Abstract
Platelet size correlates with platelet activity and can be assessed by platelet volume indices (PVI). The PVI, mean platelet volume (MPV), is universally available with routine blood counts by automated hemograms and therefore is an attractive index to study in clinical scenarios. PVI are useful in assessing the etiology of thrombocytopenia. In addition, a normal platelet distribution width in the setting of thrombocytosis is highly suggestive of a reactive etiology. Higher MPV is also associated with the presence of cardiovascular risk factors, chest pain due to acute coronary syndrome, and adverse outcome after acute coronary syndrome. Results from studies evaluating MPV in patients with peripheral artery disease, unprovoked deep vein thrombosis, and pulmonary embolism further advocate a potential role for MPV in identifying patients at high risk of thrombosis.
Nevertheless, most of these data come from retrospective studies some of which have small study populations and confounding factors influencing platelet volume. Moreover, the cut-off values derived from these retrospective studies have not been validated prospectively. Despite the potential for clinical utility evident from these studies, the above-mentioned flaws together with technical problems in measuring MPV currently limit its clinical usefulness. Our review provides a perspective on PVI's potential clinical use.
Key messages
Mean platelet volume (MPV) differs between various etiologies of thrombocytopenia, whereas platelet distribution width may help identify patients with reactive thrombocytosis.
MPV is higher in patients with acute coronary syndrome compared to those with non-cardiac chest pain, and high MPV is associated with worse prognosis both after acute coronary syndrome and after percutaneous coronary intervention.
Lack of power and methodological flaws of supporting studies, along with technical problems in platelet volume measurements and lack of cut-off values, presently limit the widespread usefulness of platelet volume indices (PVI). Future prospective studies with standardized measurements may clarify the clinical role of PVI.
Introduction
Platelet volume indices (PVI) are a group of parameters which are inexpensive and derived from routine blood counts. The mean platelet volume (MPV) and platelet distribution width (PDW) are the most validated and prominent of these and are attractive indices for research in clinical settings because of their widespread availability. The majority of the data presented in this review rely upon, and sequentially support, the notion that variations in PVI are indicative of changes in platelet function. Platelet size, measured by these parameters, correlates with platelet activity (Citation1), whether measured as aggregation, thromboxane A2 or β-thromboglobulin release, or by expression of glycoprotein Ib and IIb/IIIa receptors (Citation2–6). PVI have many potential applications in current clinical practice, which will be reviewed in this paper, especially in the fields of hematology and vascular medicine where platelets play a central role in the pathogenesis of disease.
Search strategy and selection criteria
We searched the NHI PubMed electronic database for articles published in the English language between 1 January 1966 and 31 July 2011. We performed the following initial database search: ‘mean platelet volume’ or ‘platelet distribution width’ in title or abstract, which resulted in 844 articles. We largely focused on publications from the past 10 years but also related to commonly referenced older publications. We also searched the reference lists of articles identified by this search strategy and selected those we judged relevant.
Because the majority of these studies are retrospective with small study populations, we reviewed all publication types, but elaborated more on studies which were larger and more methodologically sound.
Measurement and definition of PVI
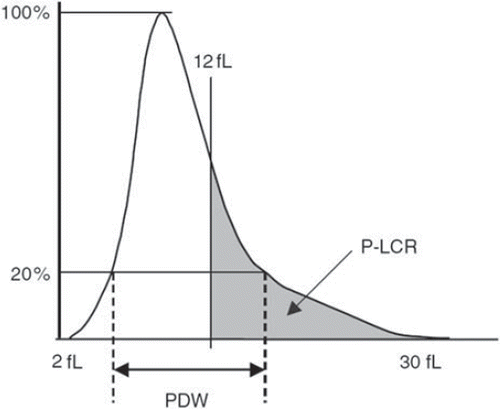
MPV and PDW are routinely measured and calculated from the platelet count by automated hematology analyzers, such as ADVIA, Coulter, or Sysmex, using either the electrical impedance or optical fluorescence method. MPV, measured in femtoliters (fL), may be calculated by the following formula, in which the plateletcrit represents the ratio of platelet volume to whole blood volume: MPV (fL) = [(plateletcrit (%) / platelet count (109/L)] × 105 (Citation7). Platelet volume and other PVI such as PDW and platelet-large cell ratio (P-LCR) can be derived from the platelet size distribution curve () (Citation8,Citation9).
Figure 1. Histogram of platelet size distribution and the definition of platelet size deviation width (PDW), and platelet-large cell ratio (P-LCR). From reference (Citation8) with permission. The distribution width at the level of 20% was defined as PDW, and the percentage of the platelets with a size of more than 12 fL was defined as P-LCR.
