Abstract
Certain bacterial infections have been demonstrated to be causative of reactive arthritis. The most common bacterial trigger of reactive arthritis is Chlamydia trachomatis. Chlamydia pneumoniae is another known cause, albeit far less frequently. Although Chlamydia-induced reactive arthritis will often spontaneously remit, approximately 30% of patients will develop a chronic course. Modern medicine has provided rather remarkable advances in our understanding of the chlamydiae, as these organisms relate to chronic arthritis and the delicate balance between host and pathogen. C. trachomatis and C. pneumoniae both have a remarkable ability to disseminate from the initial site of infection and establish persistently viable organisms in distant organ sites, namely the synovial tissue. How these persistent chlamydiae contribute to disease maintenance remains to be fully established, but recent data demonstrating that long-term combination antimicrobial treatment can not only ameliorate the symptoms but eradicate the persistent infection suggest that these chronically infecting chlamydiae are indeed a driving force behind the chronic inflammation. We are beginning to learn that this all appears possible even after an asymptomatic initial chlamydial infection. Both C. trachomatis and C. pneumoniae are a clear cause of chronic arthritis in the setting of reactive arthritis; the possibility remains that these same organisms are culpable in other forms of chronic arthritis as well.
Key words::
Key messages
Chlamydiae are a definite trigger of reactive arthritis.
Approximately 30%–50% of patients with Chlamydia-induced reactive arthritis develop a chronic course.
Viable chlamydiae have been demonstrated in the synovial tissue of patients with chronic Chlamydia-induced reactive arthritis.
In 460 BC, Hippocrates wrote, ‘a youth does not suffer from gout until sexual intercourse’ (Citation1). In the ancient medical literature, the term gout was used rather indiscriminately for any type of acute arthritis. Could Hippocrates actually have been describing Chlamydia-induced reactive arthritis (CiReA)? Moving forward in the medical literature, several noted physicians suggested a potential link between Chlamydia trachomatis and arthritis. In 1507, Pierre van Forest noted patients with ‘secondary arthritis and urethritis’ (Citation2). In 1806, Baron Yvan described a French captain who developed ‘ophthalmia’ and inflammatory arthritis of the lower extremities after a venereal infection (Citation3). Perhaps most notably, Sir Benjamin Brodie described five patients with probable CiReA in his book Pathological and surgical observations on the disease of the joints, which was published in 1818; these five patients developed conjunctivitis and arthritis after episodes of urethritis. He described the ‘train of symptoms’ that ensued in all five of these subjects, and he duly noted that four of the five patients developed chronic disease with a ‘relapsing course’ (Citation4).
Certain bacterial infections have been demonstrated unequivocally to be causative of reactive arthritis (ReA) in some proportion of individuals who are exposed to these organisms. ReA is an inflammatory arthritis that arises 1–6 weeks after certain genitourinary and gastrointestinal infections. Chlamydia trachomatis (C. trachomatis), Salmonella, Shigella, Campylobacter, and Yersinia are all known triggers of reactive arthritis (Citation5). Emerging evidence also support the fact that Chlamydia pneumoniae (C. pneumoniae) is another causative agent (Citation5). C. trachomatis is the most common cause of ReA.
Regardless of whether a patient develops post-chlamydial ReA or the post-dysentery variety, the clinical symptoms are considered to be congruent. ReA is a type of spondyloarthritis (SpA), so this condition shares many features with the other types of SpA. Patients develop an inflammatory arthritis that has a predilection for the axial skeleton, particularly the sacroiliac joints, as well as peripheral arthritis, typically the large joints of the lower extremities, although any joint can be involved. Patients can present with a mono-, oligo-, or polyarthritis, although an oligoarthritis is probably the most typical pattern. Enthesitis is another common feature of ReA. Other organs including the skin and mucous membranes are also often involved. Although the literature varies, approximately 50%–70% of cases of ReA will resolve spontaneously within the first 6 months (Citation6,Citation7), and 30%–50% of patients will develop a chronic course (5a-5b). One large retrospective study suggested that as many as 63% of patients develop chronic symptoms (5c). Oftentimes, patients with chronic disease experience a waxing and waning course (Citation8). Studies indicate that post-dysentery ReA affects males and females equally, but post-chlamydial ReA appears to have a predilection for males. This apparent predilection is probably a function, at least in part, of the disease definition, which requires a documented prior genital infection with C. trachomatis. Genital chlamydial infections are often asymptomatic/subclinical in women, thus obviating an official diagnosis of CiReA. To our knowledge, no official diagnostic criteria exist as yet for the ReA elicited by C. pneumoniae.
Chlamydial persistence
There are two main species that cause human disease, C. trachomatis and C. pneumoniae. C. trachomatis is among the most prevalent genital pathogens worldwide. The spectrum of illness from both species can range from asymptomatic infection to severe illness. The Centers for Disease Control (CDC) estimates 3 million new C. trachomatis infections in persons 15–44 years of age every year in the United States (US) (Citation9). The overall incidence of C. pneumoniae infections is unknown, but it is felt to be a more common pathogen than C. trachomatis.
