Abstract
Objective. To investigate whether blood cytokines during the perinatal period predict the risk of cerebral palsy (CP) in preterm infants.
Methods. This prospective cohort study comprised 169 children born before 32 weeks of gestation. Cord blood was drawn at birth, and 109 cytokines were analyzed using microarrays. Eleven cytokines were further measured from both cord and peripheral blood on days 1 and 7. Cerebral palsy was confirmed at 5 years of age.
Results. Cerebral palsy was diagnosed in 19 children. Five clusters of cord blood cytokines were scored using factor analysis. According to logistic regression analysis, the scores of factors 1 and 2 independently predicted the risk of CP. These cytokines included several growth factors and chemokines, and they all tended to be higher in children with CP than in children without CP. Inflammatory cytokine levels were associated with CP risk on days 1 and 7 after birth.
Conclusion. The high blood concentrations of various cytokines during the perinatal period may relate to CP, and these cytokines may influence the pathways leading to early insult in the central nervous system. The risk profile of inflammatory cytokines is different at birth than during the first week after birth.
Key words::
| Abbreviations | ||
| BBB | = | blood–brain barrier |
| CP | = | cerebral palsy |
| cPVL | = | cystic periventricular leukomalacia |
| CRIB | = | clinical risk index for babies |
| ELGAN | = | extremely low gestational age newborn (<28 weeks) |
| FU | = | fluorescence unit |
| GA | = | gestational age |
| HCA | = | histologic chorioamnionitis |
| HIF | = | hypoxia-inducible factor |
| IL | = | interleukin |
| OL | = | oligodendrocyte |
| ROC | = | receiver-operating characteristic |
| SGA | = | small for gestation |
| TNF | = | tumor necrosis factor |
Key messages
We identified a cluster of cord blood cytokines that predicted the risk of CP.
Also, the present study revealed that another cytokine predicting CP, IL-12, emerged after birth.
The present findings are compatible with the concept that antenatal and neonatal events additively predispose infants to CP.
Introduction
Cerebral palsy (CP) is a collective term for motor deficits (Citation1). It is the most common cause of physical, neurocognitive, and neurosensory disabilities in childhood, affecting the quality of life throughout the patient's life-span. The prevalence rate is about 2 in every 1,000 live births in Europe and reveals clustering to preterm infants, in whom the brain injury occurred presumably in either the peri- or neonatal period (Citation2).
Neuronal migration, synaptogenesis, vascularization, myelination, and brain folding are delicate processes that are susceptible to insult during brain maturation. Factors such as hypoxia/ischemia, inflammation/infection, and genetic factors can affect these processes (Citation3,Citation4). Extracellular cytokines that interact with cellular compartments have important roles not only in the immune response to inflammatory pathogens, but also in generating neurotrophic functions in the developing brain. Neuronal cells, such as astrocytes and oligodendrocytes (OLs), can act as providers of growth factors to neurons (Citation5). On the other hand, high levels of pro-inflammatory cytokines in the amniotic fluid, especially tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), IL-6, and IL-8, reflect immunological activation in utero and are associated with white matter damage and CP later on (Citation6,Citation7).
Periventricular leukomalacia (PVL) and periventricular hemorrhagic infarction are two of the most important focal destructive lesions associated with cerebral white matter damage in very preterm infants. The axonal injury that lies behind these lesions is partly due to the specific vulnerability of the immature brain, particularly early-stage myelin-producing OLs to hypoxia/ischemia and inflammation. Reactive free-radical species, generated in abundance during ischemia–reperfusion, and various mediators of the inflammatory cascade appear to contribute to this process. In addition to OLs, other types of cells are also affected; neuronal loss in cerebral gray matter is associated with reduced volume in the cerebral cortex as well as in the thalamus and basal ganglia (Citation8).
Infants born before 32 weeks of gestation have a 50-fold higher risk of CP than those born at term (Citation2). The perinatal events promoting the risk of CP in very preterm infants may occur antenatally or neonatally. Therefore, we designed the current study to analyze whether the presence of one or more cytokines in cord blood at the time of very premature birth would be associated with CP and whether an inflammatory cytokine during the early neonatal period would also be associated with CP. Based on investigations of the role of immunoproteins and different pathways involved in the damage of the brain structures, multiple proteins could be involved in the pathogenesis of CP. Here, we propose that infants born very prematurely are exposed to antenatal adverse events and postnatal inflammatory stress that additively increase the risk of CP.