Hematology
Thrombocytopenia
There are a number of studies () evaluating whether PVI can help differentiate between under-production (e.g. bone-marrow aplasia) and increased destruction such as idiopathic thrombocytopenic purpura (ITP). Several retrospective studies with small study populations have demonstrated higher MPV, PDW, and, to a lesser extent, P-LCR in ITP than in hypoproliferative thrombocytopenia (Citation8,Citation10–13). However, a single study with several limitations demonstrated no between-group differences in PDW and MPV in the above setting (Citation14). Bowles et al. conducted a cross-sectional study of PVI amongst 473 thrombocytopenic patients and demonstrated that only 5% of those patients with MPV above 10.5 fL had primary bone-marrow disease, compared with 75% of those with MPV below 8 fL (Citation10). Other studies have also assessed the diagnostic utility of PVI, mainly MPV, in aiding the diagnosis of ITP: sensitivity and specificity both ranged from 60% to 100% depending on the PVI cut-off values () (Citation12,Citation15). Additional volume indices such as the peak platelet volume and percentage of large platelets have been researched in a handful of small studies and similarly indicate higher platelet volumes in ITP than in hypoproliferative thrombocytopenia (Citation16,Citation17). Importantly, the above data must be reconciled with the fact that there is also a component of decreased production now recognized in ITP.
Table I. Summary of studies† evaluating the diagnostic value‡ of platelet volume indices in thrombocytopenia.
In addition, patients with inherited giant platelet disorders have higher MPV and PDW than do normal controls (Citation18,Citation19). Noris et al. evaluated the utility of MPV in the challenging task of differentiating between inherited macro-thrombocytopenias and ITP. A MPV cut-off above 12.4 fL had good diagnostic power for the diagnosis of inherited macro-thrombocytopenias (). Although promising, this cut-off was derived from only 35 patients in each group and has not been prospectively validated, and thus it cannot be utilized in clinical practice at present. This study also showed that mean platelet diameter, a non-automated platelet volume index measured by optical microscopy, had similar diagnostic power (Citation18).
One study demonstrated higher MPV values among patients with megaloblastic pancytopenia compared to non-megaloblastic pancytopenia; however, the diagnostic value of MPV was poor () (Citation20). Thrombocytopenia associated with the myelodysplastic syndrome (MDS) may cause diagnostic confusion because, like ITP and inherited macro-thrombocytopenias, it is also associated with increased MPV and PDW (Citation10). However, isolated thrombocytopenia is rarely seen (<1%) in MDS. Apart from ITP, other etiologies of hyperdestructive thrombocytopenia are infections, drugs, and disseminated intravascular coagulation, all of which may be associated with similarly high PVI but can be clinically distinguished from ITP and inherited macro-thrombocytopenia. Hypersplenism-induced thrombocytopenia results in low or normal MPV values (Citation21).
Apart from their value in determining the cause of thrombocytopenia, PVI are related to the platelets’ functional capacity (Citation1). In-vivo and in-vitro tests demonstrate that larger platelets are more active in hemostasis than smaller ones, and thus more efficient in preventing hemorrhage (Citation22–30). Larger platelets have more granules and secretion capacity than smaller ones, are activated more readily by adenosine diphosphate, collagen, and adrenaline, and contain more surface receptors such as glycoprotein IIb/IIIa and P-selectin (Citation31). Furthermore, MPV correlates positively with release of thromboxane A2, platelet factor 4, and thromboglobulin (Citation32). Notably, ITP platelets are larger and may have enhanced function, thus accounting for bleeding times which are shorter than expected based purely on the platelet count (Citation33).
Indeed, a low MPV in the setting of severe thrombocytopenia has been shown to predict a hemorrhagic diathesis (Citation34,Citation35). Eldor et al. followed the blood counts and hemorrhagic episodes (n =84) in 175 patients with hematological disorders (mainly malignancies) over a 5-month period (Citation34). The MPV among patients who suffered from hemorrhagic events was significantly lower compared with this index in patients without such tendencies (5.52±0.7fL versus 7.87±1.75 fL). A MPV cut-off value of 6.4 fL was proposed, below which bleeding episodes would have been predicted with a sensitivity of 93% and specificity of 94.6% in severely thrombocytopenic patients (<20 × 109 platelets). This correlation between lower MPV and hemorrhagic diathesis was also shown in 43 patients with Henoch–Schonlein purpura who had normal or elevated platelet counts (Citation36).
Thrombocytosis
In thrombocytosis, PVI can be used to distinguish between a primary derangement in bone-marrow thrombopoiesis as seen in myeloproliferative diseases (MPD), and a reaction secondary to an underlying cause, namely reactive thrombocytosis (RT). There are conflicting observations on the relevance of MPV in determining the cause of thrombocytosis. A number of studies have shown that MPV is significantly higher in MPD than in RT (Citation37–40), whereas others have found it to be lower in MPD than in RT (Citation41), or the same (Citation42). Of all the PVI, PDW seems to be the most reliable in differentiating between RT and MPD. The PDW is statistically significantly higher in MPD as opposed to RT but frequently could not be used as the sole factor in making a diagnosis of thrombocytosis in these studies (Citation37,Citation39–42). Van der Lelie and Von dem Borne did, however, demonstrate a negative predictive value of 99.8% for MPD in the presence of a high PDW (>17%) (Citation37), and a comparable trend was found in a similar study (Citation40). Hence, a normal PDW in thrombocytosis is indicative of a reactive origin. A single study assessing P-LCR in this setting showed that this index was significantly decreased in reactive causes in comparison with neoplastic ones (Citation43).