CiReA represents the classic interplay of host and environment. As mentioned, chlamydiae are undeniably involved in the genesis of ReA. A certain percentage of patients with the proper genetic background will develop ReA after an acute C. trachomatis infection; this is presumably the case for C. pneumoniae infection as well. The traditional description of CiReA was that of a sterile or aseptic arthritis that occurs after exposure to one of the causative organisms. It was termed a ‘sterile arthritis’ because early studies performed in the 1970s and 1980s utilizing traditional culture techniques failed to demonstrate live chlamydial organisms in the synovial fluid (Citation10,Citation11). Interestingly, electron microscope (EM) studies performed during that same time period did show what appeared to be intact chlamydial elementary bodies (EB) and/or reticulate bodies (RB)(see below) in synovial samples of patients with a prior genital C. trachomatis infection (Citation12,Citation13). The EM findings were largely ignored, perhaps because traditional culture techniques were better validated at the time. Recent techniques, including polymerase chain reaction (PCR) and real-time (RT)-PCR have demonstrated that both C. pneumoniae and C. trachomatis have the ability to disseminate from the initial site of infection and enter a long-term persistently viable state in distant organs, most notably the synovial tissue of patients with chronic CiReA. For C. pneumoniae, the vehicle of dissemination has been shown to be monocytic cells (Citation14); this is presumed to be the case also for C. trachomatis, although it has not been definitively demonstrated to date. The exact role that these synovial-based chlamydiae play in the maintenance of CiReA remains in debate, but our knowledge of the pathophysiology between the host and organism and particular arthritogenic features of the organism itself is rapidly expanding.
Early studies used hybridization probes against chlamydial DNA to screen for the presence of C. trachomatis in synovial samples from patients with presumed CiReA (Citation15–17). Later a DNA fragment was utilized as probe against C. trachomatis 16S rRNA, since these transcripts show high conservation, high transcript copy number, and good stability (Citation18,Citation19). One of these early studies initially showed that such rRNA was present in 7/9 ReA patient samples, but not in those from patients with other arthritides (Citation20); other groups found similar results (Citation21).
PCR for C. trachomatis DNA sequences offered improvements in sensitivity and specificity over hybridization systems. We and others developed PCR-based C. trachomatis and C. pneumoniae-targeted detection systems (Citation22,Citation23), most of which targeted the chlamydial omp1 gene or the C. trachomatis plasmid for amplification. Many of the initial PCR-based studies that were performed to examine if chlamydial DNA is present in synovia of ReA patients gave inconsistent results. Some showed DNA from C. trachomatis at high frequency in ReA patient samples (Citation23–25), while others found a low rate or total absence of PCR positivity (Citation26). One study showed that the source of material assayed (i.e. synovial fluid versus synovial tissue) is critical, because synovial tissue, not fluid, is the site of long-term residence for the organism in the joint (Citation22). Later studies further demonstrated that the organisms reside below the synovial lining and that monocytic cells are their primary host cells (Citation27). Further PCR-based studies by our group and others established that DNA from C. trachomatis is present at high frequency in the joints of patients with chronic CiReA (Citation28).
Chlamydiae are mucosal pathogens, and their primary target host cell type during an initial infection is epithelial or epithelial-like cells. However, C. pneumoniae has been shown to infect many other cell types, including vascular endothelial cells, smooth muscle cells, monocytic cells, and others (Citation29–31); C. trachomatis also can infect cell types other than those in epithelia, including fibroblasts (Citation32). By standard description, C. trachomatis undergoes a biphasic developmental cycle that is thought to be transcriptionally controlled (Citation33). The cycle is initiated when EB, the extracellular form of the organism, bind to the target host cell. Once bound, EB are brought into a membrane-bound cytoplasmic inclusion within which they spend their intracellular lifecycle. During the first few hours in the inclusion, each EB undergoes a transcriptionally governed developmental process yielding the vegetative growth form of the organism, the RB, which then undergoes seven to eight cell divisions. Near the termination of the cycle, most RB differentiate back to EB, which are released from the cell by exocytosis or cell lysis, thus propagating the infection to other cells (Citation34). In HeLa or HEp-2 cells, the entire cycle requires about 48 hours to complete for C. trachomatis and approximately 72 hours for C. pneumoniae.
It is important to note that this description of the chlamydial developmental cycle was derived from study of in-vitro culture systems that used permissive host cells. However, under some circumstances, including those relevant to synovial infections and possibly other organs, the developmental cycle can be arrested at a late point, obviating production and release of new EB. This state of arrested development is referred to as persistence (). The idea that both chlamydial species under discussion here undergo persistent infection is not new, but much of the evidence supporting the reality and clinical significance of chlamydial persistence has been generated during the last 10 years from in-vivo and in-vitro studies relating to CiReA (Citation35). Much of the early work on chlamydial persistence was based on studies of C. trachomatis infection of HeLa cells treated first with penicillin and later with low levels of IFN-γ (Citation36). In those studies, it was shown that infected cultures so treated contain RB-like forms displaying aberrant morphology; supernatants from treated cultures contained no, or extremely low levels of, new EB. It was also demonstrated that the oddly shaped C. trachomatis cells accumulate copies of the bacterial chromosome in the absence of cell division (Citation37). Removing IFN-γ from the medium releases the block in the lifecycle, resulting in return to normal morphology and EB production (Citation36,Citation37). Interestingly, one study demonstrated that the panel of chlamydial genes involved in the transition from acute to the persistent infection state, and those genes involved in maintenance of that state following transition, are similar to those which perform the similar functions in Mycobacterium tuberculosis (Citation38). Both C. trachomatis and M. tuberculosis utilize many genes specifying products of unknown function in the transition to and maintenance of the persistent state. The pathologic importance of persistent/latent M. tuberculosis has been known for many years; the mystery that shrouds persistent chlamydiae is beginning to be revealed.
