Abstract
Although excess body fat is a significant health hazard, estimation of body fat content with the body mass index may not adequately reflect the amount of atherogenic adipose tissue (AT), i.e. visceral and ectopic fat. As opposed to subcutaneous AT that supposedly acts as a metabolic sink buffering excess dietary energy, visceral or intra-abdominal AT depots respond to several external stimuli that trigger lipolysis and secretion of free fatty acids (FFAs). Reaching the liver, FFAs accumulate in the liver and, over time, promote a chronic condition known as non-alcoholic fatty liver disease (NAFLD). The liver of the typical NAFLD patient secretes large amounts of very-low-density lipoproteins, the lipid content of which may accumulate in additional organs (skeletal muscle, heart, and pancreas). Here, we review the evidence emerging from functional and population studies that point towards an important role of ectopic fat accumulation in the pathophysiology of type 2 diabetes and cardiovascular disease. We conclude that although patients with impaired glycemic control or type 2 diabetes are at increased cardiovascular disease (CVD) risk, estimating cardiovascular risk goes wellbeyond the assessment of glycemic control and traditional CVD risk factors, and the estimation of visceral/ectopic fat deposition via readily available imaging techniquesshould be considered.
| Abbreviations | ||
| BMI | = | body mass index |
| CAC | = | coronary artery calcium |
| CHD | = | coronary heart disease |
| CIMT | = | carotid artery intima–media thickness |
| CRP | = | C-reactive protein |
| CT | = | computed tomography |
| CVD | = | cardiovascular disease |
| EAT | = | epicardial adipose tissue |
| FFA | = | freefatty acid |
| FHBL | = | hypobetalipoproteinemia |
| HDL | = | high-density lipoprotein |
| IHD | = | ischemic heart disease |
| IL-6 | = | interleukin-6 |
| IMCL | = | intramyocellular lipid |
| LDL | = | low-density lipoprotein |
| MRI | = | magnetic resonance imaging |
| NAFLD | = | non-alcoholic fatty liver disease |
| NASH | = | non-alcoholic steatohepatitis |
| NMR | = | nuclear magnetic resonance |
| PAT | = | pericardial adipose tissue |
| PPAR-γ | = | peroxisome proliferator-activated receptor-γ |
| SAT | = | subcutaneous adipose tissue |
| VAT | = | visceral adipose tissue |
Key messages
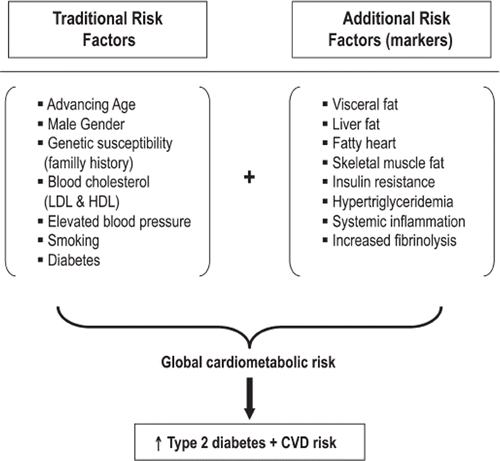
Independent of total body fat, visceral (intra-abdominal) and hepatic fat accumulation are significant predictors of a deteriorated cardiometabolic risk profile and of an increased risk for type 2 diabetes and cardiovascular disease.
Imaging techniques are now available to estimate the amount of ectopic fat accumulation in the intra-abdominal adipose depots, the liver, the heart, and the skeletal muscle.
Although patients with type 2 diabetes are characterized by an increased cardiovascular disease (CVD) risk, a better ‘phenotyping’ of these patients would require going beyond the assessment of glycemic control and traditional CVD risk factors and rather measure or at least estimate the extent of ectopic fat accumulation.
Introduction
It is now widely accepted that obesity causes prejudice to several aspects of global human health (Citation1–7). However, some individuals carrying what could be considered as a small excess of body fat may nevertheless show early signs of insulin resistance and substantial deterioration in their plasma lipoprotein-lipid profile, which are predictive of increased diabetes and cardiovascular disease (CVD) risk (Citation8). Assessing high-risk obesity in clinical practice represents a challenge to physicians and health care professionals due to its remarkable heterogeneity. Prospective and cross-sectional imaging studies published over the last 20–25 years have provided robust evidence that the regional distribution of body fat, rather than excess fatness per se, is the critical correlate of the metabolic abnormalities expected from the presence of overweight/obesity. Although waist circumference should be measured by all means beyond the body mass index (BMI) to estimate the amount of atherogenic abdominal adipose tissue accumulation, the assessment of waist circumference does not discriminate between visceral (VAT) versus subcutaneous adipose tissue (SAT) accumulation, the latter fat depot being associated with an overall more favorable cardiometabolic risk profile (Citation9). It has been proposed that VAT accumulation could represent a good marker for the presence of more fundamental neuroendocrine abnormalities leading to storage of lipids at undesired sites, such as the liver, the heart, the skeletal muscle, and the pancreas, a phenomenon described in the literature as ‘ectopic fat’ deposition (Citation10). The main objective of this paper is therefore to provide an overview of the relationship between visceral fat, liver fat, heart fat, and muscle lipids and metabolic abnormalities predisposing individuals with such ectopic fat accumulation to develop premature CVD and/or type 2 diabetes.
Abdominal fat and visceral adipose tissue
In 1947, a French physician named Jean Vague was the first to propose that excess upper body fat (a phenotype frequently found in men) was more closely associated with greater cardiovascular complications than other forms of obesity such as those often found in premenopausal women who tend to accumulate lower body fat in the gluteal-femoral areas (Citation11). In fact, it is well known that premenopausal women must accumulate a substantial amount of body fat in order to develop the metabolic abnormalities found in men.
The epidemiological evidence
Visceral fat represents approximately 10% of total body fat in women whereas this proportion may reach up to 20%–25% in men (Citation12). As abdominal fat accumulation is modulated by steroid hormones, postmenopausal women tend to develop a pattern of visceral fat accumulation which eventually approaches over years to the one found in men, a phenomenon which considerably raises their CVD and type 2 diabetes risk (Citation13,Citation14). On top of gender and steroid hormones, several other biological factors explain the regional distribution of abdominal fat such as age, ethnicity (for a given waist circumference, Asians have more visceral fat than Caucasians, who in turn have more visceral fat than blacks (Citation15–17)), stress, lack of physical activity/exercise, diet, and genetic factors (Citation18,Citation19). Although it is beyond the scope of this review article to summarize the biological mechanisms that lead to increased visceral and ectopic fat accumulation (see other timely reviews on the topic (Citation20–22)), we will briefly describe the evidence that points towards a detrimental role of visceral adiposity and ectopic fat deposition in the risk of type 2 diabetes and CVD.
Over the past decade, a few studies have investigated the relationship between VAT and incident coronary heart disease (CHD). In a prospective study including 291 men who were followed for 2.2 years, Kuk and colleagues (Citation23) have shown that the amount of VAT was an independent predictor of all-cause mortality. Importantly, the relationship between VAT and mortality was independent of other indices of body fat distribution such as SAT, liver fat, as well as waist circumference. In a sample of Japanese men followed for 10 years, those who incidentally developed CHD during follow-up had comparable BMIs as well as similar total and SAT accumulation, whereas CHD cases had approximately 22% more VAT than controls (Citation24). VAT was also found to be an independent predictor of incident type 2 diabetes in that cohort (Citation25). In women aged 70–79 years in the Health, Aging and Body Composition Study, VAT was an independent predictor of incident myocardial infarction over a 4.6-year follow-up (Citation26), the relationship between VAT and incident myocardial infarction being independent of BMI and total fat mass. Among men of the same age-range who were included in the study, there was no relationship between any of the studied adiposity markers and incident myocardial infarction. This sex-specific relationship may be attributable to a survival bias and/or to the fact that with advancing age, increased body fat accumulation may represent a ‘protective’ fuel source. However, both VAT and SAT were associated with the risk of chronic heart failure in the Health, Aging and Body Composition Study (Citation27). In the Multicultural Community Health Assessment Trial (M-CHAT), Lear and colleagues(Citation28) tested the potential cross-sectional associations between VAT and carotid artery intima–media thickness (CIMT) measured by carotid ultrasound in apparently healthy middle-aged individuals. Among the 794 participants of the study, they found that VAT was positively associated with CIMT and with total plaque area even after adjusting for potential confounders including age, ethnicity, smoking, and total body fat. In men, but not in women, VAT was associated with CIMT after further adjustment for glucose and insulin levels, homocysteine levels, blood pressure, the total cholesterol to high-density lipoprotein (HDL) ratio, low-density lipoprotein (LDL) cholesterol, triglycerides, apolipoprotein B levels, and waist circumference. Finally, in an older cohort of subjects without documented CHD, the Rancho Bernardo Study, Kramer and colleagues (Citation29) showed that abdominal obesity was an independent predictor of coronary artery calcium (CAC) progression in both sexes. In this sample of 156 men and 182 women (mean age of 67 years), results showed that waist circumference was the best predictor of CAC progression in women, whereas the ratio of VAT to SAT was the most powerful predictor in men.