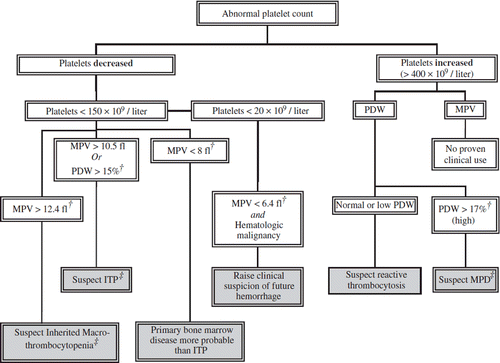
Possible clinical applications of PVI in the setting of thrombocytopenia and thrombocytosis are summarized in .
Figure 2. Possible clinical application of platelet volume indices in the diagnosis and management of abnormal platelet counts. †Many cut-off values are based on small studies and as such should be confirmed with prospective evaluation. ‡In the suitable clinical setting. ITP = idiopathic thrombocytopenic purpura; MPD = myeloproliferative disorder; MPV = mean platelet volume; PDW = platelet distribution width.
Vascular disease
A recently published large hospital-based cohort (n =206,554) evaluated the association between MPV quintiles and vascular mortality. Subjects with a MPV ≥11.01 fL (highest quintile) were at the highest risk of overall vascular mortality, when compared to patients with an MPV below 8.7 fL (hazard ratio 1.5; 95% CI 1.3–1.8). This increased risk was apparent beginning with MPV ≥9.61 fL (third, fourth, and fifth quintiles). Importantly, adjustment for possible confounders was performed only for sex, age, and platelet count (Citation44). In order better to understand the contemporary evidence regarding the association of MPV with vascular disease, and potential confounding factors, we have divided the review into various aspects of vascular medicine.
The association of MPV with risk factors for cardiovascular disease
Existing data show that increased MPV in comparison with control groups correlate with the presence of risk factors for cardiovascular disease (Citation45) such as diabetes mellitus type 2 (Citation46), impaired fasting glucose (Citation47), insulin resistance (Citation48), hypertension (Citation49–52), hyperlipidemia (Citation31,Citation53), metabolic syndrome (Citation54), and cigarette smoking (Citation55). Among diabetic patients, several reports have demonstrated conflicting results regarding the association between MPV and both hemoglobin A1C levels and the presence of diabetic micro- and macro-vascular complications (Citation46,Citation56–58). One study also demonstrated elevated P-LCR and PDW among diabetic patients in comparison with controls, and a positive correlation between PDW and micro-vascular complications (Citation58). Interestingly, improved glycemic control was associated with decreased MPV compared to baseline values (Citation57).
Regarding patients with hypertension, several studies have demonstrated a correlation of increased MPV with more severe hypertensive disease and overt target organ damage (Citation49,Citation51,Citation59). Moreover, a recent publication showed that MPV levels were also associated with the severity of subclinical target organ damage, including carotid atherosclerosis, left ventricular hypertrophy, and proteinuria, in newly diagnosed hypertensive patients (Citation60). Nevertheless, the few reports on the effect of improved hypertensive control, with either life-style modification or anti-hypertensive drugs, on MPV values showed inconclusive results (Citation61–64).
Patients with hyperlipidemia treated with rosuvastatin had significantly reduced MPV from baseline (Citation53). This effect did not correlate with a change in the lipid profile. Non-alcoholic fatty liver disease, seen in the majority of patients with the above risk factors, is also associated with higher MPV compared with control subjects after comprehensive logistic regression (Citation65,Citation66). Furthermore, in a single small study (n =95), patients with severe obstructive sleep apnea, which can lead to cardiovascular complications (Citation67), were shown to have higher MPV values than control subjects (Citation68). In a similar population, 6 months of treatment with continuous positive airway pressure resulted in a significant decrease in MPV (Citation69).
Atherosclerosis and peripheral vascular disease
Berger et al. recently studied the relationship between peripheral artery disease and MPV in 6354 subjects from a national cross-sectional survey. There was a significant association, after comprehensive multivariate analysis, between increasing MPV tertiles and a higher prevalence of peripheral artery disease, as defined by an ankle brachial index ≤0.90 in either leg. The multivariable adjusted odds ratio for peripheral artery disease, associated with an increase of each femtoliter of MPV, was 1.25 (95% CI 1.08–1.45) (Citation70). A cross-sectional study of healthy subjects demonstrated a positive correlation between MPV and arterial stiffness, measured by brachial ankle pulse wave velocity (Citation71).
In addition, in a general population cohort (n =259) MPV values were positively associated with the degree of coronary artery calcification on computerized tomography (Citation72), which in turn is related to the presence of coronary artery atherosclerosis (Citation73) and the severity of atherosclerotic disease (Citation74). Another study showed that increased MPV correlated with the severity of carotid intima-media thickness and increased high-sensitivity C reactive protein in newly diagnosed hypertensive patients (Citation60).