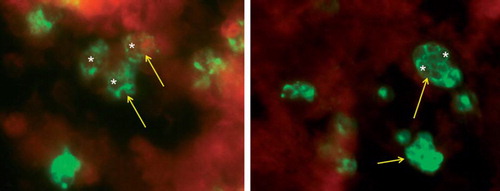
Figure 1. Left panel: Human monocytes infected with C. trachomatis for 6 days. Cells were fixed with 70% methanol, then stained for chlamydial lipopolysaccharide (Pathfinder, BioRad, Hercules, CA, USA). Green fluorescence indicates chlamydial infection (arrows). Note large circular structures which are aberrant reticulate bodies (RB) (white asterisks). These are similar in size to aberrant RB after 48 hours of infection induced by penicillin G treatment of HEp-2 cells at the time of infection (right panel). Images collected with the assistance of MsMirabelaHali, Wayne State University.
Cytokine response
As suggested by the in-vitro studies cited above, cytokine production and release play critical roles in the initiation, regulation, and resolution of inflammatory responses to pathogens (Citation39,Citation40). Several studies have indicated roles for cytokines in the growth, differentiation, and persistence of chlamydiae (Citation39,Citation41). While the Th1 cytokines, such as TNF-α, play a role in the clinical manifestations of ReA, their influence seems to be less important than that in other types of inflammatory arthritis (Citation42–44). This might be particularly true for chronic ReA, i.e. patients with disease duration of greater than 6 months (Citation42).
Temporal relationships of these different Th1 and Th2 cytokines or blunting of initial cytokine response might also be important in disease development and/or maintenance. Animal studies have demonstrated a lower initial TNF-α, IFN-γ, and interleukin-4 (IL-4) response to C. trachomatis infection leading to decreased bacterial clearance, which suggests that a blunted initial Th1 response after acute chlamydial infection might increase the likelihood of development of CiReA (Citation45). Similarly, background cytokine levels favoring a Th2 response might contribute to bacterial persistence; in-vitro data reveal that low levels of TNF-α and, as previously stated, IFN-γ help to promote the persistent state of both C. trachomatis and C. pneumoniae (Citation46–48).
Molecular biology of persistent chlamydiae
Our and others’ studies on synovial tissues from patients with CiReA have indicated that the primary synovial host cell for C. trachomatis is the monocyte/macrophage (Citation27). These studies identified only aberrant, intracellular chlamydial forms in synovial samples (Citation49) similar to those described earlier with the IFN-γ-related studies. Combined with the culture negativity of synovial tissue for C. trachomatis, these data indicated that chronic C. trachomatis-induced ReA involves persistent organisms that behave altogether differently than do organisms associated with acute infections; more recent data indicate that C. pneumoniae also is in the persistent infection state in synovial tissue of patients with ReA (Citation50).
More recent PCR and RT-PCR studies have confirmed that the molecular biology of persistent chlamydiae is easily distinguishable from that of acute chlamydial infections. The pattern of gene expression associated with persistently viable chlamydiae of both relevant species is significantly different than that seen during normal active infections, although the transcript patterns during persistence are not entirely identical for these two organisms. For example, during persistence expression of the major outer membrane protein (omp1) gene and several genes required for the cell division process are severely down-regulated for both (Citation50,Citation51); this is coupled with an up-regulation of heat shock proteins (HSPs) for C. trachomatis (Citation51). We showed recently that in relation to in-vitro model systems of persistent C. pneumoniae infection, the details of the transcript profile induced depends on the means by which persistence is elicited (Citation52). HSPs, in general, are paramount to the persistent state of both C. trachomatis and C. pneumoniae; they provide many functions involved with cell survival. The HSP-60 molecule, specifically, has many functions that appear to be important to the pathophysiology of ReA. HSP-60 has been shown to be pivotal in the inability of Chlamydia-infected cells to undergo apoptosis(Citation53). Other studies have demonstrated that HSP-60 plays a role in eliciting the host immune response(Citation54,Citation55).
The C. trachomatis and C. pneumoniae genomes each specify three HSP-60-encoding genes (Citation51,Citation56). However, in terms of HSP-60 gene expression, there are differences even within the Chlamydia genus. With C. trachomatis, the authentic HSP-60 gene, groEL, is designated Ct110 in the genome sequence and resides in an operon with groES, as in Escherichia coli and other bacteria. The two additional paralogs are designated Ct604 and Ct755. The groEL HSP-60 protein is believed to be important in synovial pathogenesis. Each of the three C. trachomatis HSP-60 genes is transcribed independently during active infection (Citation51). In acutelyinfected cells, Ct604 and Ct755 are expressed at higher levels than Ct110. However, expression of Ct110 and Ct755 is attenuated during persistence in the monocyte model, whereas transcript levels from Ct604 are increased significantly in this state. This suggests that the product of Ct604 functions to maintain persistence. Analyses of synovial biopsy samples from patients with CiReA also demonstrate high mRNA levels from Ct604 and extremely low levels from Ct755. These observations might explain, in part, the continued elicitation of inflammation in patients with chronic CiReA. They also suggest that the Ct604 gene product is involved in the transition from normal active to persistent infection. Conversely, the Ct755 gene product appears to function only during the acute infection state. It has been demonstrated that some of the transcriptional details that characterize persistent C. pneumoniae are not fully congruent with those that characterize C. trachomatis, although fewer data exist regarding the transcript profile for HSP-60-encoding or other genes in C. pneumoniae during persistence (Citation57). Although the clinical significance of these differences remains unknown, this might also explain the apparent divergent arthritogenic propensity of these two similar organisms.
Chlamydiae in patients with chronic arthritis
Interestingly many of the synovial samples obtained from patients with CiReA from these previously mentioned studies were analyzed years after the patients’ initial infection, thereby demonstrating that persistent organisms can exist in synovial tissue for extended periods of time, indeed many years. Such samples were obtained from patients with chronic CiReA, with the term chronic referring to patients with disease duration of greater than 6 months. Unfortunately, there have been no similar synovial tissue analyses on patients who experienced acute CiReA with spontaneous resolution, i.e. synovial samples obtained after remission. Such analyses would provide interesting correlation, but these existing data suggest that persistently viable synovial-based chlamydiae play at least some role in disease maintenance.