Visceral adipose tissue and the metabolic syndrome
Aiming at identifying the potential biological mechanisms that may explain the association between VAT and CVD/type 2 diabetes risk, several investigators have suggested that increased VAT is the key correlate of the metabolic syndrome (Citation30–32). The metabolic syndrome represents a constellation of CVD risk factors including abdominal obesity, hypertension, a deteriorated lipoprotein-lipid profile, a pro-inflammatory and pro-thrombotic profile, as well as insulin resistance, leading to glucose intolerance and type 2 diabetes (Citation33,Citation34). Meta-analyses conducted on the topic have shown that the presence of the metabolic syndrome increased the risk of CVD approximately 1.5–2.0-fold (Citation35,Citation36). Although the etiology of the metabolic syndrome is still a matter of debate (Citation37), cross-sectional and prospective studies have shown that the relationship between VAT and metabolic syndrome was independent of other body fat compartments such as SAT, specific measures of insulin resistance, or cardiorespiratory fitness (Citation38–42). A substantial number of cross-sectional studies have also shown that independently of anthropometric indices such as BMI, waist circumference, and SAT, the amount of VAT was positively associated with plasma levels of C-reactive protein (CRP), a marker of inflammation, which has emerged as a significant correlate of CVD risk (Citation43–45).
Subcutaneous adipose tissue: a protective body fat compartment?
Although excess abdominal fat is detrimental to human health, experimental and imaging studies have provided evidence that irrespective of VAT accumulation, some SAT depots may represent metabolic sinks buffering the energy surplus towards potentially protective body fat compartments. Among those pioneer studies, investigators of the Framingham Heart Study have recently provided convincing evidence for a cardioprotective role of increased SAT accumulation (Citation46). In that study, although both SAT and VAT were positively associated with a deteriorated cardiometabolic risk profile, a thorough classification of the study participants on the basis of both VAT and SAT accumulation revealed that among participants with the highest VAT accumulation, those with the highest SAT accumulation were characterized by the lowest triglyceride levels. Among men with the highest VAT accumulation, higher SAT levels were also associated with a lower prevalence of low HDL cholesterol levels (Citation46). Although this study was limited by its cross-sectional design, it provided evidence for a cardioprotective role of SAT. In the same study population, VAT was found to be a better correlate of specific features of insulin resistance compared to SAT (Citation47). These results, combined with those of epidemiological studies which have suggested a cardioprotective role of carrying an elevated hip circumference (an anthropometric marker of gluteo-femoral fat) for a given waist circumference, further reinforce the notion that there is a need to go beyond traditional indices of body fat distribution to appreciate better the role of regional adipose tissue distribution as a potential risk factor for cardiovascular mortality and morbidity (Citation6,Citation48). However, beyond the cardioprotective role of SAT, one must not disregard the direct impact of an increased VAT on cardiovascular health.
The biological evidence
In order to appreciate the deleterious consequences of visceral fat on various metabolic pathways, a closer look at the pathobiology of visceral adipocytes is warranted. In lean and healthy individuals, insulin has a critical role to play in maintaining optimal adipocyte function (differentiation, lipid storage, lipolysis, etc.). As VAT mass expands, as a consequence of overeating, lack of physical activity, insulin resistance, and inflammation, insulin is no longer capable of controlling adipocyte differentiation and the balance between lipid uptake and lipolysis. Despite not being able to divide, visceral adipocytes continue to remove lipids from the blood-stream and therefore become hypertrophied (Citation49). In parallel, a local state of hypoxia controls macrophage infiltration inside VAT, which further preventsperoxisome proliferator-activated receptor(PPAR)-γ-dependent fat cell differentiation through inflammatory mediators such as tumor necrosis factor-α or interleukin (IL)-6 (Citation50,Citation51). Interestingly, IL-6 also has a key role to play in promoting hepatic C-reactive protein secretion, another detrimental consequence of the portal vein system draining fat cells in the visceral adipose tissue (Citation52). Investigators who have directly studied the anatomical characteristics of abdominal adipose tissue have reached the conclusion that visceral adipocytes, e.g. those located in the omentum and around the mesentery which are drained by the portal vein, directly carry the VAT-derived secretagogues to the liver, whereas adipose cells of subcutaneous depots are drained by the peripheral vein system (Citation53). According to this ‘portal vein theory’, freefatty acids (FFAs), which are secreted by visceral adipocytes in a dose-dependent manner (increased VAT correlated with portal FFA levels), are directly conveyed to the liver where they accumulate. This phenomenon under which FFAs and triglycerides accumulate inside the liver is an important cause of liver fat infiltration, which may lead to non-alcoholic fatty liver disease(NAFLD). In a heterogeneous non-diabetic population, Thamer and colleagues (Citation56) have shown that even after adjusting for sex, age, waist-to-hip ratio, and SAT, VAT was a powerful predictor of intrahepatic lipid accumulation as measured by proton magnetic resonance spectroscopy. Other groups have also reported similar observations and further suggested that visceral fat changes over time may be associated with the onset of NAFLD (Citation57,Citation58).
Visceral fat and inflammation
Among the other potential links between excess visceral adiposity and hepatic fat accumulation, non-infectious or systemic inflammation is also recognized as a key player. It has been documented for decades that inflammation plays an important role in the pathophysiology of several chronic diseases such as CVD and type 2 diabetes (Citation61–63). However, recent data point towards VAT as an important source of pro-inflammatory and pro-thrombotic adipokines that may promote hepatic and peripheral insulin resistance and favor plaque occlusion and rupture (Citation64,Citation65). In fact, we now know that several inflammatory cells such as leukocytes and macrophages infiltrate and reside within VAT where, upon activation, they create a local state of inflammation and insulin resistance (Citation66,Citation67). Among the inflammatory markers secreted by tissues localized inside VAT, IL-6 may contribute to the local insulin resistance state inside adipose tissue as well as in the liver by reducing the expression of insulin receptor substrate-1, a key protein involved in insulin signaling (Citation68,Citation69). It has recently been shown that VAT accumulation was an independent predictor of circulating IL-6 concentrations (Citation70). A study also showed that portal vein IL-6 concentrations were twice as high as IL-6 concentrations inside the brachial artery (Citation71). As portal vein IL-6 concentrations were also closely associated with brachial artery CRP concentrations, this study provided strong experimental evidence that VAT is a sensible predictor of systemic inflammation. Accordingly, results of the Québec Cardiovascular Study have shown that the long-term relationship between IL-6 and risk of ischemic heart disease (IHD) was independent of plasma CRP levels, whereas the opposite was not found, i.e. the relationship of CRP to IHD was not independent of IL-6 (Citation72). Similar results have also recently been published in a sample of middle-aged men living in England (Citation73). On top of promoting hepatic CRP production, IL-6 increases insulin resistance in hepatocytes and stimulates hepatic gluconeogenesis (Citation69,Citation74). In contrast to IL-6, an expanded VAT area has been associated with low plasma levels of adiponectin, an anti-inflammatory and anti-diabetic adipokine (Citation75). Adiponectin is specifically secreted by adipocytes. In normal-weight individuals, adiponectin is one of the most abundant proteins in the blood-stream that might protect the vascular endothelium from lipid and monocyte infiltration (Citation76). Several investigations have also reported that adiponectin has an important role to play in hepatic lipoprotein-lipid metabolism (Citation77,Citation78). For instance, in a murine model of NAFLD, injection of recombinant adiponectin has been shown to lower hepatic steatosis, hepatic inflammation, as well as serum transaminase concentrations (Citation79). The next section will discuss the biology and the pathological consequences of hepatic fat accumulation.
Liver fat
The liver is the central organ of the lipoprotein-lipid metabolism. In genetically predisposed individuals as well as in many who adopt poor life-style habits, small droplets of triglyceride may accumulate in the liver. Accumulation of hepatic fat may eventually lead to NAFLD, which encompasses the spectrum that links the fatty liver, non-alcoholic steatohepatitis (NASH), and eventually liver cirrhosis. Under homeostatic conditions, the relative percentage of fat in the liver is below 5% of its total weight (Citation80). NAFLD is defined as the proportion of fat accumulation in the liver exceeding 5%–10% of total liver weight, as determined from the percentage of fat-laden hepatocytes by light microscopy (Citation81). It has been shown that the worldwide prevalence of fatty liver is directly correlated with the prevalence of obesity (Citation82). For instance, in the US, the prevalence of obesity (defined as BMI ≥30 kg/m2) is identical to the prevalence of NAFLD, reaching an astonishing 30% of the general population (Citation83).