Conflictingly, a large study of patients undergoing coronary angiography showed no association between MPV and the extent of coronary artery disease and carotid intima-media thickness (Citation75). Moreover, no correlation between MPV and carotid intima-media thickness was found in a smaller study of patients with non-alcoholic fatty liver disease (Citation76). This can be partially reconciled by findings by Endler et al., which similarly showed no relation between MPV and extent of coronary artery disease but still showed an association between increased MPV and MI (Citation77). These conflicting results may possibly be attributed to the different patient populations in the various studies. Another platelet volume index, PDW, was not associated with the prevalence of coronary artery disease in a prospective study of 1882 subjects undergoing coronary angiography (Citation78).
Finally, Choi et al. assessed the correlation between MPV and endothelial dysfunction, as measured by acetylcholine-induced coronary vasospasm, among patients who underwent coronary angiography for typical or atypical angina (Citation79). MPV was significantly higher in patients with acetylcholine-induced coronary vasospasm (n =183) than in control subjects (n =513) and was an independent predictor of coronary vasospasm. This the first study in this area and merits further research.
Coronary artery disease
Association of MPV with myocardial infarction (MI)
Prospective studies have shown that among patients presenting to the hospital with chest pain, higher MPV favors a diagnosis of unstable angina and myocardial infarction (MI) over stable angina or non-cardiac chest pain, after multivariate analysis () (Citation77,Citation80). In addition, there are conflicting reports on differences in MPV between acute MI and unstable angina (Citation80,Citation81). In a meta-analysis of MPV in cardiovascular disease, the estimated mean difference in MPV between patients with acute MI and all other subjects was 0.92 fL (95% CI 0.67–1.16, P <0.001) (Citation82). Interestingly, in one study there was no correlation between MPV and time from MI, implying that higher MPV represents a general pro-thrombotic state rather than an acute-phase reactant (Citation77).
Table II. Summary of studies* evaluating the diagnostic and prognostic value† of mean platelet volume in cardiovascular disease.
Recently, a cross-sectional study of 39,531 subjects from the general Danish population augmented these findings by revealing a correlation between increasing MPV and a higher risk of MI. This is the first study of this scale demonstrating this association in the general population. MPV values were divided into tertiles (first: 5.7–7.3 fL; second: 7.4–8 fL; third: 8.1–16.1 fL). With the lower tertile of MPV as a reference value, the risk of MI was increased by 37% (95% CI 18%–59%) in the middle and by 30% (12%–52%) in the upper, after robust multifactorial adjustment for known cardiovascular risk factors. An MPV of 7.4 fL was identified as the value above which there was no further increase in risk for MI. In some subjects MPV was measured after MI, but a prospective sub-analysis of subjects from the same cohort who had MPV measured before MI confirmed the above associations (Citation83).
Association of MPV with post-MI prognosis
Increased mortality due to ischemic heart disease among patients in MPV quintiles ≥9.61 fL, compared to those with MPV <8.7 fL, was demonstrated in a sub-analysis of a large cohort (n =206,554) of hospital-based patients (Citation44). In particular, there are several studies showing that an increased MPV suggests a poor outcome among survivors of myocardial infarction (Citation77,Citation84,Citation85). Several cohort studies have used different end-points, such as increased 6-month mortality and various angiographic scores of impaired reperfusion, to show higher MPV among subjects with adverse clinical and angiographic outcomes after acute MI than those with more favorable results (Citation86–89). Furthermore, Pereg et al. assessed MPV values among 122 patients undergoing thrombolysis for ST elevation myocardial infarction (STEMI). Those patients with failed thrombolysis had higher MPV than subjects with successful thrombolysis, after multivariate analysis (Citation90).
A meta-analysis of studies researching MPV in cardiovascular disease supported the aforementioned data by demonstrating increased mortality among acute MI patients with elevated MPV compared with those with normal values (11.5% versus 7.1%, OR 1.65, 95% CI 1.12–2.52, P =0.012; analysis of 3184 patients across three cohorts) (Citation82). However, it must be noted that the end-points and MPV cut-offs (>10.3fL in two and >9 fL in the third) varied between the three cohorts analyzed. Since the publication of this meta-analysis, a cohort study of patients with non-ST elevation acute coronary syndrome (n =1041) revealed that patients with MPV ≥8.9 fL were at a higher risk of reaching the primary composite outcome of cardiovascular death and recurrent MI at 1 year, than those with lower MPV (hazard ratio 1.41; 95% CI 1.06–1.89, P =0.02) (Citation91). The inclusion of MPV into a comprehensive model of risk increased the likelihood ratio for predicting both the composite end-point (P =0.004) and cardiovascular death (P =0.009). A smaller cohort study with a similar primary end-point contradicted these findings (Citation92).