The true significance of the documentation of synovial-based chlamydiae has been questioned by some because PCR and RT-PCR data demonstrating these organisms in synovial samples are not unique to patients with CiReA. Reports have described similar findings in a small percentage of patients with other types of arthritis, primarily osteoarthritis, but even some normal controls (Citation58–60). This background PCRpositivity rate has ranged from 5% (Citation59) to as high as 20% (Citation60). However, it is important to note that the prevalence of chlamydiae in the synovial tissue of patients with other conditions is significantly lower compared to synovial tissue of patients with CiReA (Citation61,Citation62). The clear difference in the prevalence of PCRpositivity for C. trachomatis in CiReA compared to control populations highlights the importance of host genetic variability, host tolerance, and important potential arthritogenic differences within the Chlamydia genus itself.
We and others have argued for many years that development of joint disease following genital or pulmonary chlamydial infection is a function of the details of host–pathogen interaction during both acute infection and, as importantly, during the establishment of persistent infection (Citation5). It remains to be established why not everyone who acquires such infections develops arthritis, but one explanation may be that individuals with certain characteristics of their genetic backgrounds are able to ‘tolerate’ persistent chlamydiae better with fewer or even no clinical sequelae. Traditionally, efforts to discover host influences that might predispose to disease development have focused on the human leukocyte antigen (HLA)-B27. Although data suggest HLA-B27-positive patients exposed to C. trachomatis might be more likely to develop CiReA, more specifically, such individuals might develop more robust clinical symptoms with acute CiReA (Citation63,Citation64). Other data also suggest that these individuals are more likely to develop a chronic course (Citation65). However, patients that are HLA-B27 negative can clearly develop ReA or CiReA, specifically, both the acute and chronic form. Neither HLA-B27 nor any other single or specific host feature has been uncovered that holds the unique key to disease susceptibility.
Pathogen as the key to disease susceptibility
Recent data regarding disease susceptibility to CiReA force reconsideration of traditional paradigms. These data suggest that the pathogen itself might hold the clue to disease susceptibility in this classic host–pathogen interaction. Because C. trachomatis-induced ReA is triggered by a genital infection, it was logical to assume that the genital strains of C. trachomatis were causative. However, there are several serovars of C. trachomatis. Serovars A, B, Ba, and C are ocular (trachoma) and are etiologic for trachoma, a blinding disease that remains highly prevalent in parts of the world. The remaining serovars (serovars D through K and biovarlymphogranulosum venereum (LGV)) are causative of genital infections. Remarkably, a recent study analyzing the C. trachomatis serovars of 36 subjects with known CiReA demonstrated that all 36 synovial tissue samples were positive for ocular serovars, not genital serovars (Citation66). It is known that genital infections with the ocular strains do occur, but they are rare (Citation67,Citation68). These data suggest that about 1%–5% of genital infections with C. trachomatis will include inoculation with ocular serovars. These surprising data strongly suggest that the infrequent rate of genital infections with the ocular strain might explain the low attack rate of ReA in patients with acute C. trachomatis infections in general. It should also be noted that the previously cited studies which demonstrated the rare presence of synovially based persistent C. trachomatis in arthritides other than CiReA, such as osteoarthritis, did not include serovar typing. Such analyses might prove enlightening, particularly if non-ocular serovars are discovered in such patients.
Molecular genetic studies have discovered variations within these chlamydial strains, i.e. ocular versus genital serovars, which might account for these apparent differences in propensity to disseminate, tissue tropism, and pathogenesis. Studies have demonstrated that some DNA sequence variation in omp1 is present within each serovar (Citation69,Citation70). In addition to differences at omp1, ocular and genital serovars have non-identical deletions around the cytotoxin gene (toxB, Ct166) (Citation69). Further, while genital serovars have functional products from the trpA gene, encoding one component of the tryptophan synthase enzyme, ocular serovars have deletions in that gene which produce a non-functional product (Citation70). Other studies showed that differences in genomic structure among C. trachomatis ocular serovars result in varying IFN-γ sensitivity, growth rate in vitro, and virulence within that serovar group (Citation71). It is also interesting to note that the LGV strains of C. trachomatis are generally felt to be non-arthritogenic.
Can persistent chlamydiae be eradicated?
The best treatment for CiReA remains open to debate, although this is a topic beyond the scope of this review. However, given the well documented state of persistently viable chlamydial cells in the synovial tissue of patients, the role these organisms appear to play in disease maintenance of CiReA correlates with that of persistent M. tuberculosis, and the in-vitro response of these persistent chlamydiae to blunting of the major arm of the Th1 response (TNF-α and IFN-γ), biologic response modifiers, such as anti-TNF therapy, should be used with great caution, and perhaps not at all. However, there is a recent retrospective report of ten patients with ReA (both post-dysentery and post-chlamydial) suggesting that anti-TNF therapy improved the clinical symptoms in the majority of these ten subjects (Citation72). It should be noted that seven of these ten patients had acute ReA (disease duration 6 months or less), and, as stated, acute ReA spontaneously remits in most patients. Obviously, more studies are needed, but even if anti-TNF therapy can ameliorate the symptoms, there is no reasonable expectation that it can eradicate the underlying persistent chlamydial infection. Although an initial study suggested chronic antimicrobial therapy might prove efficacious for CiReA (Citation73), several follow-up studies provided disappointing results (Citation74–77). However, we recently completed a double-blind placebo-controlled trial demonstrating that a 6-month course of combination antibiotics (either doxycycline and rifampin or azithromycin and rifampin) was superior to placebo not only at improving the clinical symptoms of CiReA but also at clearing the PCR-documented C. trachomatis or C. pneumoniae infection underlying the disease (Citation78). This further supports the growing body of evidence that CiReA should no longer be considered a ‘sterile arthritis’ and these synovial-based organisms can be cleared with proper treatment resulting in abrogation of the clinical sequelae that result from these persistently infecting organisms.