Several studies have shown that liver fat is closely associated with the components as well as with the development of the metabolic syndrome. Recently, Kotronen and colleagues have provided strong evidence supporting the idea that liver fat is a hall-mark of type 2 diabetes (Citation84). After having carefully matched patients with type 2 diabetes with non-diabetic patients for age and anthropometric indices such as BMI or waist circumference, patients with type 2 diabetes had 80% more liver fat than non-diabetic patients, while showing 16% more visceral fat. However, because of the cross-sectional design of this study, a causal relationship between liver fat and type 2 diabetes could not be established. However, one could suggest from these results that liver fat should also be considered as a prevalent marker/feature of type 2 diabetes. Another study conducted among patients with type 2 diabetes has revealed that insulin resistance, dyslipidemia, and systemic inflammation were only found among those who were characterized by excess liver fat (Citation85). Therefore, the direct measurement of liver fat by proton magnetic resonance spectroscopy may be helpful as a sensitive predictor of type 2 diabetes, independent of waist circumference and/or liver enzymes. However, it is not clear whether visceral fat, liver fat, or both, should be considered to provide a sensitive prognosis of either CVD or type 2 diabetes risk. In this regard, it has been shown that both VAT and liver fat predicted a deteriorated metabolic risk profile among men, independently of other covariates such as SAT and cardiorespiratory fitness (Citation86). In a sample of premenopausal women, VAT but not liver fat was a powerful predictor of a deteriorated metabolic risk profile (Citation87). This discrepancy in the association of visceral and ectopic fat with cardiometabolic risk across these studies may be attributable to the low prevalence of liver fat in premenopausal women compared to men. Following menopause, however, the prevalence of NAFLD increases to a point that it can reach the levels observed in men. On the other hand, a recent study by Fracanzani et al. (Citation88) has shown that NAFLD could be present even in individuals with a low waist circumference. However, investigators of this study estimated visceral fat by measuring waist circumference rather than obtaining a direct measurement with imaging techniques. In subjects with type 2 diabetes, VAT and liver fat (but not SAT) were both independently associated with basal hepatic insulin resistance and residual endogenous glucose production measured during the glucose-insulin clamp procedure (Citation89). Similar to VAT accumulation, ethnic differences in liver fat accumulation can be observed. In a recent population-based study, it was found that liver fat accumulation was closely linked to visceral adiposity, regardless of ethnicity, in a large group of adults (Citation90). However, in this study, for a given body mass, African-Americans tended to accumulate less VAT than either Caucasians or Hispanics.
From the above studies, it is difficult to determine which factor has the most important role to play in the etiology of insulin resistance. In that regard, Amaro et al. (Citation91) have recently studied the question in patients with familial hypobetalipoproteinemia (FHBL), a genetic disorder that impairs triglyceride-rich lipoprotein secretion from the liver. Given that patients with FHBL had similar hepatic and muscle insulin sensitivity indices to lean individuals, indices that were, in both cases, considerably higher than those of individuals matched for hepatic fat content but without FHBL (patients with NAFLD), it could be suggested that liver fat in isolation cannot cause insulin resistance. However, in the setting of a dysmetabolic environment such as what can be observed in individuals with increased VAT accumulation, liver fat may associate with local and peripheral insulin resistance.
The fatty heart
In 1933, Smith and Willius (Citation92) conducted a series of pioneering autopsy studies,the results of which suggested that obesity is frequently associated with lipid infiltration in various cavities of the heart and may therefore represent an important cause of dilated cardiomyopathy and associated CVD risk in humans. In the following decades, extensive investigations have shown that fat infiltration throughout the heart may represent an important but often forgotten cause of non-ischemic dilated cardiomyopathy in humans and a direct cause of atherosclerosis (Citation93). There are three major fat depots in the heart: pericardial fat, epicardial fat, and intracellular fat (Citation94). Pericardial adipose tissue (PAT) is located outside the pericardium, whereas epicardial adipose tissue (EAT) is located at the surface of the heart where it closely surrounds coronary arteries even as they penetrate the myocardium, in the absence of any discernable fascia separating EAT from the vessel wall (Citation95). Intracellular fat is packaged in lipid droplets inside the cytoplasm of the cardiac muscle (Citation96). Altogether, adipose tissue may cover up to 80% of the heart's surface and may encompass up to 20% of its total weight (Citation97). Although the long-term relationship between lipid infiltration of the heart (in any depot) and CVD and/or type 2 diabetes risk is largely unknown, a few cross-sectional studies have reported a potential association between the fatty heart and CVD. For instance, Djaberi et al. (Citation98) have measured the volume of EAT in 190 patients undergoing multi-slicecomputed tomography (CT) coronary angiography and reported an independent association between EAT and atherosclerosis burden as estimated by the coronary artery calcium score. Similar associations were reported by Gorder et al. (Citation99), although this finding appeared to be limited to lean individuals. In another cross-sectional study, EAT thickness as well as the extent of coronary atherosclerosis measured by quantitative coronary angiography were obtained in 527 patients undergoing their first coronary angiographic procedure (Citation100). Results of this study showed that EAT was close to 3-fold thicker in patients with documented coronary artery disease compared to those without. Results of this study also showed that EAT was more closely associated with VAT compared to either BMI or waist circumference. Additional studies have reported significant associations between pericardial fat and health hazards. For instance, using dual source CT angiography to measure simultaneously PAT and coronary atherosclerosis burden in 286 consecutive patients, Greif et al. (Citation101) have recently shown that patients without plaques had low PAT whereas patients with any kind of plaques (calcified, non-calcified, or a mixture of all plaques) had approximately twice as much PAT. In that study, compared to well established CVD risk factors such as smoking, arterial hypertension, hypercholesterolemia, diabetes mellitus, and an elevated Framingham risk score, PAT was by far the most important risk factor for coronary atherosclerosis. For instance, compared with patients with a pericardial fat volume lower than 300 cm3, those with a pericardial fat volume above 300 cm3 had a relative risk for coronary atherosclerosis of 4.1 (95% CI 3.6–4.3). It has also been suggested that PAT was positively associated to the extent of coronary atherosclerosis in African-Americans with type 2 diabetes and that ethnic differences in PAT accumulation may explain to a certain extent the ethnic differences in susceptibility to atherosclerosis (Citation102).
The relationship between PAT, VAT, and CAC was also investigated in 1,155 asymptomatic individuals of the Framingham Heart Study (Citation103). Similar to what has been previously reported with EAT, PAT was positively correlated with BMI and waist circumference, but the correlation with VAT accumulation was of slightly higher magnitude. PAT was associated with a whole set of cardiometabolic risk factors related to the metabolic syndrome independently of BMI and waist circumference, but not independently of VAT. Although the relationship of PAT with coronary artery calcium was independent of VAT, VAT was closely associated with the prevalence of type 2 diabetes, impaired fasting glucose, hypertension, and metabolic syndrome at any level of PAT. Another recent report from Framingham that included individuals with CVD (n = 1,267) suggested that independently of BMI and waist circumference both PAT and VAT were associated with CVD, with VAT again showing correlations with CVD of higher magnitude than PAT (Citation104). Similar findings were observed when magnetic resonance imaging (MRI) measures of left ventricular mass and left ventricular end-diastolic volume were considered as outcomes (Citation105). Finally, using proton magnetic resonance spectroscopy, McGavock et al. (Citation106) have shown that the lipid content of cardiomyocytes was another hall-mark feature of type 2 diabetes that was closely associated with both serum and hepatic triglyceride levels in humans. This study confirmed findings that have been previously reported in the Zucker diabetic fatty rat which revealed several cardiac abnormalities such as eccentric left ventricular remodeling, increased left ventricular pressure, and decreased systolic efficiency, most of which were reversed with thiazolidinedione treatment (Citation107,Citation108). In humans, Kankaanpaa et al. have also reported that myocardial triglyceride content is associated with plasma FFA levels, overall ectopic fat deposition, and left ventricular hypertrophy (Citation109). On the impact of exercise training on EAT and VAT, Kim et al. (Citation110) have shown that this type of intervention promoted a profound loss of EAT (twice as high as the relative percentage loss of either waist circumference orBMI) and that this loss in EAT was positively associated with loss of VAT. Moreover, as recently pointed out by Sacks (Citation111), it is tempting to suggest that EAT promotes the secretion of a VAT-like adipokine pattern. However, this has not been proven in humans. The literature on the topic is somewhat scarce, and more studies on this topic are needed, especially in apparently healthy non-obese humans. In this context, a study showed that visceral but not epicardial fat is associated with blood pressure indices and severity of insulin resistance in a small sample of non-diabetic men (Citation112).
Similar to VAT, fat depots located in the heart may contribute to the low-grade inflammatory state that characterizes individuals with ectopic fat deposition/metabolic syndrome (Citation113). For instance, patients with documented coronary artery disease exhibit higher levels of IL-6 gene expression while showing lower levels of adiponectin expression (Citation114). Similar findings have been observed in patients with hypertension (Citation115). Interestingly, a recent study conducted in a population with metabolic syndrome and/or type 2 diabetes has documented the benefits of pioglitazone in reducing the genetic expression of a number of pro-inflammatory mediators such as IL-1β, IL-1Ra, and IL-10 (Citation116). Taken together, these observations suggest that ectopic fat deposition in various regions of the heart is detrimental to energy homeostasis. EAT, PAT, and intramyocellular lipids (IMCL) may be important causes of local insulin resistance and promote the secretion of several adipokines that may strongly impact the heart's ability to remove plasma lipids and use them as an energy source.