Patients and methods
The current study is part of a prospective longitudinal cohort study of infants born very preterm (< 32 weeks) in Oulu University Hospital, the regional perinatal center of Northern Finland (Citation9,Citation10). The total number of children born alive during the study period (November 1998 to November 2002) was 232. Complete follow-up information was available for 162 children. An additional seven very preterm participants were born in Tampere University Hospital during March 2000 to January 2001. The definition of gestational age was based on an ultrasonographic examination before 20 weeks of gestation, performed as a Finnish standard. Informed consent was signed by the parents. The study was approved by the local ethics committees of Oulu and Tampere University Hospitals.
The diagnosis and classification of CP was confirmed at the age of 5 years by a child neurologist, based on the criteria established by the Surveillance of Cerebral Palsy in Europe (SCPE) network (Citation11). Eleven children had bilateral spastic CP; six had unilateral spastic CP; and two children dyskinetic CP, of which one child died at the age of 3 years and 11 months but was included in this analysis because of a definite diagnosis of CP.
Cytokine analysis from umbilical cord blood
After double-clamping the umbilical cord at birth, arterial blood was drawn into a sterile tube. Cord blood serum was separated at 3,000 rpm for 15 min, and the samples were stored frozen at −70°C. Peripheral arterial blood was collected on days 1 and 7 after birth, and after centrifugation it was stored at −70°C until analysis.
Cord blood cytokines were analyzed using antibody-based protein microarrays with DNA amplification (Citation12). Monoclonal antibodies (R&D Systems, Minneapolis, MN, USA; PharMingen, San Diego, CA, USA) were dispensed onto the microarrays. After incubation, the captured proteins were detected by secondary antibodies containing an oligonucleotide DNA primer to generate a fluorescent signal (GenePix; Axon Instruments, Foster City, CA, USA), which was used to quantify specific proteins. Altogether 109 different cytokines were analyzed, including adhesion molecules, chemokines, growth factors, hematopoietin family cytokines, interleukins, metalloproteinases, and tumor necrosis factor family (TNF) cytokines (). The concentrations are reported as fluorescence units (FU).
Table I. The cytokine groups analyzed from umbilical cord blood obtained at the time of birth.
Eleven cytokines, interleukin (IL)-1β, IL-3, IL-4, IL-6, CXCL8/IL-8, IL-10, IL-12p70, granulocyte colony stimulating factor (G-CSF), granulocyte-macrophage colony stimulating factor (GM-CSF), CCL11/eotaxin, and TNF-α, were measured from umbilical cord blood collected at birth and from peripheral blood collected 1 and 7 days after birth using the Cytometric Bead Array Kit (BD Biosciences, San Diego, CA, USA). Bead populations with distinct fluorescence intensities for specific soluble proteins were measured with flow cytometry. The concentrations are reported as pg/mL.
Clinical assessment
The following pre- and postnatal clinical parameters were included in this study: multiple pregnancy, parity, maternal fever, pre-eclampsia (Citation13), histologic placental inflammation (Citation14), gestational age at birth, gender, infant being small for gestation (SGA) (Citation15), 5-min Apgar score, Clinical Risk Index for Babies (CRIB) I score (Citation16), lowest mean arterial blood pressure in the first 24 hours after birth, duration of mechanical ventilation support, requirement for additional oxygen at the age of 36 weeks of gestation, and blood culture-positive sepsis during the neonatal period. Brain ultrasounds were performed using the HDI 5000 (Advanced Technology Laboratories Ultrasound, Botwell, WA, USA) with a curved-array 5–8 MHz transducer. Intraventricular hemorrhage (IVH) was classified according to Papile (Citation17), and cystic periventricular leukomalacia (cPVL) according to de Vries (Citation18).