Chlamydiae as etiologic agents for other types of chronic arthritis
As mentioned, CiReA is a type of spondyloarthritis (SpA). Could chlamydiae also be etiologic for other types of spondyloarthritis? Several epidemiologic studies are in agreement that undifferentiated spondyloarthritis (uSpA) is the second most commonly diagnosed type of SpA after ankylosing spondylitis (Citation79,Citation80). Indeed, one study suggested it to be the most commonly diagnosed type of SpA (Citation81). We performed a recent study analyzing the synovial tissue of patients with uSpA for C. trachomatis and C. pneumoniae by using PCR. Interestingly, in 62% of the uSpA patients the synovial tissue was PCR-positive for one or both species compared to 12% of the osteoarthritis controls (P < 0.0001) (Citation62). This strongly suggests that chlamydiae are also common etiologic agents for uSpA. Perhaps patients diagnosed with uSpA in the general population represent patients who do not present with the ‘classic triad’ of ReA, or it is also possible these patients are HLA-B27-negative; both scenarios might lead to the under-diagnosis of CiReA. None of the uSpA patients in our study had the ‘classic triad’ of ReA, and only 16% were HLA-B27-positive. Interestingly, in this same study less than half of the uSpA patients had a known chlamydial infection at any point in their life. It is well known that acute chlamydial infections are often asymptomatic. The fact that an asymptomatic chlamydial infection might trigger CiReA renders the clinical diagnosis extremely difficult using the current definition of the disease. Taken together these data suggest that chlamydiae might be causative of a much wider scope of inflammatory arthritis than currently believed.
Conclusion
The field of Chlamydia and chronic arthritis is evolving rapidly. It has been known for about 50 years that C. trachomatis is a definite trigger of ReA. More recently C. pneumoniae has been implicated as another causative organism. Molecular biology studies have taught us that these organisms are able to disseminate from their initial site of infection and establish a persistent infection in target organs, namely the synovial tissue. These persistent chlamydial organisms are viable although in an aberrant state. Clearly, CiReA does not represent a ‘sterile arthritis’ as it has been described, and recent advances demonstrate that this terminology should be abandoned. How these persistent chlamydiae contribute to disease perpetuation and maintenance remains to be fully established, but recent data demonstrating that long-term combination antimicrobial treatment can not only ameliorate the symptoms but eradicate the persistent infection suggest that these persistently viable chlamydiae are indeed a driving force behind the chronic inflammation.
Modern medicine has provided remarkable advances in our understanding of the chlamydiae as it relates to chronic arthritis and the delicate balance between host and pathogen. These organisms are clearly culpable in the genesis of ReA. They have a remarkable ability to disseminate from the initial site of infection and establish persistently viable cells in distant organ sites with apparent variable tissue tropism. The ocular strains of C. trachomatis might be uniquely capable of such actions, at least in the setting of chronic arthritis and related sequelae. All of this appears to be possible even after an asymptomatic initial chlamydial infection, thereby creating a stealthy pathway to disease. In an ironic twist of fate, Hippocrates might have documented the very first case of CiReA approximately 2,500 years ago when he wrote, ‘a youth does not suffer from gout until sexual intercourse’. He astutely made the connection that a sexually transmitted disease might cause a secondary inflammatory arthritis, but he missed the precise diagnosis. Now do we make the diagnosis of chronic inflammatory arthritis but oftentimes miss the etiologic agent?
Declaration of interest: The authors state no conflict of interest and have received no payment in preparation of this manuscript.
References
- Hippocrates: The Genuine Works of Hippocrates, vol I and II. New York: Pelican Books; 1978. 229.
- Sharp JT. Reiter's syndrome. Hollander JH, McCarthy DJ. Arthritis and allied conditions. 8th Philadelphia: Lea and Febiger; 1979. 1223–9.
- Yvan AU. Observation surunemetastase de gonorrhee. Ann Soc Med Prat de Montpellier. 1806:119–25.
- Brodie BC. Pathological and surgical observations on diseases of the joints. London: Longman; 1818. 54.
- Carter JD, Hudson AP. Reactive arthritis: clinical aspects and medical management. Rheum Dis Clin North Am. 2009;35:21–44.
- Laasila K, Laasonen L, Leirisalo-Repo M. Antibiotic treatment and long term prognosis of reactive arthritis. Ann Rheum Dis. 2003;62:655–8.
- Yli-Kerttula T, Luukkainen R, Yli-Kerttula U, Möttönen T, Hakola M, Korpela M, . Effect of a three month course of ciprofloxacin on the late prognosis of reactive arthritis. Ann Rheum Dis. 2003;62:880–4.
- Michet CJ, Machado EB, Ballard DJ, McKenna CH. Epidemiology of Reiter's syndrome in Rochester, Minnesota: 1950–1980. Arthritis Rheum. 1988;31:428–31.
- Groseclose SL, Zaidi AA, Delisle SJ, Levine WC, St Louis ME. Estimated incidence and prevalence of genital Chlamydia trachomatis infections in the United States, 1996. Sex Transm Dis. 1999;26:339–44.
- Gordon FB, Quan AL, Steinman TI, Philips RN. Chlamydial isolates from Reiter's syndrome. Br J Vener Dis. 1973;49:376–8.