Intramyocellular lipids
Muscular infiltration of lipids is not limited to the heart muscle as virtually all skeletal muscles are subject to lipid infiltration, which may impair energy homeostasis and create a local state of muscular insulin resistance. Two fat depots are found in skeletal muscle: intramyocellular ‘intramuscular’ fat, which is fat infiltration within myocytes, and extramyocellular ‘intermuscular’ fat, which is the small adipose depot interspersed between muscle fibers. Although lipid droplets inside the skeletal muscle may be an important source of FFAs to be oxidized by surrounding cells, an elevated IMCL content assessed by proton nuclear magnetic resonance (NMR) spectroscopy has been shown to be a close correlate of insulin resistance in several studies (Citation117,Citation118). The ability to distinguish between the two depots may be important since studies have suggested that extramyocellular fat is also associated with higher fasting glucose (Citation119), impaired glucose tolerance, insulin resistance, type 2 diabetes (Citation120), and metabolic syndrome (Citation121), independent of overall obesity. Interestingly, a recent longitudinal study has shown that skeletal muscle fat infiltration increases with aging and decreases with exercisetraining. Notably, such changes are mostly independent of changes in body-weight(Citation122). However, for the moment, there are few data available on a potential role for IMCL as an important risk factor for CVD or type 2 diabetes. As IMCL is usually correlated with intrahepatic fat accumulation or VAT, the deteriorated cardiometabolic risk profile has been proposed to be most likely attributable to these ectopic deposition sites rather than to IMCL per se (Citation123). The benefits of PPAR-γ activation have also been reported in a study by Bajaj et al. (Citation124) who have shown that following pioglitazone treatment in a population of patients with type 2 diabetes, intramyocellular fat content (measured by proton NMR) decreased by approximately 40%.
Pancreatic fat
In the natural development of type 2 diabetes, insulin secretion generally increases as insulin resistance develops until the pancreas can no longer secrete enough insulin to maintain glycemia to a normal level. However, ectopic lipid accumulation in the pancreas may represent a key feature of obesity-driven type 2 diabetes and could also explain to a certain extent such impairment in insulin secretion (Citation125). A number of studies have recently investigated the potential relationship between pancreas lipid accumulation and β-cell dysfunction. Animal studies have shown that increased pancreatic lipid accumulation was associated with deterioration of both β-cell mass and function (Citation126,Citation127). In humans, lipid-induced β-cell damage could be present for more than a decade before the onset of type 2 diabetes. Pancreatic lipid content increases with deterioration of glucometabolic state, as shown by van der Zijl et al. (Citation128). Another study in humans has also shown that increased pancreatic fat directly associates with impaired insulin secretion in subjects with impaired fasting glucose (Citation129). Interestingly, it has been shown that mice with specific inactivation of ATP binding-cassette A-1 (ABCA1), a cellular cholesterol transporter, in the β-cell have impaired glucose tolerance and that,ex vivo, the islets of ABCA1-/- mice showed impaired insulin secretion (Citation130). In humans, carriers of loss-of-function mutations in ABCA1 have impaired pancreatic β-cell function, which suggests that the cholesterol metabolism may play a significant role in the etiology of insulin resistance (Citation131). In humans, the concept of lipotoxicity as a causal factor for β-cell dysfunction remains unclear and warrants further investigation (Citation132).
In comparison with other fat sites, fat infiltrated in the heart, skeletal muscle, and the pancreas accounts for a very small proportion of total body fat (less than 5%). However, the local toxic effects may play a very important pathophysiological role in the various systems that regulate energy homeostasis. Consequently, removal or utilization of lipids in these ectopic sites may become an important target of therapy to prevent the onset of chronic diseases such as type 2 diabetes, CVD, and hypertension (). Experimental and clinical studies are clearly needed to test this hypothesis. Additionally, although the relationships between fat accumulation in various tissues are often correlated to oneanother, studies have suggested that this may not always be the case (Citation106). One of the biggest challenges of the field will be to validate whether or not ectopic fat in the above-discussed depots will predict CVD and/or type 2 diabetes independent of each other.
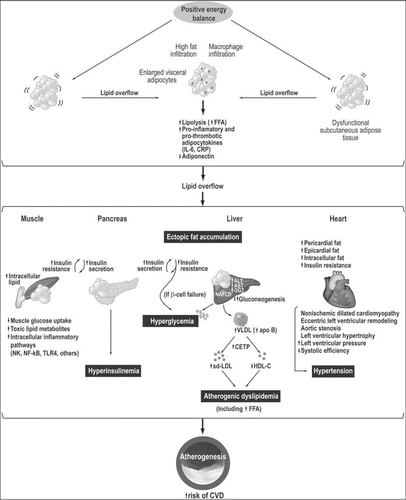
Figure 1. Schematic representation of abnormal visceral adipose tissue development leading to the storage of lipids at undesired sites. This phenomenon has been described as ‘ectopic fat’ deposition. According to this model, when energy intake is greater than energy expenditure, a dysfunctional subcutaneous adipose tissue may be unable to store appropriately the dietary energy excess, eventually leading to lipid overflow and to visceral fat deposition. This excess of visceral adiposity is associated with an altered free fatty acid metabolism with the release of adipokines as well as with adipocyte hypertrophy and increased fat storage in non-adipose organs, notably liver and skeletal muscle, and in other organs such as the pancreas and the heart. Excess adipocyte hypertrophy leads to ectopic fat storage and metabolic abnormalities such as insulin resistance, atherogenic dyslipidemia, hypertension, systemic inflammation, and eventually increases the risk of CVD.
Assessing body fat distribution with imaging techniques
Several imaging techniques such as CT and MRI have been proven to be useful for the determination of total and regional body composition. At the time being, these methods are the most accurate tools available for directly quantifying body composition at a tissue level. Both methods provide cross- sectional images that can be used to determine SAT and VAT accumulation in vivo. CT uses ionizing radiation and differences in tissue X-ray attenuation to produce cross-sectional images of the body, which can be compared to determine the surface of various fat depots (Citation12,Citation133). Although excess VAT accumulation is increasingly recognized as a risk factor for type 2 diabetes and CVD, there is currently no consensus with regard to optimal cut-offs for identifying individuals with the high-risk obesity phenotype. Currently, suggested cut-offs at the L4–L5 level range from 100 to 130 cm2. Nevertheless, regardless of optimal cut-off values, an increase in VAT is a hall-mark feature of increased cardiometabolic risk, even among normal-weight individuals. However, as CT is expensive and involves radiation exposure, it is currently not advised to measure routinely the VAT using CT or MRI in clinical practice. Health professionals are therefore advised to focus on measuring waist circumference on a regular basis as waist circumference is, for the time being, the best surrogate measure currently available. CT can also be used to assess lipid infiltration in other peripheral tissues such as muscle and the liver (Citation134). Since fat has a lower density than both water and protein, visceral, muscle, or liver fat infiltration can be reflected and quantified by a lower liver density and thus lower attenuation values.
Glycemic control and CVD risk: are we aiming at the right target?
There is now considerable evidence that type 2 diabetes is a significant risk factor for CVD. Although this is not a consistent finding, some prospective studies have reported that individuals with type 2 diabetes without CVD may carry the same risk of incident CVD as individuals without diabetes that have previously suffered a myocardial infarction (Citation135,Citation136). Clinicians are aware of the deleterious vascular consequences of a poorly controlled hyperglycemic state. For instance, a poor glycemic control reflected by high plasma glycated hemoglobin levels is associated with microcirculatory complications which may lead to retinopathies, nephropathies, and neuropathic complications (Citation137,Citation138). These microcirculatory damages explain why individuals with poor glycemic control are at increased risk for blindness and end-stage renal disease, which may lead to dialysis, as well as amputations (Citation139).
On the basis of clinical trials that have documented the protective effects of a better glycemic control on the microcirculation, physicians are required to use a broad pharmacological arsenal and, if needed, exogenous insulin to protect patients against these complications (Citation140). However, the literature providing evidence for a strong protective effect of better glycemic control on atherosclerotic macrovascular disease is limited. For instance, although the pharmacological management of concomitant risk factors such as hypertension and LDL-cholesterol levels has clearly produced cardiovascular benefits in patients with type 2 diabetes, three recently reported trials have failed to report clear cardiovascular benefits of a better glycemic control achieved by intensive pharmacotherapy (Citation141–143). Although results coming from these large clinical studies may be disappointing at first sight, they have led the scientific and medical community to seek additional risk factors for CVD and other therapeutic targets in patients with diabetes. In this regard, we and other groups around the world have shown that the high triglyceride–low HDL cholesterol atherogenic dyslipidemia, which is part of a broader constellation of risk factors including visceral obesity and inflammation, is predictive of a further increase in CVD risk in patients with type 2 diabetes (Citation144–146). Therefore, even if the population of patients with type 2 diabetes is, as a whole, characterized by an increased CVD risk, a better ‘phenotyping’ of patients with type 2 diabetes would require going beyond the assessment of glycemic control and of traditional CVD risk factors. In this regard, studies have shown that approximately 15% of patients with type 2 diabetes are neither viscerally obese nor dyslipidemic and are therefore at much lower risk of CVD than the majority of patients (85%) with type 2 diabetes who are likely to carry a certain amount of VAT and/or ectopic fat (Citation147). Furthermore, within the latter group, we have proposed that the greater the amount of VAT/ectopic fat, the greater will be the risk of CVD among patients with type 2 diabetes (Citation144). It is increasingly recognized that type 2 diabetes is a very heterogeneous condition and that those patients with type 2 diabetes that are at higher CVD risk are likely to be those with deleterious body fat distribution patterns. These patients nowadays unfortunately represent the vast majority of patients with type 2 diabetes.