The ratio between MPV and platelet count has also been studied regarding outcome after MI, albeit less so than MPV alone. Previous studies have demonstrated an inverse relationship between MPV and platelet count in a normal population (Citation93,Citation94). This finding led to a claim that the MPV and platelet count need to be interpreted as a ratio, rather than separate variables. In a recent study, the MPV/platelet ratio, but not MPV alone, was shown to be an independent predictor of 4-year mortality after STEMI, whereby the highest and lowest ratio tertile had worse outcomes than the middle one (Citation95). The authors propose two different hypotheses to explain the worse prognosis in these two extremes of MPV tertiles.
Data on the prognostic value of MPV in cardiovascular disease are summarized in .
Association of PVI with post-PCI outcomes
Although the majority of research pertains to patients with acute coronary syndromes, Goncalves et al. recently published the largest study to date of the correlation between pre-procedural MPV and percutaneous coronary intervention (PCI) outcomes in a general population cohort (n =1432). Among patients undergoing PCI for a variety of indications, there was an increased frequency of the primary end-point of mortality or MI at 1 year (9.0%, 4.5%, and 3.5%, P <0.01) across increasing MPV tertiles (MPV >9.1 fL, 9.1 ≥ MPV ≥8.1, and MPV <8.1, respectively), after comprehensive logistic regression accounting for anti-platelet treatment and risk factors for atherosclerosis. An elevated MPV was a strong independent predictor of long-term outcome after PCI, with an adjusted odds ratio (OR) of 2.48 (95% CI 1.44–4.27) (Citation96). An association between unfavorable angiographic results and high MPV was also demonstrated among patients with stable angina undergoing percutaneous coronary intervention (PCI) (Citation97).
Stent thrombosis A small retrospective case–control study of subjects with acute coronary syndromes who had bare-metal stents implanted during PCI demonstrated higher MPV, measured before stent implantation, in the group of subjects with early stent thrombosis. However, these findings were not subjected to multivariate analysis, and no such correlation was found for PDW (Citation98).
Restenosis A retrospective study of 174 patients with stable and unstable angina pectoris who underwent elective percutaneous transluminal coronary angiography demonstrated an increased pre-procedural MPV among those with restenosis shown in angiographic follow-up within 6 months of the initial procedure, as compared to those without restenosis (Citation99). A meta-analysis of pooled data from five heterogeneous cohorts of 430 patients undergoing coronary angioplasty for various indications showed higher MPV among patients who developed restenosis (mean MPV of 8.67 fL, 95% CI 8.44–8.87) than among those who did not (mean difference of 0.98 fL, 95% CI 0.74–1.21, P <0.001) (Citation82).
Other fields of cardiovascular medicine
In addition to the research in coronary artery disease, PVI have been researched in other fields of cardiac medicine. In a retrospective study of 207 patients with heart failure, patients with decompensated heart failure had higher MPV than patients with stable heart failure () (Citation100). An increase in MPV has been shown in subjects with a left ventricular thrombus associated with myocardial infarction (Citation101) and dilated cardiomyopathy (Citation102), in comparison to those without left ventricular thrombi. These, however, are small studies, without multivariate analysis stratifying for other significant risk factors of thrombus formation. A high MPV tertile (≥8.9fL) was a predictive marker of ischemic stroke among 200 subjects with atrial fibrillation, compared to the lowest one (<8.0fL). This association was enhanced among subjects with a low to medium thromboembolic risk and remained after multivariate analysis (Citation103). On the other hand, a retrospective study of 205 patients with persistent atrial fibrillation demonstrated that MPV and PDW had no value in predicting left atrial thrombus, after multivariate analysis (Citation104).
Finally, in an attempt to suggest to a role of platelet activation in systemic thromboembolism associated with mitral and aortic valve stenosis, several small studies showed increased MPV among subjects with mitral or aortic stenosis in sinus rhythm, in comparison with normal controls (Citation105–107).
Stroke
The association of MPV with stroke was investigated in a post-hoc analysis of 3134 subjects with recorded MPV values, from a prospective trial assessing the effect of blood pressure-lowering agents on stroke in subjects with prior cerebrovascular disease. Increasing MPV was positively and independently associated with the risk of suffering an ischemic stroke (n =301), but not with the risk of hemorrhagic stroke (n =59) or stroke of unknown type (n =42), after multivariate logistic regression analysis (Citation63). Similarly, a recent prospective study of 384 patients with ischemic stroke who had no prior history of cerebrovascular disease demonstrated higher MPV than in normal controls, after adjustment for confounding factors (Citation108). Conversely some studies, albeit with smaller study populations, have shown similar (Citation109) or decreased (Citation110,Citation111) MPV values among patients with ischemic strokes in comparison with controls. There are also conflicting reports on the connection between MPV on hospital admission and the severity and subtype of ischemic stroke and functional outcome (Citation108,Citation112–115). Moreover, in a subgroup analysis of a large cohort of hospital-based patients (n =206,554) there was no association between MPV and cerebrovascular mortality (Citation44).