Statistical analysis
All statistical analyses were performed with the SPSS 15.0 program (SPSS Inc., Chicago, IL, USA). There is not sufficient power to evaluate the role of each individual cytokine as a risk factor. Therefore, we used factor analysis as the primary statistical method to investigate the interdependencies in the variation of the complete set of altogether 109 cord blood cytokines (n = 120 children). This analysis considers the variation of the individual cytokines and does not consider the differences in the absolute values of individual cytokines. Factor analysis combines the pattern of variation of individual cytokine variables that correlate with each other into a single factor. This factor is a linear combination of variables in a (multidimensional) scatter plot into which an orthogonal regression line (or a plane) has been fitted. Varimax rotation was then used to maximize the variability of a factor. The factor extracting was halted after 60% of the variability of the cytokines was explained. Altogether five factors were identified. Factor scores from factors 1 to 5 were then used in a multiple logistic regression model as independent predictors of predicted outcome, after adjustment for well known antenatal risk factors, gestational age at birth (GA), and histologic chorioamnionitis (HCA).
The chi-square or Fisher's exact tests were used for categorical variables to find differences in characteristics between CP and non-CP children. The cut-off values for the maximum accuracy of cytokines from peripheral blood in predicting the risk of CP were derived from the receiver-operating characteristic (ROC) curve. The Kruskal–Wallis test and Mann–Whitney U test were used to determine the difference in continuous variables between CP and non-CP children. All of the tests were two-tailed. The significance of P values was set at < 0.05.
Results
The final study group comprised 169 children. Thirty-nine children were from multiple pregnancies; one of these children had CP. The clinical data of children with CP (n = 19) and without CP (n = 150) are shown in . Fifty children were born extremely preterm (extremely low gestational age newborn (ELGAN) group; gestational age <28 weeks at birth), and nine (18%) of them developed CP.
Table II. Characteristics of children with and without CP who were born at <32 weeks of gestation.
To investigate several groups of interrelated cytokines and their relationship to a predicted outcome, CP, we performed factor analysis. Factor analysis sorts groups of variables with distinct factor loadings and eigenvalues describing correlations and variability inside factors. The cut-off of factor loading was set at 0.5. Altogether five factors were built. The cytokines that were loaded onto each of the five factors and explanations for the variations are presented in . The total variance explained by the five factors was 60.7%.
Table III. Summary of factor analysis.
Next, we used a multiple logistic regression analysis to investigate the relationship between the factor scores at the time of birth and CP after adjustment for GA and HCA. Scores from factors 1–5 were used as continuous variables presenting the weighted mean scores of cytokines for a given factor. Factor scores for factors 1 and 2 independently predicted CP (odds ratio (OR) 2.8, 95% confidence interval (CI) 1.5–5.5, P = 0.002 and OR 2.2, 95% CI 1.2–4.1, P = 0.014, respectively). The ORs were higher when considering, as an outcome, only the 11 CP children who were born after spontaneous onset of labor (OR 4.8, 95% CI 1.8–12.5, P = 0.001 and OR 2.8, 95% CI 1.2–6.4, P = 0.014, respectively). In contrast, GA, HCA, and scores for factors 3–5 did not explain the risk of CP. However, CP children tended to be born at a lower GA (), and most of the umbilical cord blood cytokines in factors 1 and 2 were higher in HCA compared to non-HCA pregnancies (data not shown). The levels of all of the cord blood cytokines in factors 1 and 2 tended to be higher in CP children compared to very preterm children without CP ().
Table IV. Differences in cord blood cytokines comprising factor 1 or factor 2 between children with and without CP.
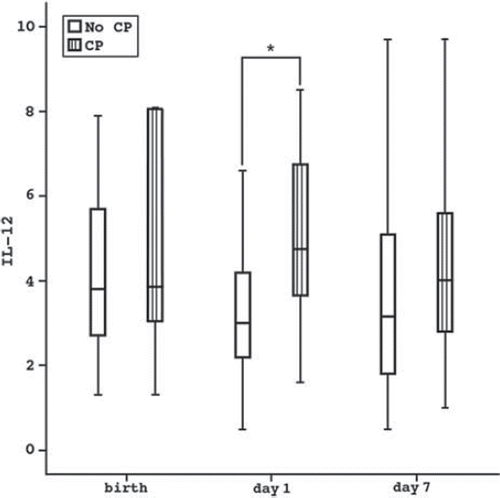
The concentrations of the eleven inflammatory cytokines were measured from cord blood at birth and from peripheral blood on days 1 and 7 (; Patients and methods). None of these cytokines measured from cord blood differed between children with and without CP (data not shown). The concentration of IL-12p70 on day 1, but not on day 7, was higher among CP children compared to children without CP (median 4.8 pg/mL, range 1.6–8.5 and median 3.0 pg/mL, range 0.5–10.3, respectively; P = 0.023) (). The concentration of IL-8 on day 7 was higher among CP children compared to children without CP (median 77.4 pg/mL, range 12.8–312.5 and median 27.9 pg/mL, range 1.4–422.4, respectively; P = 0.034); this difference was not detectable on day 1. None of the other cytokines from peripheral blood associated with CP (data not shown). Cerebral palsy of the 11 children who were born after spontaneous onset of labor was additionally considered. These CP children did not have higher levels of the pro-inflammatory cytokines after birth than children without CP.