In order to decrease the odds of apparently healthy individuals with visceral obesity/ectopic fat converting to type 2 diabetes or having a vascular event, increasing daily energy expenditure though regular physical activity/exercise is warranted in most if not all individuals. In this context, the results of the Diabetes Prevention Program have shown that increasing physical activity levels delays the onset of both type 2 diabetes (Citation148) and metabolic syndrome (Citation149). Interestingly, the recently published results of the LOOK-AHEAD trial (Citation150) may suggest that increasing physical activity levels improves cardiovascular risk factors in obese patients with type 2 diabetes. On top of life-style therapy, careful management of parameters of the lipoprotein-lipid profile is advised in this high-risk population, and both statin and fibrate therapy have been proven effective in patients with metabolic syndrome or those with the hightriglyceride–low HDL cholesterol atherogenic dyslipidemia (Citation151–154).
Conclusions
Although mortality from CVD appears to have recently decreased in several developed countries, there is evidence that the mosaic of modifiable risk factors for CVD has evolved with an increased prevalence of type 2 diabetes that is likely to be attributable to the increased prevalence of individuals characterized by high amounts of visceral fat (Citation155,Citation156). As the worldwide prevalence of patients characterized by a deteriorated cardiometabolic risk profile is steadily increasing (Citation157,Citation158), there is an urgent need to develop new therapeutic approaches where the loss of visceral/ectopic fat would become an additional therapeutic target which would be as important if not more as achieving optimal glycemic control (). With the arsenal of non-invasive imaging techniques to assess not only body fat distribution, there is a remarkable opportunity to decipher better the key drivers of cardiometabolic risk in a rapidly expanding group of individuals with poor life-style habits and with an excess of visceral/ectopic fat.
Acknowledgements
The work of the authors has been supported by research grants from the Canadian Institutes of Health Research, the Heart and Stroke Foundation, and by the Foundation of the Quebec Heart Institute. Dr Larose is a researchscholar of the Fonds de la Recherche en Santé du Québec (FRSQ). Dr Després is the Scientific Director of the International Chair on Cardiometabolic Risk, which is based at the Université Laval. Benoit J. Arsenault is supported by a post-doctoral fellowhip from the Fonds de la Recherche en Santé du Québec and the Fondation de l’Institut universitaire de cardiologie et de pneumologie de Québec.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- McGee DL. Body mass index and mortality: a meta-analysis based on person-level data from twenty-six observational studies. Ann Epidemiol. 2005;15:87–97.
- Balkau B, Deanfield JE, Després JP, Bassand JP, Fox KA, Smith SC Jr, . International Day for the Evaluation of Abdominal Obesity (IDEA): a study of waist circumference, cardiovascular disease, and diabetes mellitus in 168,000 primary care patients in 63 countries. Circulation. 2007; 116:1942–51.
- Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, . Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004; 364:937–52.
- Ohlson LO, Larsson B, Svardsudd K, Welin L, Eriksson H, Wilhelmsen L, . The influence of body fat distribution on the incidence of diabetes mellitus. 13.5 years of follow-up of the participants in the study of men born in 1913. Diabetes. 1985;34:1055–8.
- Pischon T, Lahmann PH, Boeing H, Friedenreich C, Norat T, Tjonneland A, . Body size and risk of colon and rectal cancer in the European Prospective Investigation Into Cancer and Nutrition (EPIC). J Natl Cancer Inst. 2006;98:920–31.
- Zhang C, Rexrode KM, van Dam RM, Li TY, Hu FB. Abdominal obesity and the risk of all-cause, cardiovascular, and cancer mortality: sixteen years of follow-up in US women. Circulation. 2008;117:1658–67.
- Brown LK. A waist is a terrible thing to mind: central obesity, the metabolic syndrome, and sleep apnea hypopnea syndrome. Chest. 2002;122:774–8.
- Poirier P, Giles TD, Bray GA, Hong Y, Stern JS, Pi-Sunyer FX, . Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss: an update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2006;113:898–918.
- Lemieux I, Drapeau V, Richard D, Bergeron J, Marceau P, Biron S, . Waist girth does not predict metabolic complications in severely obese men. Diabetes Care. 2006;29:1417–9.
- Rasouli N, Molavi B, Elbein SC, Kern PA. Ectopic fat accumulation and metabolic syndrome. Diabetes Obes Metab. 2007;9:1–10.
- Vague J. La différenciation sexuelle : facteur déterminant des formes de l’obesité. Presse Med. 1947;55:339–40.
- Kvist H, Sjostrom L, Tylen U. Adipose tissue volume determinations in women by computed tomography: technical considerations. Int J Obes. 1986;10:53–67.
- Krotkiewski M, Bjorntorp P, Sjostrom L, Smith U. Impact of obesity on metabolism in men and women. Importance of regional adipose tissue distribution. J Clin Invest. 1983;72:1150–62.
- Katzmarzyk PT, Bray GA, Greenway FL, Johnson WD, Newton RL Jr, Ravussin E, . Racial differences in abdominal depot-specific adiposity in white and African American adults. Am J Clin Nutr. 2010;91:7–15.
- Sniderman AD, Bhopal R, Prabhakaran D, Sarrafzadegan N, Tchernof A. Why might South Asians be so susceptible to central obesity and its atherogenic consequences? The adipose tissue overflow hypothesis. Int J Epidemiol. 2007;36:220–5.
- Kadowaki T, Sekikawa A, Murata K, Maegawa H, Takamiya T, Okamura T, . Japanese men have larger areas of visceral adipose tissue than Caucasian men in the same levels of waist circumference in a population-based study. Int J Obes (Lond). 2006;30:1163–5.
- Hoffman DJ, Wang Z, Gallagher D, Heymsfield SB. Comparison of visceral adipose tissue mass in adult African Americans and whites. Obes Res. 2005;13:66–74.
- Rice T, Pérusse L, Bouchard C, Rao DC. Familial clustering of abdominal visceral fat and total fat mass: the Québec Family Study. Obes Res. 1996;4:253–61.
- Romaguera D, Angquist L, Du H, Jakobsen MU, Forouhi NG, Halkjaer J, . Dietary determinants of changes in waist circumference adjusted for body mass index—a proxy measure of visceral adiposity. PLoS ONE. 2010;5:e11588.
- Arner P. Human fat cell lipolysis: biochemistry, regulation and clinical role. Best Pract Res Clin Endocrinol Metab. 2005;19:471–82.
- Jensen MD. Role of body fat distribution and the metabolic complications of obesity. J Clin Endocrinol Metab. 2008;93(11 Suppl 1):S57–63.
- Stefan N, Machann J, Schick F, Claussen CD, Thamer C, Fritsche A, . New imaging techniques of fat, muscle and liver within the context of determining insulin sensitivity. Horm Res. 2005;64 Suppl 3:38–44.
- Kuk JL, Katzmarzyk PT, Nichaman MZ, Church TS, Blair SN, Ross R. Visceral fat is an independent predictor of all-cause mortality in men. Obesity (Silver Spring). 2006;14:336–41.
- Fujimoto WY, Bergstrom RW, Boyko EJ, Chen KW, Leonetti DL, Newell-Morris L, . Visceral adiposity and incident coronary heart disease in Japanese-American men. The 10-year follow-up results of the Seattle Japanese-American Community Diabetes Study. Diabetes Care. 1999;22:1808–12.
- Boyko EJ, Fujimoto WY, Leonetti DL, Newell-Morris L. Visceral adiposity and risk of type 2 diabetes: a prospective study among Japanese Americans. Diabetes Care. 2000; 23:465–71.
- Nicklas BJ, Penninx BW, Cesari M, Kritchevsky SB, Newman AB, Kanaya AM, . Association of visceral adipose tissue with incident myocardial infarction in older men and women: the Health, Aging and Body Composition Study. Am J Epidemiol. 2004;160:741–9.
- Nicklas BJ, Cesari M, Penninx BW, Kritchevsky SB, Ding J, Newman A, . Abdominal obesity is an independent risk factor for chronic heart failure in older people. J Am Geriatr Soc. 2006;54:413–20.
- Lear SA, Humphries KH, Kohli S, Frohlich JJ, Birmingham CL, Mancini GB. Visceral adipose tissue, a potential risk factor for carotid atherosclerosis: results of the Multicultural Community Health Assessment Trial (M-CHAT). Stroke. 2007;38:2422–9.
- Kramer CK, von Muhlen D, Gross JL, Barrett-Connor E. A prospective study of abdominal obesity and coronary artery calcium progression in older adults. J Clin Endocrinol Metab. 2009;94:5039–44.
- Carr MC, Brunzell JD. Abdominal obesity and dyslipidemia in the metabolic syndrome: importance of type 2 diabetes and familial combined hyperlipidemia in coronary artery disease risk. J Clin Endocrinol Metab. 2004;89: 2601–7.
- Després JP. Is visceral obesity the cause of the metabolic syndrome? Ann Med. 2006;38:52–63.
- Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365:1415–28.
- Alberti KG, Zimmet P, Shaw J. The metabolic syndrome—a new worldwide definition. Lancet. 2005;366:1059–62.
- Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, . Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–52.
