33). In addition, in a follow-up study among 342,815 healthy subjects, high PP (i.e. high SBP and low DBP) associated with an increased CVD risk among men aged 45–57 years (Citation34). In another study, coronary heart disease risk was found to associate with lower DBP at any level of SBP ≥ 120 mmHg especially among the middle-aged and elderly (Citation35). In agreement, we found a stronger association between elevated PP and impaired C1 and C2 compared to that between SBP and arterial elasticity indices among middle-aged men with MetS. Since both elevated PP and impaired large arterial elasticity have been found to associate with MetS, the stiffening of large arteries may contribute to the increased CVD risk among MetS subjects (Citation24,Citation25,Citation36). The question of whether the arterial elasticity measurement is superior to PP in assessing the changes in large arterial function, and especially in predicting future CVD events, needs to be elucidated in a prospective study.
The results of the multivariate analysis of C1 support previous studies reporting elevated glucose level and hypertension to correlate with impaired large arterial elasticity (Citation24,Citation25). However, opposite to what was expected (Citation24,Citation25), waist circumference was positively associated with C1 in the present study. This finding may be due to the selected study group containing only men with MetS and increased waist circumference. Among a highly selected study group, generally accepted risk factors may not act in an expected way in statistical analyses.
Elevated levels of oxLDL have been reported to associate with different MetS components (Citation17). In the multivariate analysis of oxLDL in the present study, only triglycerides of the MetS components as well as physical inactivity and LDL cholesterol related positively to increased oxLDL levels. The effect of these risk factors remained significant even after adjustment for statin medication and other risk factors assessed in the study. Elevated levels of LDL cholesterol and triglycerides are known to be independent risk factors for CVD events (Citation37,Citation38). Triglycerides boost the production of small, dense LDL particles more prone to oxidation (Citation17). Regular aerobic exercise, on its part, has been reported to reduce the level of oxidative stress (Citation39). Since physical activity associated with lower oxLDL levels in the present study, regular aerobic exercise should be recommended for those at high CVD risk.
We calculated the 10-year CVD risk score only among those without established CVD because the risk score models are designed to assess the need for primary prevention. There were also a relatively large number of those with statin medication but without established CVD. These patients had been estimated to be at high risk for future CVD events by their physician, and the primary prevention with statin therapy had been initiated according to recommendations. Because of this, and the fact that statins affect lipid levels and may affect arterial elasticity as well (Citation40), we also excluded subjects with statin medication from the analyses including risk score models. However, to ensure a greater number of subjects in this relatively small study, all study subjects were included in the multivariate analyses as well as in the analyses comparing subjects with different number of MetS variables. In these analyses, the effect of CVD and statin medication was taken into account by adjusting the results for these covariates.
Linear regression model, used in the multivariate analyses, has limitations. Since it assumes the effects of covariates to be linear, a covariate with non-linear effect may be disregarded from the model. In addition, the stepwise method is often indiscriminate and may produce models with incorrect covariates. Furthermore, the regression model assumes that covariates do not have strong correlations with each other. We selected only established CVD risk factors, statin medication, and previously diagnosed CVD as covariates into the analyses. To avoid shortcomings, only the variables without strong correlations with each other were included as covariates into the multivariate analyses. The linear relations of continuous covariates to oxLDL and arterial elasticity were verified. In addition, the results were reassured by automatic forward and backward methods as well as manually by the enter method. Finally, the relevance of the models was confirmed visually.
The non-invasive pulse wave analysis has been criticized since the model is based on a number of theoretical approximations and there are no studies on the possible predictive value on future CVD events (Citation41). However, values obtained by the non-invasive pulse contour analysis have been found to correlate tightly with those assessed invasively (Citation20). In addition, C1 has been reported to relate significantly to MRI-determined aortic distensibility, and C2, on its part, to endothelial function assessed by flow-mediated dilation (Citation42,Citation43). Furthermore, impaired arterial elasticity, assessed by this method, has been found to associate with hypertension, symptomatic coronary artery disease, severity of peripheral vascular disease, microalbuminuria, erectile dysfunction, as well as with MetS, all known as high-risk conditions for future CVD events (Citation20,Citation24,Citation25,Citation44–46). PWV, the golden standard for measuring arterial elasticity, may also be inaccurate among subjects with MetS, abdominal obesity, and diabetes (Citation41). For these reasons, the pulse contour analysis, a reproducible method to assess systemic arterial stiffness, was used in the present study (Citation41,Citation47).
The relatively small number of subjects is a limitation of the study. In addition, subjects were not randomly selected from a certain population. As subjects without MetS were not recruited as well, the effect of the presence of MetS per se could not be assessed. However, the main aim of the study was to compare MetS subjects with high estimated CVD risk to those with lower risk, not the differences between MetS and non-MetS subjects. Previously, we and others have reported increased oxLDL levels and impaired arterial elasticity among subjects with MetS compared to those without (Citation13,Citation24,Citation25,Citation31).