Venous thromboembolism
The above data pertain purely to arterial thromboembolism. There are limited such data concerning venous thromboembolism (VTE). Braekkan et al. complemented these abovementioned facts by assessing the influence of platelet counts and MPV values on the incidence of VTE in a large population-based cross-sectional study including 25,923 patients with no prior history of VTE (Citation116). The primary end-point of any first lifetime VTE was detected over a mean follow-up period of 10.8 years. No significant association was found between MPV and the incidence of all types of VTE (provoked and unprovoked). However, a sub-analysis demonstrated that an increased MPV (≥9.5fL) was significantly associated with a 1.5-fold increased risk of unprovoked VTE.
In a prospective study of 192 patients with acute pulmonary embolism, MPV on hospital admission was an independent predictor of all-cause mortality after 7 and 30 days, after multivariate analysis correcting for a broad range of confounding factors. Accordingly MPV was positively associated with right ventricular dysfunction and elevated troponin levels (Citation117). Interestingly, there was no difference in MPV compared with matched controls without pulmonary embolism; however, this was contradicted by another study which demonstrated elevated MPV among subjects with pulmonary embolism compared with controls (Citation118). In addition, a small study (n =60) of patients with Behcet's disease showed increased MPV among subjects with venous thrombosis relative to the MPV in patients without such complications (Citation119).
Association with anti-platelet treatment
Many of the patients in the above-mentioned studies were taking anti-platelet therapy. Therefore, in order properly to analyze some of the above data, the effect of anti-platelet therapy on mean platelet volume should be understood. Among hypertensive patients, regular aspirin treatment was associated with increased MPV (Citation49,Citation63); however, it is not clear whether the high MPV results from the aspirin treatment itself or is secondary to the coexistence of more cardiovascular risk factors. On the other hand, 30 post-MI patients who were treated with aspirin for a 6-month period had no significant change in MPV values (Citation120). Multivariate analysis including aspirin treatment did not modify the association shown between MPV and risk of MI in a large cross-sectional study, indirectly suggesting little effect of aspirin on MPV (Citation83). This is supported by an in-vitro study which showed no effect of aspirin on the MPV (Citation121).
A large cohort (n =1432) of patients undergoing PCI revealed a significantly lower odds ratio (OR) for clopidogrel treatment among subjects with MPV >9.1, compared to those with lower values, after logistic regression analysis (OR 0.63, 95% CI 0.48–0.83, P =0.001) (Citation96). The higher MPV had worse long-term prognosis. No such difference was found for aspirin treatment. This, together with supportive in-vitro findings (Citation122), suggests a possible role for clopidogrel in the inhibition of increased platelet size and its resulting sequelae. This hypothesis and the possibility that MPV may be a surrogate marker for clopidogrel responsiveness both warrant further research. We found no studies primarily investigating the effect of glycoprotein IIb/IIIa antagonists on MPV.
An interesting question is whether higher MPV values denote a group of patients in need of aggressive anti-platelet treatment. This hypothesis received preliminary support from Huczek et al., who found that the administration of the IIb/IIIa antagonist, abciximab, to patients with STEMI undergoing primary PCI significantly reduced mortality only in patients in the highest MPV tertile (Citation87).
Other disciplines
Obstetrics
The role of MPV during pregnancy has been evaluated in a handful of studies. Higher MPV levels were demonstrated in women with gestational diabetes compared with healthy pregnant women (Citation123,Citation124). In addition, an increment in MPV during pregnancy intimates a higher risk for development of pre-eclampsia (Citation125). A similar trend was demonstrated in a cohort study of blood counts and parameters in 1338 pregnancies which identified 107 cases of pre-eclampsia. The MPV in pre-eclamptic pregnancies was significantly higher than in normal pregnancies, with the increase occurring from the 24th week of gestation, several weeks prior to the diagnosis of pre-eclampsia (Citation126). These findings were not demonstrated in a much smaller study comparing pregnant women with and without pre-eclampsia (Citation127). Furthermore, amongst 57 women with high-risk pregnancies per maternal-fetal Doppler velocimetry measurements, higher MPV was significantly associated with adverse fetal outcome (Citation128).
Inflammatory disease
Several studies have presented data which allude to a correlation between higher MPV values and active inflammatory disease (Citation129–134). A handful of small studies conducted on patients with rheumatoid arthritis have demonstrated a correlation between elevated MPV and increased disease activity and inflammatory markers (Citation129,Citation134). A similar correlation was shown in ankylosing spondylitis, with a decrease in MPV after treatment (Citation133). In the aforementioned studies, the MPV among patients with rheumatoid arthritis and ankylosing spondylitis were higher than controls. Another study, however, contradicted the above findings in rheumatoid arthritis (Citation135) and ankylosing spondylitis (Citation136), showing lower MPV in patients with active disease and an increase in MPV after treatment for rheumatoid arthritis (Citation135). A recent study also showed increased MPV among patients with psoriasis as compared with controls and revealed a positive correlation between MPV, disease severity, and presence of arthritis (Citation131). A small retrospective study of patients with infective endocarditis showed a correlation between MPV and disease activity and also demonstrated an increased MPV among subjects with embolic and other complications and death compared with those without such complications (Citation137).