Figure 1. IL-12 at birth, on day 1, and on day 7 in children with CP compared to children without CP. *P = 0.023.
Infants with cPVL had a higher IL-12p70 concentration on day 1 (median 4.5 pg/mL, range 3.1–8.5) compared to infants without cPVL (median 3.0 pg/mL, range 0.5–10.3) (P = 0.039). Infants with cPVL had a higher IL-8 concentration on day 1 (median 327.9 pg/mL, range 47.1–532.8) compared to infants without cPVL (median 85.9 pg/mL, range 12.1–1,425.9) (P = 0.020). Other analyses of inflammatory cytokine levels in cord blood or in peripheral blood revealed no association with the risk of cPVL.
For IL-12p70 on day 1, the cut-off value of 4.2 pg/mL or higher predicted CP with a sensitivity of 75% and a specificity of 73%. In a multiple logistic regression model dichotomized IL-12p70 on day 1 predicted CP (OR 6.75, 95% CI 1.11–41.0; P = 0.038). The clinical variables (GA < 28 weeks, CRIB I, duration of mechanical ventilation, and additional oxygen at 36 weeks of GA), together with dichotomized IL-12p70, did not predict CP. IL-8 on day 7 was also modeled as a dichotomized variable (cut-off value 30.3 pg/mL or higher, predicting CP with a sensitivity of 71% and specificity of 53%) and put into a multiple logistic regression model. In this analysis, only the duration of mechanical ventilation emerged as a significant factor predicting the risk of CP (OR 1.047, 95% CI 1.019–1.076; P = 0.001).
Finally, we conducted a multiple logistic regression analysis including continuous variables GA at birth, factor scores for factors 1 and 2; dichotomized IL-12p70 on day 1, and IL-8 on day 7, as independent predictors, whereas CP was an outcome variable. Factor scores for factor 1 and IL-12p70 on day 1 independently predicted CP (OR 7.07, 95% CI 1.47–33.96; P = 0.015 and OR 1.97, 95% CI 1.14–3.40; P = 0.015, respectively).
Discussion
The present prospective study of preterm infants was designed to investigate whether children who went on to develop CP could be identified on the basis of blood immunoproteins collected during the perinatal period. After considering the known major risk factors, certain combinations of cytokines in cord blood, mainly growth factors and chemokines, significantly predicted the CP risk. IL-12p70 additively contributed to the CP risk one day after birth. The present findings further explain the concept that perinatal events predispose infants to CP.
The present study revealed two high-risk clusters of proteins (factor 1 and factor 2 shown in ) involving mainly chemokines, growth factors, TNF-family cytokines, and hematopoietin family cytokines. These cytokines have a range of functional characteristics. Some of them may be involved in neural damage affecting OLs, neurons, or microglia. Others may be associated with the repair of such injuries, while some cytokines may have either several or no known roles in damage/repair. Previous studies of cytokine profiles in both term and preterm children with CP support the current finding that clusters of blood proteins are associated with or predispose infants to CP (Citation19–21).
Although the central nervous system is considered to be an immune-privileged space, not least because of tight junctions interconnected with endothelial cells lining the vasculature, some cytokines that are activated during a deleterious process, such as inflammation, influence the integrity of the blood–brain barrier (BBB) (Citation22). Hypoxia/ischemia also affects blood–brain integrity; up-regulation of vascular endothelial growth factor (VEGF) may have a role in leakage of the BBB (Citation23). Moreover, in response to hypoxia, astrocytes may lose their supportive function in the brain vasculature and thus contribute to BBB breakdown (Citation24). A deficient BBB is a likely contributing factor that would either allow detrimental blood constituents to enter brain structures or allow leakage of proteins that are important in the function of the central nervous system. In the current study, birth asphyxia was not more common among infants who developed CP, nor was there a direct association between CP and chorioamnionitis. However, most of the very preterm children with CP were born after spontaneous preterm labor, which is commonly associated with antenatal infection and inflammatory cytokines (Citation25). We therefore propose that several of the immunoproteins identified in this study may have a role in mediating an early deleterious pathway that leads to the development of CP. The precise mechanism(s) enabling the immunoproteins to cross the BBB remains unclear.