Indices of pulse wave analysis have been found to correlate differently among men and women (Citation48). The present study assessed for the first time whether statistically high CVD risk among MetS associated with both impaired arterial elasticity and increased oxLDL. To avoid internal biases caused by gender, only men were included. Results cannot therefore be generalized to women and subjects without MetS.
The pathophysiological process between MetS and CVD is not fully understood. Since markers of inflammation and insulin resistance indices may explain the connection between metabolic abnormalities and increased CVD risk, their association to oxLDL and arterial elasticity would have been interesting to study (Citation49,Citation50). In addition, the value of oxLDL and arterial elasticity in predicting future CVD events among MetS subjects, additive to the traditional CVD risk score models, needs to be evaluated in prospective studies.
In conclusion, asymptomatic men with MetS and high 10-year CVD risk had increased oxLDL and impaired arterial elasticity compared to those with lower risk. Hence, 10-year CVD risk estimation, especially when extrapolated to age 60, seems to differentiate MetS subjects with respect to markers of subclinical atherosclerosis.
Acknowledgements
We appreciate the professional technical aid of Sanna Haavisto, Jaana Heikkinen, Kirsti Inkilä, Matti Kataja, Paula Lahtinen, and Kirsti Räsänen. The authors gratefully acknowledge the co-operation of the clinical staff at Kanta-Häme Central Hospital, Linnan Klinikka, and Mehiläinen.
This study was supported by grants from the Ministry of Social Affairs and Health in Finland through the Medical Research Fund of Kanta-Häme Central Hospital, the Häme Regional Fund under the auspices of the Finnish Cultural Foundation, Paavo Ilmari Ahvenainen Foundation and Hilkka and Väinö Kiltti Foundation.
Trial registration: ClinicalTrials.gov NCT0111 9404.
Declaration of interest: The authors state no conflict of interest and have received no payment in preparation of this manuscript.
References
- Vartiainen E, Laatikainen T, Salomaa V, Jousilahti P, Peltonen M, Puska P. The FINRISK FUNCTION: Estimation of the risk of coronary events and stroke in the Finnish population. SLL. 2007;62:4507–13. Available at: www.fimnet.fi/cgi-cug/brs/artikkeli.cgi?docn=000029286) (in Finnish; abstract and functions in English).
- Conroy RM, Pyörälä K, Fitzgerald AP, Sans S, Menotti A, De Backer G, . Estimation of ten-year risk of fatal cardiovascular disease in Europe: the SCORE project. Eur Heart J. 2003;24:987–1003.
- Stocker R, Keaney JF Jr. Role of oxidative modifications in atherosclerosis. Physiol Rev. 2004;84:1381–478.
- Holvoet P, Jenny N, Schreiner P, Tracy R, Jacobs D. The relationship between oxidized LDL and other cardiovascular risk factors and subclinical CVD in different ethnic groups: The Multi-Ethnic Study of Atherosclerosis (MESA). Atherosclerosis. 2007;194:245–52.
- Meisinger C, Baumert J, Khuseyinova N, Loewel H, Koenig W. Plasma oxidized low-density lipoprotein, a strong predictor for acute coronary heart disease events in apparently healthy, middle-aged men from the general population. Circulation. 2005;112:651–7.
- Boutouyrie P, Tropeano I, Asmar R, Gautier I, Benetos A, Lacolley P, . Aortic stiffness is an independent predictor of primary coronary events in hypertensive patients. A longitudinal study. Hypertension. 2002;39:10–15.
- Meaume S, Benetos A, Henry OF, Rudnichi A, Safar ME. Aortic pulse wave velocity predicts cardiovascular mortality in subjects > 70 years of age. Arterioscler Thromb Vasc Biol. 2001;21:2046–50.
- Lakka H-M, Laaksonen D, Lakka T, Niskanen L, Kumpusalo E, Tuomilehto J, . The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA. 2002;288:2709–16.
- Bonora E. The metabolic syndrome and cardiovascular disease. Ann Med. 2006;38:64–80.
- Kullo IJ, Cassidy AE, Peyser PA, Turner ST, Sheedy PF 2nd, Bielak LF. Association between metabolic syndrome and subclinical coronary atherosclerosis in asymptomatic adults. Am J Cardiol. 2004;94:1554–8.
- Koskinen J, Kähönen M, Viikari J, Taittonen L, Laitinen T, Rönnemaa T, . Conventional cardiovascular risk factors and metabolic syndrome in predicting carotid intima-media thickness progression in young adults: The cardiovascular risk in young Finns study. Circulation. 2009;120:229–36.