While the majority of reports showed elevated MPV in active inflammatory disease, a small retrospective study demonstrated lower MPV among patients with active familial Mediterranean fever than among those with inactive disease (Citation138). A similar correlation between lower MPV and disease activity has been shown in ulcerative colitis (Citation130,Citation139). Also, in a recent study, non-smoking patients with chronic obstructive pulmonary disease had higher traditional inflammatory markers and a lower MPV than did control subjects (Citation140).
Gasparyan et al. attempted to explain this contradiction by hypothesizing that high-grade inflammatory diseases, such as active rheumatoid arthritis or attacks of familial Mediterranean fever, result in low levels of MPV, while low-grade inflammatory diseases have the opposite effect on MPV (Citation141). Nevertheless, pending further investigation, the aforementioned findings still appear contradictory, and thus the nature of MPV as an inflammatory marker remains controversial. Furthermore, the authors of some of the above studies have hypothesized that increased MPV in patients with inflammatory disease may represent an increased risk for cardiovascular disease. However, it must be stressed that many of the studies implicating increased MPV in cardiovascular disease excluded patients with inflammatory disorders, and therefore generalizations cannot be made and additional research is required.
General
Several recent isolated studies have presented preliminary findings of increased MPV, compared with controls, in pulmonary arterial hypertension (Citation142), subclinical hypothyroidism (Citation143), and celiac disease (Citation144). In the latter two, MPV decreased after treatment. These findings warrant reproduction and further research.
Technical limitations of platelet volume measurement
Although PVI are useful in specific study settings, as shown above, there are several technical issues limiting their clinical use. The main concern is the influence exerted by three components of the measuring process: the type of hematology analyzer, the anticoagulant applied, and the time from sampling to analysis.
There are potential discrepancies in platelet counts between the impedance and optical methods, and thus validation by immuno-platelet procedures may be required when making important clinical decisions (Citation145,Citation146). Each hematology analyzer utilizes one or both of these methods. Instrument-dependent differences between platelet counts may result in different PVI. These inaccuracies are especially seen in severe thrombocytopenia (Citation146). When using impedance counting, the recommended anticoagulant for complete blood count, ethylenediamine tetra-acetic acid (EDTA), causes gradual platelet swelling of up to 13.4% at 24 hours after sampling, most of which occurs during the first 6 hours (Citation10). On the other hand, when MPV is measured by optical light scatter instruments, it decreases over time by nearly 10%, possibly by dilution of cytoplasmatic contents (Citation147). Samples collected in citrate produce smaller MPV than EDTA samples (Citation148), and alternative anticoagulant solutions have been investigated in an attempt to minimize time-related changes in volume (Citation149). Still, the vast majority of the studies reviewed in this paper utilized EDTA. An optimal measuring time of 120 minutes after venipuncture has been suggested for both types of samples (Citation150). The bulk of the aforementioned studies analyzed blood samples within 2 hours of collection.
In a healthy cohort in Turkey, the mean MPV was 8.9±1.4 fL, and 95% of the subjects had MPV values between 7.2 and 11.7 fL (Citation151). A reference range of 10%–17.9% (CI of 95%) and a normal median PDW of 13.3% was calculated by Farias et al. in 231 healthy subjects in Brazil (Citation9). However, due to the above-mentioned factors, many laboratories do not report the PVI to clinicians. All hematology analyzers have manufacturer-assigned ranges for PVI, which differ significantly between different analyzers depending on the technology used (Citation152), but no external quality assessment schemes are currently available (Citation7). Therefore, external quality assessment schemes are warranted for measuring PVI. Until such schemes are in place, we recommend analysis of blood samples within 2 hours of venipuncture, in keeping with the methods used in most of the above studies.
Discussion and summary of clinical applications
PVI, especially MPV, have potential prognostic and diagnostic value especially in hematology and cardiovascular medicine, while other fields have shown association with PVI in smaller studies. Although MPV has previously been reviewed in the field of cardiovascular medicine (Citation82,Citation153), there is no contemporary literature review assessing the use of PVI in all the above clinical disciplines. From the aforementioned hematology studies it is evident that MPV, PDW, and P-LCR are increased in hyperdestructive thrombocytopenia in comparison with hypoproductive etiologies. Although their independent diagnostic value is questionable (), they could potentially be a useful addition to a diagnostic score for thrombocytopenia (Citation154). One area in which MPV has promising diagnostic value is in differentiating between ITP and macro-thrombocytopenia. When assessing thrombocytosis, PDW is higher in MPD than RT, and a normal PDW is highly suggestive of a reactive etiology.
There is also consistent evidence in cardiology that MPV is higher in patients with risk factors for atherosclerosis than in subjects without these risk factors. Patients with chest pain due to acute coronary syndrome have higher MPV than those with non-cardiac chest pain or stable angina. Moreover, patients with higher MPV have a worse prognosis both after acute coronary syndrome and after PCI performed for various indications. Interesting areas for future investigation in the field of cardiology are whether the reduction of MPV in the individual patient will lead to a change in cardiovascular outcome and whether a high-risk subgroup of patients with acute coronary syndrome and elevated MPV warrant more aggressive treatment. There are data from Huczek et al. on the efficacy of abciximab in patients with STEMI and the highest MPV tertile (Citation87) which supports the latter notion, but both of the above hypotheses still warrant further research. Another topic for future research is the correlation between MPV and clopidogrel treatment.