Oxidative damage to cellular structures contributes to pathogenesis in both the immature and mature nervous system (Citation26). Immature OLs are the cells that are most vulnerable to deleterious processes such as inflammation, hypoxia/ischemia, and oxidant injury. Cytokines participating in these processes, e.g. TNF and interferon family members, have been shown to cause damage to OLs (Citation27,Citation28). In previous studies, two members of the TNF family of cytokines, TRAIL-R1 and FASL, induced death in OLs isolated from human brain (Citation28,Citation29). These cytokines were associated with CP in the current study.
Cytokines may further contribute to the production of toxic substances, such as nitric oxide and free oxygen radicals (Citation30,Citation31), and to the up-regulation of hypoxia-inducible factor (HIF) (Citation32). HIF-1α is a transcription factor that regulates the expression of the erythropoietin (EPO) gene (Citation33). Hypoxia induces phosphorylation of HIF-1α, which leads to transcriptional activation of a number of hypoxia- responsive genes (Citation34). Some growth factors, e.g. TGF-α, IGF1, IGF2, and PDGF, increase expression of HIF-1α (Citation32). Ischemia and hypoxia followed by reperfusion may lead to apoptosis and mitochondrial cell death. Two inflammatory chemokines, CTACK/CCL27 and fractalkine/CX3CL1, that are known to be up-regulated in response to mitochondrial damage (Citation35), and seven members of the TNF family, TRAIL-R1, CD30, CD27, FASL, HVEM, RANK, and TRAIL-R4, that are known to participate in apoptosis (Citation28,Citation36–38), were included in the cytokine clusters predicting CP.
Low gestational age is an important risk factor of CP. Infants born before 32 weeks of gestation account for 20%–25% of all CP cases, although they represent only 0.9%–1.5% of all live births (Citation2,Citation39). The current study exclusively focused on this high-risk population. The known environmental risk factors, GA and antenatal inflammation, had no detectable association to risk in this population.
On the basis of two independent analyses, we did not detect an association between cord blood pro-inflammatory cytokines and CP. This somewhat unexpected finding may be due to early referral and active treatment of pregnant mothers with suspected antenatal infection. However, the inflammatory cytokine profiles from peripheral blood after birth were revealing. We found that IL-12p70 (day 1) and IL-8 (day 7) were higher in children with CP compared to children without CP. Consistently with the previous study, IL-12p70 levels measured from cord blood at birth did not predict the development of CP (Citation19). IL-12 is a pro-inflammatory cytokine that also induces another pro-inflammatory cytokine, IFN-γ, in peripheral lymphocytes. Circulating IFN-γ levels were found to be associated with white matter damage in very preterm children (Citation40). Postnatal inflammation in very preterm infants also correlates with reduced cortical growth (Citation41).
In this prospective cohort study we identified a group of cord blood cytokines that were associated with the development of CP in children born very preterm (). This group included several growth factors and chemokines that have a proposed role in the growth, differentiation, or the injury of the nervous system. All these cytokines tended to be higher in fetuses developing CP than in the gestation controls. The inflammatory cytokine profile changed after birth, and elevated levels of specific inflammatory cytokines were associated with an increased risk of CP, both singly and additively with the protein cluster defined at birth in cord blood. We propose that these serum proteins serve as biomarkers of the early pathways, resulting in neuronal cell death and white matter injury (Citation28,Citation29,Citation36–38). Further clinical and experimental studies are required to confirm and extend the current knowledge on the role of specific cytokines in pathogenesis and prevention of CP.
Acknowledgements
We thank Reetta Vuolteenaho for some of the cytokine analyses.
Declaration of interest: This investigation is supported by the Foundation for Pediatric Research (T.K.), the Sigrid Jusélius Foundation (M.H.), Oulu University Hospital Research Fund (M.H., T.K.), and The Finnish Academy (M.H.). The authors declare no conflict of interest.