- Galassi A, Reynolds K, He J. Metabolic syndrome and risk of cardiovascular disease: a meta-analysis. Am J Med. 2006;119:812–9.
- Gami AS, Witt BJ, Howard DE, Erwin PJ, Gami LA, Somers VK, . Metabolic syndrome and risk of incident cardiovascular events and death: a systematic review and meta-analysis of longitudinal studies. J Am Coll Cardiol. 2007;49:403–14.
- Kahn R, Buse J, Ferrannini E, Stern M. The metabolic syndrome: time for a critical appraisal: joint statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2005;28:2289–304.
- Carr DB, Utzschneider KM, Hull RL, Kodama K, Retzlaff BM, Brunzell JD, . Intra-abdominal fat is a major determinant of the National Cholesterol Education Program Adult Treatment Panel III criteria for the metabolic syndrome. Diabetes. 2004;53:2087–94.
- Tong J, Boyko EJ, Utzschneider KM, McNeely MJ, Hayashi T, Carr DB, . Intra-abdominal fat accumulation predicts the development of the metabolic syndrome in non-diabetic Japanese-Americans. Diabetologia. 2007;50: 1156–60.
- Arsenault BJ, Lachance D, Lemieux I, Alméras N, Tremblay A, Bouchard C, . Visceral adipose tissue accumulation, cardiorespiratory fitness, and features of the metabolic syndrome. Arch Intern Med. 2007;167:1518–25.
- Fox CS, Massaro JM, Hoffmann U, Pou KM, Maurovich-Horvat P, Liu CY, . Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation. 2007;116:39–48.
- Smith SR, Lovejoy JC, Greenway F, Ryan D, deJonge L, de la Bretonne J, . Contributions of total body fat, abdominal subcutaneous adipose tissue compartments, and visceral adipose tissue to the metabolic complications of obesity. Metabolism. 2001;50:425–35.
- Pou KM, Massaro JM, Hoffmann U, Vasan RS, Maurovich-Horvat P, Larson MG, . Visceral and subcutaneous adipose tissue volumes are cross-sectionally related to markers of inflammation and oxidative stress: the Framingham Heart Study. Circulation. 2007;116:1234–41.
- Cartier A, Lemieux I, Alméras N, Tremblay A, Bergeron J, Després JP. Visceral obesity and plasma glucose-insulin homeostasis: contributions of interleukin-6 and tumor necrosis factor-alpha in men. J Clin Endocrinol Metab. 2008;93:1931–8.
- Piché ME, Lemieux S, Weisnagel SJ, Corneau L, Nadeau A, Bergeron J. Relation of high-sensitivity C-reactive protein, interleukin-6, tumor necrosis factor-alpha, and fibrinogen to abdominal adipose tissue, blood pressure, and cholesterol and triglyceride levels in healthy postmenopausal women. Am J Cardiol. 2005;96:92–7.
- Porter SA, Massaro JM, Hoffmann U, Vasan RS, O’Donnel CJ, Fox CS. Abdominal subcutaneous adipose tissue: a protective fat depot? Diabetes Care. 2009;32:1068–75.
- Preis SR, Massaro JM, Robins SJ, Hoffmann U, Vasan RS, Irlbeck T, . Abdominal subcutaneous and visceral adipose tissue and insulin resistance in the Framingham Heart Study. Obesity (Silver Spring). 2010;18:2191–8.
- Canoy D, Boekholdt SM, Wareham N, Luben R, Welch A, Bingham S, . Body fat distribution and risk of coronary heart disease in men and women in the European Prospective Investigation Into Cancer and Nutrition in Norfolk cohort: a population-based prospective study. Circulation. 2007;116:2933–43.
- Tchernof A. Visceral adipocytes and the metabolic syndrome. Nutr Rev. 2007;65(6 Pt 2):S24–9.
- Yudkin JS, Kumari M, Humphries SE, Mohamed-Ali V. Inflammation, obesity, stress and coronary heart disease: is interleukin-6 the link? Atherosclerosis. 2000;148:209–14.
- Hube F, Hauner H. The role of TNF-alpha in human adipose tissue: prevention of weight gain at the expense of insulin resistance? Horm Metab Res. 1999;31:626–31.
- Arnaud C, Burger F, Steffens S, Veillard NR, Nguyen TH, Trono D, . Statins reduce interleukin-6-induced C-reactive protein in human hepatocytes: new evidence for direct antiinflammatory effects of statins. Arterioscler Thromb Vasc Biol. 2005;25:1231–6.
- Nielsen S, Guo Z, Johnson CM, Hensrud DD, Jensen MD. Splanchnic lipolysis in human obesity. J Clin Invest. 2004;113:1582–8.
- Frayn KN, Arner P, Yki-Jarvinen H. Fatty acid metabolism in adipose tissue, muscle and liver in health and disease. Essays Biochem. 2006;42:89–103.
- Verrijken A, Francque S, Mertens I, Talloen M, Peiffer F, Van Gaal L. Visceral adipose tissue and inflammation correlate with elevated liver tests in a cohort of overweight and obese patients. Int J Obes (Lond). 2010;34:899–907.
- Thamer C, Machann J, Haap M, Stefan N, Heller E, Schnodt B, . Intrahepatic lipids are predicted by visceral adipose tissue mass in healthy subjects. Diabetes Care. 2004;27:2726–9.
- Eguchi Y, Eguchi T, Mizuta T, Ide Y, Yasutake T, Iwakiri R, . Visceral fat accumulation and insulin resistance are important factors in nonalcoholic fatty liver disease. J Gastroenterol. 2006;41:462–9.
- Koda M, Kawakami M, Murawaki Y, Senda M. The impact of visceral fat in nonalcoholic fatty liver disease: cross-sectional and longitudinal studies. J Gastroenterol. 2007;42:897–903.
- Wallace TM, Utzschneider KM, Tong J, Carr DB, Zraika S, Bankson DD, . Relationship of liver enzymes to insulin sensitivity and intra-abdominal fat. Diabetes Care. 2007;30:2673–8.
- Park BJ, Kim YJ, Kim DH, Kim W, Jung YJ, Yoon JH, . Visceral adipose tissue area is an independent risk factor for hepatic steatosis. J Gastroenterol Hepatol. 2008;23:900–7.
- Libby P. Inflammation and cardiovascular disease mechanisms. Am J Clin Nutr. 2006;83:456S–60S.
- Ross R, Glomset JA. The pathogenesis of atherosclerosis N Engl J Med. 1976;295:369–77.
- Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–7.
- Lafontan M, Girard J. Impact of visceral adipose tissue on liver metabolism. Part I: heterogeneity of adipose tissue and functional properties of visceral adipose tissue. Diabetes Metab. 2008;34(4 Pt 1):317–27.
- Rocha VZ, Libby P. The multiple facets of the fat tissue. Thyroid. 2008;18:175–83.
- Nishimura S, Manabe I, Nagasaki M, Seo K, Yamashita H, Hosoya Y, . In vivo imaging in mice reveals local cell dynamics and inflammation in obese adipose tissue. J Clin Invest. 2008;118:710–21.
- Kintscher U, Hartge M, Hess K, Foryst-Ludwig A, Clemenz M, Wabitsch M, . T-lymphocyte infiltration in visceral adipose tissue: a primary event in adipose tissue inflammation and the development of obesity-mediated insulin resistance. Arterioscler Thromb Vasc Biol. 2008;28:1304–10.
- Rotter V, Nagaev I, Smith U. Interleukin-6 (IL-6) induces insulin resistance in 3T3-L1 adipocytes and is, like IL-8 and tumor necrosis factor-alpha, overexpressed in human fat cells from insulin-resistant subjects. J Biol Chem. 2003; 278:45777–84.
- Senn JJ, Klover PJ, Nowak IA, Mooney RA. Interleukin-6 induces cellular insulin resistance in hepatocytes. Diabetes. 2002;51:3391–9.
- Cartier A, Lemieux I, Alméras N, Tremblay A, Bergeron J, Després JP. Visceral obesity and plasma glucose-insulin homeostasis: contributions of interleukin-6 and tumor necrosis factor-alpha in men. J Clin Endocrinol Metab. 2008;93:1931–8.
- Fontana L, Eagon JC, Trujillo ME, Scherer PE, Klein S. Visceral fat adipokine secretion is associated with systemic inflammation in obese humans. Diabetes. 2007;56:1010–3.
- St-Pierre AC, Cantin B, Bergeron J, Pirro M, Dagenais GR, Després JP, . Inflammatory markers and long-term risk of ischemic heart disease in men. A 13-year follow-up of the Quebec Cardiovascular Study. Atherosclerosis. 2005; 182:315–21.
- Patterson CC, Smith AE, Yarnell JW, Rumley A, Ben-Shlomo Y, Lowe GD. The associations of interleukin-6 (IL-6) and downstream inflammatory markers with risk of cardiovascular disease: the Caerphilly Study. Atherosclerosis. 2010;209:551–7.
- Tsigos C, Papanicolaou DA, Kyrou I, Defensor R, Mitsiadis CS, Chrousos GP. Dose-dependent effects of recombinant human interleukin-6 on glucose regulation. J Clin Endocrinol Metab. 1997;82:4167–70.