- Ford ES. Risks for all-cause mortality, cardiovascular disease, and diabetes associated with the metabolic syndrome. A summary of the evidence. Diabetes Care. 2005;28:1769–78.
- Pohjantähti-Maaroos H, Palomäki A, Kankkunen P, Laitinen R, Husgafvel S, Oksanen K. Circulating oxidized low-density lipoproteins and arterial elasticity: comparison between men with metabolic syndrome and physically active counterparts. Cardiovasc Diabetol. 2010;9:41.
- Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Final report. Circulation. 2002;106:3143–421.
- Aittasalo M, Miilunpalo S, Suni J. The effectiveness of physical activity counseling in a work-site setting. A randomized controlled trial. Patient Educ Couns. 2004;55:193–202.
- Ainsworth B, Haskell W, Whitt M, Irwin M, Swartz A, Strath S, . Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32:498–516.
- Sigurdardottir V, Fagerberg B, Hulthe J. Circulating oxidized low-density lipoprotein (LDL) is associated with risk factors of the metabolic syndrome and LDL size in clinically healthy 58-year-old men (AIR study). J Intern Med. 2002;252:440–7.
- Holvoet P, Kritchevsky SB, Tracy RP, Mertens A, Rubin SM, Butler J, . The metabolic syndrome, circulating oxidized LDL, and risk of myocardial infarction in well-functioning elderly people in the health, aging, and body composition cohort. Diabetes. 2004;53:1068–73.
- Nathan DM, Kuenen J, Borg R, Zheng H, Schoenfeld D, Heine RJ, . Translating the A1C assay into estimated average glucose values. Diabetes Care. 2008;31:1473–8.
- Cohn J, Finkelstein S, McVeigh G, Morgan D, LeMay L, Robinson J, . Non-invasive pulse wave analysis for the early detection of vascular disease. Hypertension. 1995;26:503–8.
- Hae Guen Song, Eung Ju Kim, Hong Seog Seo, Seong Hwan Kim, Chang Gyu Park, Seong Woo Han, . Relative contributions of different cardiovascular risk factors to significant arterial stiffness. Int J Cardiol. 2010;139:263–8.
- Holvoet P, Harris TB, Tracy RP, Verhamme P, Newman AB, Rubin SM, . Association of high coronary heart disease risk status with circulating oxidized LDL in the well-functioning elderly. Findings from the Health, Aging and Body Composition Study. Arterioscler Thromb Vasc Biol. 2003; 23:1444–8.
- Ueba T, Nomura S, Nishikawa T, Kajiwara M, Yamashita K. Circulating oxidized LDL, measured with FOH1a/DLH3 antibody, is associated with metabolic syndrome and the coronary heart disease risk score in healthy Japanese. Atherosclerosis. 2009;203:243–8.
- Ge JY, Li XL, Zhang HF, Xu Q, Tong M, Wang JG. Elasticity indices of large and small arteries in relation to the metabolic syndrome in Chinese. Am J Hypertens. 2008;21:143–7.
- Fjeldstad A, Fjeldstad C, Acree L, Nickel KJ, Montgomery PS, Comp PC, . The relationship between arterial elasticity and metabolic syndrome features. Angiology. 2007;58:5–10.
- ; Emerging Risk Factors CollaborationSarwar N, Gao P, Seshasai SR, Gobin R, Kaptoge S, Di Angelantonio E, . Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375:2215–22.
- Mente A, Yusuf S, Islam S, McQueen MJ, Tanomsup S, Onen CL, . Metabolic syndrome and risk of acute myocardial infarction a case-control study of 26,903 subjects from 52 countries. Am J Coll Cardiol. 2010;55:2390–8.
- Graham I, Atar D, Borch-Johnsen K, Boysen G, Burell G, Cifkova R, .; European Society of Cardiology (ESC) Committee for Practice Guidelines (CPG). European guidelines on cardiovascular disease prevention in clinical practice: executive summary: Fourth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (Constituted by representatives of nine societies and by invited experts). Eur Heart J. 2007;28:2375–414.
- Vartiainen E, Laatikainen T, Peltonen M, Juolevi A, Männistö S, Sundvall J, . Thirty-five year trends in cardiovascular risk factors in Finland. Int J Epidemiol. 2010;39:504–18.
- Bhopal R, Fischbacher C, Vartiainen E, Unwin N, White M, Alberti G. Predicted and observed cardiovascular disease in South Asians: application of FINRISK, Framingham and SCORE models to Newcastle Heart Project data. J Public Health. 2005;27:93–100.