References
- Mutch L, Alberman E, Hagberg B, Kodama K, Perat MV. Cerebral palsy epidemiology: where are we now and where are we going? Dev Med Child Neurol. 1992;34:547–51.
- Himmelmann K, Hagberg G, Beckung E, Hagberg B, Uvebrant P. The changing panorama of cerebral palsy in Sweden. IX. Prevalence and origin in the birth-year period 1995–1998. Acta Paediatr. 2005;94:287–94.
- Gibson CS, MacLennan AH, Dekker GA, Goldwater PN, Sullivan TR, Munroe DJ, . Candidate genes and cerebral palsy: A population-based study. Pediatrics. 2008;122: 1079–85.
- Wu Y, Croen LA, Torres AR, Van De Water J, Grether JK, Hsu NN. Interleukin-6 genotype and risk for cerebral palsy in term and near-term infants. Ann Neurol. 2009;66:663–70.
- Du Y, Dreyfus CF. Oligodendrocytes as providers of growth factors. Mini-Review. J Neurosci Res. 2002;68:647–54.
- Yoon BH, Jun JK, Romero R, Park KH, Gomez R, Choi JH, . Amniotic fluid inflammatory cytokines (interleukin-6, interleukin-1beeta, and tumor necrosis factor alpha), neonatal brain white matter lesions, and cerebral palsy. Am J Obstet Gynecol. 1997;177:19–26.
- Yoon BH, Romero R, Park JS, Kim CJ, Kim SH, Choi JH, . Fetal exposure to an intra-amniotic inflammation and the development of cerebral palsy at the age of three years. Am J Obstet Gynecol. 2000;182:675–81.
- Volpe JJ. Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet Neurol. 2009;8:110–24.
- Kaukola T, Tuimala J, Herva R, Kingsmore S, Hallman M. Cord immunoproteins as predictors of respiratory outcome in preterm infants. Am J Obstet Gynecol. 2009;200:100.e1–8.
- Paananen R, Husa AK, Vuolteenaho R, Herva R, Kaukola T, Hallman M. Blood cytokines during perinatal period in very preterm infants: relationship of inflammatory response and bronchopulmonary dysplasia. J Pediatr. 2009;154:39–43.
- Surveillance of cerebral palsy in Europe (SCPE): a collaboration of cerebral palsy surveys and registers. Dev Med Child Neurol. 2000;42:816–24.
- Schweitzer B, Roberts S, Grimwade B, Shao W, Wang M, Fu Q, . Multiplexed protein profiling on microarrays by rolling-circle signal amplification. Nat Biotechnol. 2002; 20:359–65.
- ACOG technical bulletin. Hypertension in pregnancy. Number 219, January 1996. Committee on Technical Bulletins of the American College of Obstetricians and Gynecologits. Int J Gynaecol Obstet. 1996;53:175–83.
- Salafia CM, Weigl C, Silberman L. The prevalence and distribution of acute placental inflammation in uncomplicated term pregnancies. Obstet Gynecol. 1989;73:383–9.
- Pihkala J, Hakala T, Voutilainen P, Raivio K. Characteristic of recent fetal growth curves in Finland. Duodecim. 1989; 105:1540–6.
- The CRIB (clinical risk index for babies) score: a tool for assessing initial neonatal risk and comparing performance of neonatal intensive care units. The international neonatal network. Lancet. 1993;342:193–8.
- Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr. 1978;92:529–34.
- de Vries LS, Eken P, Dubowitz LM. The spectrum of leukomalacia using cranial ultrasound. Behav Brain Res. 1992;49:1–6.
- Nelson KB, Dambrosia JM, Grether JK, Phillips TM. Neonatal cytokines and coagulation factors in children with cerebral palsy. Ann Neurol. 1998;44:665–75.
- Nelson KB, Grether JK, Dambrosia JM, Walsh E, Kohler S, Satyanarayana G, . Neonatal cytokines and cerebral palsy in very preterm infants. Pediatr Res. 2003;53:600–7.
- Kaukola T, Satyaraj E, Patel DD, Tchernev VT, Grimwade BG, Kingsmore SF, . Cerebral palsy is characterized by protein mediators in cord serum. Ann Neurol. 2004;55: 186–94.
- Anthony D, Dempster R, Fearn S, Clements J, Wells G, Perry VH, . CXC chemokines generate age-related increases in neutrophil-mediated brain inflammation and blood-brain barrier breakdown. Curr Biol. 1998;8:923–6.