- Côté M, Mauriège P, Bergeron J, Alméras N, Tremblay A, Lemieux I, . Adiponectinemia in visceral obesity: impact on glucose tolerance and plasma lipoprotein and lipid levels in men. J Clin Endocrinol Metab. 2005;90: 1434–9.
- Okamoto Y, Kihara S, Funahashi T, Matsuzawa Y, Libby P. Adiponectin: a key adipocytokine in metabolic syndrome. Clin Sci (Lond). 2006;110:267–78.
- Kadowaki T, Yamauchi T, Kubota N, Hara K, Ueki K, Tobe K. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J Clin Invest. 2006;116:1784–92.
- Matsuzawa Y, Funahashi T, Kihara S, Shimomura I. Adiponectin and metabolic syndrome. Arterioscler Thromb Vasc Biol. 2004;24:29–33.
- Xu A, Wang Y, Keshaw H, Xu LY, Lam KS, Cooper GJ. The fat-derived hormone adiponectin alleviates alcoholic and nonalcoholic fatty liver diseases in mice. J Clin Invest. 2003;112:91–100.
- van Herpen NA, Schrauwen-Hinderling VB. Lipid accumulation in non-adipose tissue and lipotoxicity. Physiol Behav. 2008;94:231–41.
- Neuschwander-Tetri BA, Caldwell SH. Nonalcoholic steatohepatitis: summary of an AASLD Single Topic Conference. Hepatology. 2003;37:1202–19.
- Lazo M, Clark JM. The epidemiology of nonalcoholic fatty liver disease: a global perspective. Semin Liver Dis. 2008; 28:339–50.
- Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, . Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40:1387–95.
- Kotronen A, Juurinen L, Hakkarainen A, Westerbacka J, Corner A, Bergholm R, . Liver fat is increased in type 2 diabetic patients and underestimated by serum alanine aminotransferase compared with equally obese nondiabetic subjects. Diabetes Care. 2008;31:165–9.
- Kelley DE, McKolanis TM, Hegazi RA, Kuller LH, Kalhan SC. Fatty liver in type 2 diabetes mellitus: relation to regional adiposity, fatty acids, and insulin resistance. Am J Physiol Endocrinol Metab. 2003;285:E906–16.
- Nguyen-Duy TB, Nichaman MZ, Church TS, Blair SN, Ross R. Visceral fat and liver fat are independent predictors of metabolic risk factors in men. Am J Physiol Endocrinol Metab. 2003;284:E1065–71.
- Kuk JL, Nichaman MZ, Church TS, Blair SN, Ross R. Liver fat is not a marker of metabolic risk in lean premenopausal women. Metabolism. 2004;53:1066–71.
- Fracanzani AL, Valenti L, Bugianesi E, Vanni E, Grieco A, Miele L, . Risk of nonalcoholic steatohepatitis and fibrosis in patients with nonalcoholic fatty liver disease and low visceral adiposity. J Hepatol. 2011;54:1244-9.
- Gastaldelli A, Cusi K, Pettiti M, Hardies J, Miyazaki Y, Berria R, . Relationship between hepatic/visceral fat and hepatic insulin resistance in nondiabetic and type 2 diabetic subjects. Gastroenterology. 2007;133:496–506.
- Guerrero R, Vega GL, Grundy SM, Browning JD. Ethnic differences in hepatic steatosis: an insulin resistance paradox? Hepatology. 2009;49:791–801.
- Amaro A, Fabbrini E, Kars M, Yue P, Schechtman K, Schonfeld G, . Dissociation between intrahepatic triglyceride content and insulin resistance in familial hypobetalipoproteinemia. Gastroenterology. 2010;139:149–53.
- Smith HL, Willius FA. Adiposity of the heart. Arch Int Med. 1933;52:811–931.
- McGavock JM, Victor RG, Unger RH, Szczepaniak LS. Adiposity of the heart, revisited. Ann Intern Med. 2006;144:517–24.
- Rabkin SW. Epicardial fat: properties, function and relationship to obesity. Obes Rev. 2007;8:253–61.
- Iacobellis G, Sharma AM. Epicardial adipose tissue as new cardio-metabolic risk marker and potential therapeutic target in the metabolic syndrome. Curr Pharm Des. 2007;13: 2180–4.
- Roy PE. Lipid droplets in the heart interstitium: concentration and distribution. Recent Adv Stud Cardiac Struct Metab. 1975;10:17–27.
- Shirani J, Berezowski K, Roberts WC. Quantitative measurement of normal and excessive (cor adiposum) subepicardial adipose tissue, its clinical significance, and its effect on electrocardiographic QRS voltage. Am J Cardiol. 1995;76:414–8.
- Djaberi R, Schuijf JD, van Werkhoven JM, Nucifora G, Jukema JW, Bax JJ. Relation of epicardial adipose tissue to coronary atherosclerosis. Am J Cardiol. 2008;102:1602–7.
- Gorter PM, de Vos AM, van der Graaf Y, Stella PR, Doevendans PA, Meijs MF, . Relation of epicardial and pericoronary fat to coronary atherosclerosis and coronary artery calcium in patients undergoing coronary angiography. Am J Cardiol. 2008;102:380–5.
- Ahn SG, Lim HS, Joe DY, Kang SJ, Choi BJ, Choi SY, . Relationship of epicardial adipose tissue by echocardiography to coronary artery disease. Heart. 2008; 94:e7.
- Greif M, Becker A, von Ziegler F, Lebherz C, Lehrke M, Broedl UC, . Pericardial adipose tissue determined by dual source CT is a risk factor for coronary atherosclerosis. Arterioscler Thromb Vasc Biol. 2009;29:781–6.
- Divers J, Wagenknecht LE, Bowden DW, Carr JJ, Hightower RC, Register TC, . Ethnic differences in the relationship between pericardial adipose tissue and coronary artery calcified plaque: African-American-diabetes heart study. J Clin Endocrinol Metab. 2010;95:5382–9.
- Rosito GA, Massaro JM, Hoffmann U, Ruberg FL, Mahabadi AA, Vasan RS, . Pericardial fat, visceral abdominal fat, cardiovascular disease risk factors, and vascular calcification in a community-based sample: the Framingham Heart Study. Circulation. 2008;117:605–13.
- Mahabadi AA, Massaro JM, Rosito GA, Levy D, Murabito JM, Wolf PA, . Association of pericardial fat, intrathoracic fat, and visceral abdominal fat with cardiovascular disease burden: the Framingham Heart Study. Eur Heart J. 2009;30:850–6.
- Fox CS, Gona P, Hoffmann U, Porter SA, Salton CJ, Massaro JM, . Pericardial fat, intrathoracic fat, and measures of left ventricular structure and function: the Framingham Heart Study. Circulation. 2009;119:1586–91.
- McGavock JM, Lingvay I, Zib I, Tillery T, Salas N, Unger R, . Cardiac steatosis in diabetes mellitus: a 1H-magnetic resonance spectroscopy study. Circulation. 2007;116: 1170–5.
- Zhou YT, Grayburn P, Karim A, Shimabukuro M, Higa M, Baetens D, . Lipotoxic heart disease in obese rats: implications for human obesity. Proc Natl Acad Sci U S A. 2000;97:1784–9.
- Paradise NF, Pilati CF, Payne WR, Finkelstein JA. Left ventricular function of the isolated, genetically obese rat's heart. Am J Physiol. 1985;248(4 Pt 2):H438–44.
- Kankaanpaa M, Lehto HR, Parkka JP, Komu M, Viljanen A, Ferrannini E, . Myocardial triglyceride content and epicardial fat mass in human obesity: relationship to left ventricular function and serum free fatty acid levels. J Clin Endocrinol Metab. 2006;91:4689–95.
- Kim MK, Tomita T, Kim MJ, Sasai H, Maeda S, Tanaka K. Aerobic exercise training reduces epicardial fat in obese men. J Appl Physiol. 2009;106:5–11.
- Sacks HS. Weight loss in obesity reduces epicardial fat thickness; so what? J Appl Physiol. 2009;106:1–2.
- Sironi AM, Gastaldelli A, Mari A, Ciociaro D, Positano V, Buzzigoli E, . Visceral fat in hypertension: influence on insulin resistance and beta-cell function. Hypertension. 2004;44:127–33.
- Mazurek T, Zhang L, Zalewski A, Mannion JD, Diehl JT, Arafat H, . Human epicardial adipose tissue is a source of inflammatory mediators. Circulation. 2003;108:2460–6.
- Eiras S, Teijeira-Fernandez E, Shamagian LG, Fernandez AL, Vazquez-Boquete A, Gonzalez-Juanatey JR. Extension of coronary artery disease is associated with increased IL-6 and decreased adiponectin gene expression in epicardial adipose tissue. Cytokine. 2008;43:174–80.
- Teijeira-Fernandez E, Eiras S, Grigorian-Shamagian L, Fernandez A, Adrio B, Gonzalez-Juanatey JR. Epicardial adipose tissue expression of adiponectin is lower in patients with hypertension. J Hum Hypertens. 2008;22:856–63.
- Sacks HS, Fain JN, Cheema P, Bahouth SW, Garrett E, Wolf RY, . Inflammatory genes in epicardial fat contiguous with coronary atherosclerosis in the metabolic syndrome and type 2 diabetes: changes associated with pioglitazone. Diabetes Care.2011;34:730–3.