- Lapointe A, Couillard C, Piché ME, Weisnagel SJ, Bergeron J, Nadeau A, . Circulating oxidized LDL is associated with parameters of the metabolic syndrome in postmenopausal women. Atherosclerosis. 2007;191:362–8.
- Hamburg NM, Larson MG, Vita JA, Vasan RS, Keyes MJ, Widlansky ME, . Metabolic syndrome, insulin resistance and brachial artery vasodilator function in Framingham offspring participants without clinical evidence of cardiovascular disease. Am J Cardiol. 2008;101:82–8.
- McVeigh GE, Hamilton PK, Morgan DR. Evaluation of mechanical arterial properties: clinical, experimental and therapeutic aspects. Clin Sci (Lond). 2002;102:51–67.
- Domanski M, Mitchell G, Pfeffer M, Neaton J, Norman J, Svendsen K, . Pulse pressure and cardiovascular disease-related mortality. Follow-up study of multiple risk factor intervention trial (MRFIT). JAMA. 2002;287:2677–83.
- Franklin S, Khan S, Wong N, Larson M, Levy D. Is pulse pressure useful in predicting risk for coronary heart disease? The Framingham Heart Study. Circulation. 1999;100:354–60.
- Mulé G, Nardi E, Cottone S, Cusimano P, Incalcaterra F, Palermo A, . Relationship of metabolic syndrome with pulse pressure in patients with essential hypertension. Am J Hypertens. 2007;20:197–203.
- Ballantyne C, Arroll B, Shepherd J. Lipids and CVD management: towards a global consensus. Eur Heart J. 2005;26: 2224–31.
- Van Hateren KJ, Landman GW, Kleefstra N, Logtenberg SJ, Groenier KH, Kamper AM, . The lipid profile and mortality risk in elderly type 2 diabetic patients: A ten-year follow-up study (ZODIAC-13). PLoS One. 2009;4:e8464.
- Elosua R, Molina L, Fito M, Arquer A, Sanchez-Quesada JL, Covas MI, . Response of oxidative stress biomarkers to a 16-week aerobic physical activity program, and to acute physical activity, in healthy young men and women. Atherosclerosis. 2003;167:327–34.
- Leibovitz E, Hazanov N, Zimlichman R, Shargorodsky M, Gavish D. Treatment with atorvastatin improves small artery compliance in patients with severe hypercholesterolemia. Am J Hypertens. 2001;14:1096–8.
- Laurent S, Cockroft J, Van Bortel L, Boutoutyrie P, Giannattasio C, Hayoz D, . Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27:2588–605.
- Resnick LM, Militianu D, Cunnings AJ, Pipe JG, Evelhoch JL, Soulen RL, . Pulse waveform analysis of arterial compliance: relation to other techniques, age, and metabolic variables. Am J Hypertens. 2000;13:1243–9.
- Wilson AM, O'Neal D, Nelson CL, Prior DL, Best JD, Jenkins AJ. Comparison of arterial assessments in low and high vascular disease groups. Am J Hypertens. 2004;17:285–91.
- Duprez DA, De Buyzere ML, De Bruyne L, Clement DL, Cohn JN. Small and large arterial elasticity indices in peripheral arterial occlusive disease (PAOD). Vasc Med. 2001; 6:211–4.
- Li X, Qiong XU, Tong M, Lu X, Zhang H, Zhou Y, . Microalbuminuria associated with systolic blood pressure and arterial compliance in Chinese metabolic syndrome patients. Chin Med J. 2007;120:1395–9.
- Prisant LM, Loebl DH, Waller JL. Arterial elasticity and erectile dysfunction in hypertensive men. J Clin Hypertens. 2006;8:768–74.
- Zimlichman R, Shargorodsky M, Boaz M, Duprez D, Rahn KH, Rizzoni D, . Determination of arterial compliance using blood pressure waveform analysis with the CR-2000 system: reliability, repeatability, and establishment of normal values for healthy European population—the seven European sites study (SESS). Am J Hypertens. 2005;18:65–71.
- Duprez D, Kaiser D, Whitwam W, Finkelstein S, Belalcazar A, Patterson R, . Determinants of radial artery pulse wave analysis in asymptomatic individuals. Am J Hypertens. 2004;17:647–53.
- Kressel G, Trunz B, Bub A, Hülsmann O, Wolters M, Lichtinghagen R, . Systemic and vascular markers of inflammation in relation to metabolic syndrome and insulin resistance in adults with elevated atherosclerosis risk. Atherosclerosis. 2009;202:263–71.
- Bonora E, Kiechl S, Willeit J, Oberhollenzer F, Egger G, Meigs JB, . Insulin resistance as estimated by homeostasis model assessment predicts incident symptomatic cardiovascular disease in Caucasian subjects from the general population: the Bruneck study. Diabetes Care. 2007;30:318–24.