- Sivakumar V, Lu J, Ling EA, Kaur C. Vascular endothelial growth factor and nitric oxide production in response to hypoxia in the choroid plexus in neonatal brain. Brain Pathol. 2008;18:71–85.
- Mani N, Khaibullina A, Krum JM, Rosenstein JM. Astrocyte growth effects of vascular endothelial growth factor (VEGF) application to perinatal neocortical explants: receptor mediation and signal transduction pathways. Exp Neurol. 2005;192:394–406.
- Challis JR, Lockwood CJ, Myatt L, Norman JE, , Strauss JF 3rdPetraglia F. Inflammation and pregnancy. Reprod Sci. 2009;16:206–15.
- Palmer C. Hypoxic-ischemic encephalopathy. Therapeutic approaches against microvascular injury, and role of neutrophils, PAF, and free radicals. Clin Perinatol. 1995;22: 481–517.
- Buntinx M, Gielen E, Van Hummelen P, Raus J, Ameloot M, Steels P, . Cytokine-induced cell death in human oligodendroglial cell lines. II: Alterations in gene expression induced by interferon gamma and tumor necrosis factor-alpha. J Neurosci Res. 2004;76:846–61.
- Matysiak M, Jurewicz A, Jaskolski D, Selmaj K. TRAIL induces death of human oligodendrocytes isolated from adult brain. Brain. 2002;125:2469–80.
- Li W, Maeda Y, Ming X, Cook S, Chaplin J, Husar W, . Apoptotic death following Fas activation in human oligodendrocyte hybrid cultures. J Neurosci Res. 2002;69: 189–96.
- Saliba E, Marret S. Cerebral white matter damage in the preterm infant: pathophysiology and risk factors. Semin Neonatol. 2001;6:121–33.
- Johnston MV, Hoon AH. Cerebral palsy. Neuromolecular Med. 2006;8:435–50.
- Ke Q, Costa M. Hypoxia-inducible factor-1 (HIF-1). Mol Pharmacol. 2006;70:1469–80.
- Goldberg MA, Dunning SP, Bunn HF. Regulation of the erythropoietin gene: evidence that the oxygen sensor is a heme protein. Science. 1988;242:1412–5.
- Fan X, Heijnen CJ, van der Kooij MA, Groenendaal F, Van Bel F. The role and regulation of hypoxia-inducible factor-1α expression in brain development and neonatal hypoxic-ischemic brain injury. Brain Res Rev. 2009;62: 99–108.
- Haines BA, Mehta SL, Pratt SM, Warden CH, Li PA. Deletion of mitochondrial uncoupling protein-2 increases ischemic brain damage after transient focal ischemia by altering gene expression patterns and enhancing inflammatory cytokines. J Cereb Blood Flow Metab. 2010;30: 1825–33.
- Choi C, Benveniste EN. Fas ligand/Fas system in the brain: regulator of immune and apoptotic responses. Brain Res Brain Res Rev. 2004;44:65–81.
- Hase H, Kanno Y, Kojima H, Morimoto C, Okumura K, Kobata T. CD27 and CD40 inhibit p53-independent mitochondrial pathways in apoptosis of B cells induced by B cell receptor ligation. J Biol Chem. 2002;277:46950–8.
- Pasero C, Barbarat B, Just-Landi S, Bernard A, Aurran-Schleinitz T, Rey J, . A role for HVEM, but not lymphotoxin-beta receptor, in LIGHT-induced tumor cell death and chemokine production. Eur J Immunol. 2009;39: 2502–14.
- Hagberg B, Hagberg G, Beckung E, Uvebrant P. Changing panorama of cerebral palsy in Sweden. VIII. Prevalence and origin in the birth year period 1991–1994. Acta Paediatr. 2001;90:271–7.
- Hansen-Pupp I, Harling S, Berg AC, Cilio C, Hellström-Westas L, Ley D. Circulating interferon-gamma and white matter brain damage in preterm infants. Pediatr Res. 2005; 58:946–52.
- Kaukola T, Kapellou O, Laroche S, Counsell SJ, Dyet LE, Allsop JM, . Severity of perinatal illness and cerebral cortical growth in preterm infants. Acta Paediatr. 2009;98: 990–5.