- Jacob S, Machann J, Rett K, Brechtel K, Volk A, Renn W, . Association of increased intramyocellular lipid content with insulin resistance in lean nondiabetic offspring of type 2 diabetic subjects. Diabetes. 1999;48:1113–9.
- Thamer C, Machann J, Bachmann O, Haap M, Dahl D, Wietek B, . Intramyocellular lipids: anthropometric determinants and relationships with maximal aerobic capacity and insulin sensitivity. J Clin Endocrinol Metab. 2003;88:1785–91.
- Yim JE, Heshka S, Albu J, Heymsfield S, Kuznia P, Harris T, . Intermuscular adipose tissue rivals visceral adipose tissue in independent associations with cardiovascular risk. Int J Obes (Lond). 2007;31:1400–5.
- Goodpaster BH, Krishnaswami S, Resnick H, Kelley DE, Haggerty C, Harris TB, . Association between regional adipose tissue distribution and both type 2 diabetes and impaired glucose tolerance in elderly men and women. Diabetes Care. 2003;26:372–9.
- Goodpaster BH, Krishnaswami S, Harris TB, Katsiaras A, Kritchevsky SB, Simonsick EM, . Obesity, regional body fat distribution, and the metabolic syndrome in older men and women. Arch Intern Med. 2005;165:777–83.
- Delmonico MJ, Harris TB, Visser M, Park SW, Conroy MB, Velasquez-Mieyer P, . Longitudinal study of muscle strength, quality, and adipose tissue infiltration. Am J Clin Nutr. 2009;90:1579–85.
- Kotronen A, Seppala-Lindroos A, Bergholm R, Yki-Jarvinen H. Tissue specificity of insulin resistance in humans: fat in the liver rather than muscle is associated with features of the metabolic syndrome. Diabetologia. 2008;51:130–8.
- Bajaj M, Baig R, Suraamornkul S, Hardies LJ, Coletta DK, Cline GW, . Effects of pioglitazone on intramyocellular fat metabolism in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab.2010;95:1916–23.
- Bollheimer LC, Skelly RH, Chester MW, McGarry JD, Rhodes CJ. Chronic exposure to free fatty acid reduces pancreatic beta cell insulin content by increasing basal insulin secretion that is not compensated for by a corresponding increase in proinsulin biosynthesis translation. J Clin Invest. 1998;101:1094–101.
- Unger RH, Zhou YT. Lipotoxicity of beta-cells in obesity and in other causes of fatty acid spillover. Diabetes. 2001;50 Suppl 1:S118–21.
- Lee Y, Hirose H, Ohneda M, Johnson JH, McGarry JD, Unger RH. Beta-cell lipotoxicity in the pathogenesis of non-insulin-dependent diabetes mellitus of obese rats: impairment in adipocyte-beta-cell relationships. Proc Natl Acad Sci U S A. 1994 8;91:10878–82.
- van der Zijl NJ, Goossens GH, Moors CC, van Raalte DH, Muskiet MH, Pouwels PJ, . Ectopic fat storage in the pancreas, liver, and abdominal fat depots: impact on beta-cell function in individuals with impaired glucose metabolism. J Clin Endocrinol Metab. 2011;96:459–67.
- Heni M, Machann J, Staiger H, Schwenzer NF, Peter A, Schick F, . Pancreatic fat is negatively associated with insulin secretion in individuals with impaired fasting glucose and/or impaired glucose tolerance: a nuclear magnetic resonance study. Diabetes Metab Res Rev. 2010;26:200–5.
- Brunham LR, Kruit JK, Pape TD, Timmins JM, Reuwer AQ, Vasanji Z, . Beta-cell ABCA1 influences insulin secretion, glucose homeostasis and response to thiazolidinedione treatment. Nat Med. 2007;13:340–7.
- Vergeer M, Brunham LR, Koetsveld J, Kruit JK, Verchere CB, Kastelein JJ, . Carriers of loss-of-function mutations in ABCA1 display pancreatic beta-cell dysfunction. Diabetes Care. 2010;33:869–74.
- van Raalte DH, van der Zijl NJ, Diamant M. Pancreatic steatosis in humans: cause or marker of lipotoxicity? Curr Opin Clin Nutr Metab Care. 2010;13:478–85.
- Paré A, Dumont M, Lemieux I, Brochu M, Alméras N, Lemieux S, . Is the relationship between adipose tissue and waist girth altered by weight loss in obese men? Obes Res. 2001;9:526–34.
- Longo R, Ricci C, Masutti F, Vidimari R, Croce LS, Bercich L, . Fatty infiltration of the liver. Quantification by 1H localized magnetic resonance spectroscopy and comparison with computed tomography. Invest Radiol. 1993;28:297–302.
- Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339: 229–34.
- Dagenais GR, St-Pierre A, Gilbert P, Lamarche B, Després JP, Bernard PM, . Comparison of prognosis for men with type 2 diabetes mellitus and men with cardiovascular disease. CMAJ. 2009;180:40–7.
- Saydah S, Tao M, Imperatore G, Gregg E. GHb level and subsequent mortality among adults in the U.S. Diabetes Care. 2009;32:1440–6.
- Nathan DM. Long-term complications of diabetes mellitus. N Engl J Med. 1993;328:1676–85.
- Orasanu G, Plutzky J. The pathologic continuum of diabetic vascular disease. J Am Coll Cardiol. 2009;53(5 Suppl):S35–42.
- Creager MA, Luscher TF, Cosentino F, Beckman JA. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: Part I. Circulation. 2003;108:1527–32.
- Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, . Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560–72.
- Gerstein HC, Miller ME, Byington RP, Goff DC Jr, Bigger JT, Buse JB, . Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545–59.
- Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, . Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360:129–39.
- Blackburn P, Lemieux I, Lamarche B, Bergeron J, Perron P, Tremblay G, . Type 2 diabetes without the atherogenic metabolic triad does not predict angiographically assessed coronary artery disease in women. Diabetes Care. 2008;31:170–2.
- Turner RC, Millns H, Neil HA, Stratton IM, Manley SE, Matthews DR, . Risk factors for coronary artery disease in non-insulin dependent diabetes mellitus: United Kingdom Prospective Diabetes Study (UKPDS: 23). BMJ. 1998;316:823–8.
- Lehto S, Ronnemaa T, Haffner SM, Pyorala K, Kallio V, Laakso M. Dyslipidemia and hyperglycemia predict coronary heart disease events in middle-aged patients with NIDDM. Diabetes. 1997;46:1354–9.
- Alexander CM, Landsman PB, Teutsch SM, Haffner SM. NCEP-defined metabolic syndrome, diabetes, and prevalence of coronary heart disease among NHANES III participants age 50 years and older. Diabetes. 2003;52:1210–4.
- Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, . Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403.
- Orchard TJ, Temprosa M, Goldberg R, Haffner S, Ratner R, Marcovina S, . The effect of metformin and intensive lifestyle intervention on the metabolic syndrome: the Diabetes Prevention Program randomized trial. Ann Intern Med. 2005;142:611–9.
- Wing RR. Long-term effects of a lifestyle intervention on weight and cardiovascular risk factors in individuals with type 2 diabetes mellitus: four-year results of the Look AHEAD trial. Arch Intern Med. 2010;170: 1566–75.
- Deedwania P, Barter P, Carmena R, Fruchart JC, Grundy SM, Haffner S, . Reduction of low-density lipoprotein cholesterol in patients with coronary heart disease and metabolic syndrome: analysis of the Treating to New Targets study. Lancet. 2006;368:919–28.
- Ballantyne CM, Olsson AG, Cook TJ, Mercuri MF, Pedersen TR, Kjekshus J. Influence of low high-density lipoprotein cholesterol and elevated triglyceride on coronary heart disease events and response to simvastatin therapy in 4S. Circulation. 2001;104:3046–51.
- Scott R, O’Brien R, Fulcher G, Pardy C, D’Emden M, Tse D, . Effects of fenofibrate treatment on cardiovascular disease risk in 9,795 individuals with type 2 diabetes and various components of the metabolic syndrome: the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study. Diabetes Care. 2009;32:493–8.
- ACCORD Study Group; Ginsberg HN, Elam MB, Lovato LC, Crouse JR 3rd, Leiter LA, Linz P, . Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med.2010;362:1563–74.
- Hunink MG, Goldman L, Tosteson AN, Mittleman MA, Goldman PA, Williams LW, . The recent decline in mortality from coronary heart disease, 1980–1990. The effect of secular trends in risk factors and treatment. JAMA. 1997;277:535–42.
- Ford ES, Ajani UA, Croft JB, Critchley JA, Labarthe DR, Kottke TE, . Explaining the decrease in U.S. deaths from coronary disease, 1980–2000. N Engl J Med. 2007;356:2388–98.
- Hossain P, Kawar B, El Nahas M. Obesity and diabetes in the developing world—a growing challenge. N Engl J Med. 2007;356:213–5.
- Olshansky SJ, Passaro DJ, Hershow RC, Layden J, Carnes BA, Brody J, . A potential decline in life expectancy in the United States in the 21st century. N Engl J Med. 2005;352:1138–45.