- Keat A, Thomas B, Dixcey J, Osborne M. Sonnex C, Taylor-Robinson D. Chlamydia trachomatis and reactive arthritis: the missing link. Lancet. 1987;1:72–4.
- Norton WL, Lewis D, Ziff M. Light and electron microscopic observation on the synovitis of Reiter's disease. Arthritis Rheum. 1966;9:747–57.
- Ishikawa H, Ohno O, Yamasaki K, Ikuta S, Hirohata K. Arthritis presumably caused by chlamydia in Reiter's syndrome. Case report with electron microscopic studies. J Bone Joint SurgAm. 1986;68:777–9.
- Moazed TC, Kuo CC, Grayston JT, Campbell LA. Evidence of systemic dissemination of Chlamydia pneumoniae via macrophages in the mouse. J Infect Dis.1998;177:1322–5.
- Horn JE, Hammer ML, Falkow S, Quinn TC. Detection of Chlamydia trachomatis intissue culture and cervical scrapings by in situ hybridization. J Infect Dis. 1986;153:1155–9.
- Hyypia T, Larsen SH, Stahlberg T, Terho P. Analysis and detection of chlamydial DNA. J Gen Microbiol. 1984;130:3159–64.
- Naher H, Petzoldt D, Sethi KK. Evaluation of non-radioactive in situ hybridization method to detect Chlamydia trachomatis in cell culture. Genitourin Med. 1988;64:162–4.
- Cheema MA, Schumacher HR, Hudson AP. RNA-directed molecular hybridization screening: evidence for inapparent chlamydial infection. Am J Med Sci. 1991;302:261–8.
- Palmer L, Falkow S. 16S ribosomal RNA genes of Chlamydia trachomatis. Oriel D, Ridgway G, Schachter J, Taylor-Robinson D, Ward M. Chlamydial infections. New York: Cambridge University Press; 1986. 89–92.
- Rahman MU, Cheema MA, Schumacher HR, Hudson AP. Molecular evidence for the presence of chlamydia in the synovium of patients with Reiter's syndrome. Arthritis Rheum.1992;35:521–9.
- Hammer M, Nettelnbreker E, Hopf S, Schmitz E, Pörschke K, Zeidler H. Chlamydial RNA in the joints of patients with Chlamydia-induced arthritis and undifferentiated arthritis. ClinExptl Rheumatol.1992;10:63–6.
- Branigan PJ, Gérard HC, Hudson AP, Schumacher HR. Comparison of synovial tissue and fluid as sources for nucleic acids for detection of C. trachomatis by polymerase chain reaction. Arthritis Rheum. 1996;39:1740–6.
- Taylor-Robinson D, Gilroy CB, Thomas BJ, Keat ACS. Detection of Chlamydia trachomatis DNA in joints of reactive arthritis patients by PCR. Lancet.1992;340:81–2.
- Bas S, Griffais R, Kvien TK, Glennås A, Melby K, Vischer TL. Amplification of plasmid and chromosome Chlamydia DNA in synovial fluid of patients with reactive arthritis and undifferentiated seronegative oligoarthropathies. Arthritis Rheum. 1995;38:1005–13.
- Wilkinson NZ, Kingsley GH, Sieper J, . Detection of C pneumoniae and C trachomatis in the synovium of patients with a range of rheumatic diseases. Wilkinson NZ, Kingsley GH, Sieper J, Braun J, Ward ME. Proceedings of the European Society for Chlamydia Research. Bologna, Italy: Societa Editrice Esculapio; 1996. 189.
- Wordsworth BP, Hughes RA, Allan I, Keat AC, Bell JI. Chlamydial DNA is absent from the joints of patients with sexually-acquired arthritis. Br J Rheumatol.1990;29:208–10.
- Beutler AM, Whittum-Hudson JA, Nanagara R, Schumacher HR, Hudson AP. Intracellular location of inapparently infecting Chlamydia in synovial tissue from patients with Reiter's syndrome. Immunol Res. 1994;13:163–71.
- Gerard HC, Branigan PJ, Schumacher HR Jr, Hudson AP. Synovial chlamydia trachomatis in patients with reactive arthritis/Reiter's syndrome are viable but show aberrant gene expression. J Rheumatol. 1998;25:734–42.
- Kol A, Bourcier T, Lichtman AH, Libby P. Chlamydial and human heat shock protein 60s activate human vascular endothelium, smooth muscle cells, and macrophages. J Clin Invest. 1999;103:571–7.
- Rödel J, Lehmann M, Vogelsang H, Straube E. Chlamydia pneumoniae infection of aortic smooth muscle cells reduces platelet-derived growth factor receptor-beta expression. FEMS Immunol Med Microbiol. 2007;51:363–71.
- Borel N, Summersgill JT, Mukhopadhyay S, Miller RD, Ramirez JA, Pospischil A. Evidence for persistent Chlamydia pneumoniae infection of human coronary atheromas. Atherosclerosis. 2008;199:154–61.
- Beutler AM, Schumacher HR, Whittum-Hudson JA, Salameh WS, Hudson AP. In situ hybridization for detection of inapparent infection with C trachomatis in synovial tissue of a patient with Reiter's syndrome. Am J Med Sci. 1995;310:206–13.
- Nicholson TL Olinger L, Chong K, Schoolnik, Stephens RS. Global stage-specific gene regulation during the developmental cycle of Chlamydia trachomatis. J Bacteriol. 2003;185: 3179–89.
- Hackstadt T. Cell biology. Stephens RS. Chlamydia—intracellular biology, pathogenesis, and immunity. Washington DC: ASM Press; 1999. 101–38.
- Moulder JW. Interaction of Chlamydiae and host cells in vitro. Microbiol Rev. 1991;55:143–90.
- Beatty WL, Byrne GI, Morrison RP. Morphologic and antigenic characterization of interferon gamma mediated persistent C trachomatis infection. Proc Natl Acad Sci USA.1993; 90:3998–4002.
- Beatty WL, Morrison RP, Byrne GI. Reactivation of persistent Chlamydia trachomatis infection in cell culture. Infect Immun.1995;63:199–205.
- Gérard HC, Whittum-Hudson JA, Schumacher HR, Hudson AP. Synovial Chlamydia trachomatis up regulates expression of a panel of genes similar to that transcribed by Mycobacterium tuberculosis during persistent infection. Ann Rheum Dis. 2006;65:321–7.
- Köhler L, Nettelnbreker E, Hudson AP, Ott N, Gérard HC, Branigan PJ, . Ultrastructural and molecular analysis of the persistence of Chlamydia trachomatis (serovar K) in human monocytes. MicrobPathog. 1997;22:133–42.
- Carter LL, Dutton RW. Type 1 and Type 2: a fundamental dichotomy for all T cell subsets. Curr Opin Immunol. 1996;8: 336–42.
- Fitzpatrick DR, Wie J, Webb D, Bonfiglioli R, Gardner ID, Mathews JD, . Preferential binding of C trachomatis to subsets of human lymphocytes and induction of interleukin-4 and interferon gamma. Immunol Cell Biol.1991;69:337–48.
- Braun J, Yin Z, Spiller I, Siegert S, Rudwaleit M, Liu L, . Low secretion of tumor necrosis factor alpha, but no other Th1 or Th2 cytokines, by peripheral blood mononuclear cells correlates with chronicity in reactive arthritis. Arthritis Rheum. 1999;42:2039–44.
- Thiel A, Wu P, Lauster R, Braun J, Radbruch A, Sieper J. Analysis of the antigen-specific T cell response in reactive arthritis by flow cytometry. Arthritis Rheum. 2000;43:2834–42.
- Yin Z, Braun J, Neure L, Wu P, Liu L, Eggens U, . Crucial role of interleukin-10/interleukin-12 balance in the regulation of the type 2 T helper cytokine response in reactive arthritis. Arthritis Rheum. 1997;40:1788–97.
- Inman RD, Chiu B. Early cytokine profiles in the joint define pathogen clearance and severity of arthritis in Chlamydia-induced arthritis in rats. Arthritis Rheum. 2006;54:499–507.
- Ishihara T, Aga M, Hino K, Ushio C, Taniguchi M, Iwaki K, . Inhibition of chlamydia trachomatis growth by human interferon-alpha: mechanisms and synergistic effect with interferon-gamma and tumor necrosis factor-alpha. Biomed Res. 2005;26:179–85.
- Perry LL, Feilzer K, Caldwell HD. Immunity to Chlamydia trachomatis is mediated by T helper 1 cells through IFN-gamma-dependent and –independent pathways. J Immunol. 1997;158:3344–52.
- Takano R, Yamaguchi H, Sugimoto S, Nakamura S, Friedman H, Yamamoto Y. Cytokine response of lymphocytes persistent infected with Chlamydia pneumoniae. CurrMicrobiol. 2005;50:160–6.
- Nanagara R, Li F, Beutler AM, Hudson AP, Schumacher HR. Alteration of C trachomatis biological behavior in synovial membranes: suppression of surface antigen production in reactive arthritis and Reiter's syndrome. Arthritis Rheum. 1995;38:1410–7.
- Byrne GI, Ouellette SP, Wang Z, Rao JP, Lu L, Beatty WL, . Chlamydia pneumoniae expresses genes required for DNA replication but not cytokinesis during persistent infection of HEp-2 cells. Infect Immun. 2001;69:5423–9.
- Gerard HC, Whittum-Hudson JA, Schumacher HR, Hudson AP. Differential expression of three Chlamydia trachomatis hsp60-encoding genes in active vs. persistent infections. Microb Pathog. 2004;36:35–9.
- Klos A, Thalmann J, Peters J, Gérard HC, Hudson AP. The transcript profile of persistent Chlamydophila (Chlamydia) pneumoniae in vitro depends on the means by which persistence is induced. FEMS Microbiol Lett. 2009;291:120–6.
- Dean D, Powers VC. Persistent Chlamydia trachomatis infections resist apoptotic stimuli. Infect Immun. 2001;69: 2442–7.
- Zugel U, Kaufmann SH. Role of heat shock proteins in protection from and pathogenesis of infectious diseases. Clin Microbiol Rev. 1999;12:19–39.
- Curry AJ, Portig I, Goodall JC, Kirkpatrick PJ, Gaston JS. T lymphocyte lines isolated from atheromatous plaque contain cells capable of responding to Chlamydia antigens. Clin Exp Immunol.2000;121:261–9.
- Read TD, Brunham RC, Shen C, Gill SR, Heidelberg JF, White O, . Genome sequences of Chlamydia trachomatis MoPn and Chlamydia pneumoniae AR39. Nucleic Acids Res. 2000;28:1397–406.
- Gerard HC, Wang Z, Whittum-Hudson JA, El-Gabalawy H, Goldbach-Mansky R, Bardin T, . Cytokine and chemokine mRNA produced in synovial tissue chronically infected with Chlamydia trachomatis and C. pneumoniae. J Rheumatol. 2002;29:1827–35.
- Wilkinson NZ, Kingsley GH, Jones HW, Sieper J, Braun J, Ward ME, . The detection of DNA from a range of bacterial species in the joints of patients with a variety of arthritides using a nested, broad-range polymerase chain reaction. Rheumatology (Oxford). 1999;38:260–6.
- Schumacher HR, Arayssi T, Crane M, Lee J, Gérard HC, Hudson AP, . Chlamydia trachomatis nucleic acids can be found in synovium of some asymptomatic volunteers. Arthritis Rheum. 1999;42:1281–4.
- Olmez N, Wang GF, Li Y, Zhang H, Schumacher HR. Chlamydial nucleic acids in synovium in osteoarthritis: what are the implications? J Rheumatol. 2001;28:1874–80.
- Siala M, Jaulhac B, Gdoura R, Sibilia J, Fourati H, Younes M, . Analysis of bacterial DNA in synovial tissue of Tunisian patients with reactive and undifferentiated arthritis by broad-range PCR, cloning and sequencing. Arthritis Res Ther. 2008;10:R40.
- Carter JD, Gérard HC, Espinoza LR, Ricca LR, Valeriano J, Snelgrove J, . Chlamydiae as etiologic agents in chronic undifferentiated spondylarthritis. Arthritis Rheum. 2009;60:1311–6.
- Sonkar GK, Usha. Role of HLA B27 in diagnosis of seronegative spondyloarthropathies. Indian J Pathol Microbiol. 2007;50:908–13.
- Schiellerup P, Krogfelt KA, Locht H. A comparison of self-reported joint symptoms following infection with different enteric pathogens: effect of HLA-B27. J Rheumatol. 2008;35:480–7.
- Kaarela K, Jäntti JK, Kotaniemi KM. Similarity between chronic reactive arthritis and ankylosing spondylitis. A 32–35-year follow-up study. Clin Exp Rheumatol. 2009;27:325–8.
- Gerard HC, Stanich JA, Whittum-Hudson JA, Schumacher HR, Carter JD, Hudson AP. Patients with Chlamydia-associated arthritis have ocular (trachoma), not genital, serovars of C. trachomatis in synovial tissue. Microb Pathog. 2010;48:62–8.
- Mittal A. Serovar distribution of Chlamydia trachomatis isolates collected from the cervix: use of the polymerase chain reaction and restriction endonuclease digestion. Br J Biomed Sci. 1998;55:179–83.
- Workowski KA, Stevens CE, Suchland RJ, Holmes KK, Eschenback DA, Pettinger MB, . Clinical manifestations of genital infection due to Chlamydia trachomatis in women: differences related to serovar. Clin Infect Dis. 1994;19:756–60.
- Carlson JH, Hughes S, Hogan D, Cieplak G, Sturdevant DE, McClarty G, . Polymorphisms in the C trachomatis cytotoxin locus associated with ocular and genital isolates. Infect Immun. 2004;72:7063–72.
- Caldwell HD, Wood H, Crane D, Bailey R, Jones RB, Mabey D, . Polymorphisms in C trachomatis tryptophan synthase genes differentiate between genital and ocular serovars. J Clin Invest. 2003;111:1757–69.
- Kari L, Whitmire WM, Carlson JH, Crane DD, Reveneau N, Nelson DE, . Pathogenic diversity among C trachomatis ocular strains in nonhuman primates is affected by subtle genomic variations. J Infect Dis. 2008;197:449–56.
- Meyer A, Chatelus E, Wendling D, Berthelot JM, Dernis E, Houvenagel E, . Safety and efficacy of anti-tumor necrosis factor α therapy in ten patients with recent-onset refractory reactive arthritis. Arthritis Rheum. 2011;63:1274–80.
- Lauhio A, Leirisalo-Repo M, Lähdevirta J, Saikku P, Repo H. Double-blind, placebo-controlled study of three-month treatment with lymecycline in reactive arthritis, with special reference to Chlamydia arthritis. Arthritis Rheum. 1991;34:6–14.
- Smieja M, MacPherson DW, Kean W, Schmuck ML, Goldsmith CH, Buchanan W, . Randomised, blinded, placebo controlled trial of doxycycline for chronic seronegative arthritis. Ann Rheum Dis. 2001;60:1088–94.
- Wakefield D, McCluskey P, Verma M, Aziz K, Gatus B, Carr G. Ciprofloxacin treatment does not influence course or relapse rate of reactive arthritis and anterior uveitis. Arthritis Rheum. 1999;42:1894–7.
- Yli-Kerttula T, Luukkainen R, Yli-Kerttula U, Möttönen T, Hakola M, Korpela M, . Effect of a three month course of ciprofloxacin on the outcome of reactive arthritis. Ann Rheum Dis. 2000;59:565–70.
- Kvien TK, Gaston JS, Bardin T, Butrimiene I, Dijkmans BA, Leirisalo-Repo M, . Three-month treatment of reactive arthritis with azithromycin: A EULAR double-blind, placebo-controlled study. Ann Rheum Dis. 2004;63:1113–9.
- Carter JD, Espinoza LR, Inman RD, Sneed KB, Ricca LR, Vasey FB, . Combination antibiotics as a treatment for chronic Chlamydia-induced reactive arthritis: a double-blind, placebo-controlled, prospective study. Arthritis Rheum. 2010;62:1298–307.
- Olivieri I, van Tubergen A, Salvarani C, van der Linden S. Seronegative spondyloarthritides. Best Pract Res Clin Rheumatol. 2002;16:723–39.
- Braun J, Bollow M, Remlinger G, Eggens U, Rudwaleit M, Distler A, . Prevalence of spondyloarthropathies in HLA-B27 positive and negative blood donors. Arthritis Rheum. 1998;41:58–67.
- Boyer GS, Templin DW, Bowler A, Lawrence RC, Heyse SP, Everett DF, . Spondyloarthropathy in the community: clinical syndromes and disease manifestations in Alaskan Eskimo populations. J Rheumatol. 1999;26:1537–44.