MPV seems to have no clear prognostic value in strokes, due to conflicting study results. Other interesting findings from recent studies are higher MPV among patients with peripheral artery disease and unprovoked deep vein thrombosis, and a correlation between increased MPV and mortality in acute pulmonary embolism.
Although a cause–effect relationship has been suggested in retrospective studies in the field of vascular medicine (Citation83), the nature of this association must be elucidated by prospective cause–effect analysis. Recent genome-wide studies have identified loci involved with MPV (Citation155), and these data will hopefully lead to research revealing the connection between platelet size and cardiovascular disease.
Limitations
Many of the above studies have several limitations which preclude the use of PVI as independent markers of cardiovascular risk and the cause of thrombocytopenia in clinical practice. These include: the limited sample size of most studies to date, the post-hoc analysis used in some cases, the overlap between various clinical syndromes, confounding cardiovascular risk factors which influence platelet volume, technical limitations in measuring PVI, differences between study populations, and varying PVI cut-off values with inadequate diagnostic sensitivity and specificity.
Specifically, the majority of the above-mentioned studies relate to a relatively increased MPV in one study group as compared to another group with different characteristics, and not an elevation above the normal MPV range. Although cut-off values have been suggested by individual studies, these are either purely retrospective studies or cross-sectional studies, and the few with prospective follow-up of outcomes still rely upon retrospective analysis of MPV status. As a result, the clinical usefulness of PVI is greatly limited by the lack of prospective evaluation of these proposed cut-off values in different cohorts from the original derivative cohort of patients.
Throughout our review, we have related specifically to the main methodological flaws of important studies. In addition, the majority of the studies reviewed excluded patients with severe renal and hepatic impairment, malignancy, and in some cases excluded patients treated with oral anticoagulants. Subjects with hematological disorders were also excluded, apart from studies in the field of hematology.
Conclusions
PVI have potential for clinical utility, supported by compelling evidence in certain clinical scenarios, especially in hematology and vascular medicine, and the large number of studies published on the topic. However, at present the clinical utility of PVI is significantly limited by variability in measurement of PVI and lack of definitive cut-off values. Therefore an emphasis should be placed on the evaluation of retrospectively derived cut-off values in new prospective cohorts and standardization of PVI measurement. These studies should also assess other less-researched PVI, such as P-LCR and PDW, in addition to MPV. Moreover, the above evidence raises several interesting questions, such as the role of MPV in risk stratification in acute coronary syndrome, which also warrant further investigation in prospective studies.
References
- van der Loo B, Martin JF. A role for changes in platelet production in the cause of acute coronary syndromes. Arterioscler Thromb Vasc Biol. 1999;19:672–9.
- Thompson CB, Eaton KA, Princiotta SM, Rushin CA, Valeri CR. Size dependent platelet subpopulations: relationship of platelet volume to ultrastructure, enzymatic activity, and function. Br J Haematol. 1982;50:509–19.
- Jakubowski JA, Thompson CB, Vaillancourt R, Valeri CR, Deykin D. Arachidonic acid metabolism by platelets of differing size. Br J Haematol. 1983;53:503–11.
- Martin JF, Trowbridge EA, Salmon G, Plumb J. The biological significance of platelet volume: its relationship to bleeding time, platelet thromboxane B2 production and megakaryocyte nuclear DNA concentration. Thromb Res. 1983;32:443–60.
- Tschoepe D, Roesen P, Kaufmann L, Schauseil S, Kehrel B, Ostermann H, . Evidence for abnormal platelet glycoprotein expression in diabetes mellitus. Eur J Clin Invest. 1990;20:166–70.
- Giles H, Smith RE, Martin JF. Platelet glycoprotein IIb-IIIa and size are increased in acute myocardial infarction. Eur J Clin Invest. 1994;24: 69–72.
- Briggs C. Quality counts: new parameters in blood cell counting. Int J Lab Hematol. 2009;31:277–97.
- Kaito K, Otsubo H, Usui N, Yoshida M, Tanno J, Kurihara E, . Platelet size deviation width, platelet large cell ratio, and mean platelet volume have sufficient sensitivity and specificity in the diagnosis of immune thrombocytopenia. Br J Haematol. 2005;128:698–702.
- Farias MG, Schunck EG, Dal Bo S, de Castro SM. Definition of reference ranges for the platelet distribution width (PDW): a local need. Clin Chem Lab Med. 2010;48:255–7.
- Bowles KM, Cooke LJ, Richards EM, Baglin TP. Platelet size has diagnostic predictive value in patients with thrombocytopenia. Clin Lab Haematol. 2005;27:370–3.
- Ntaios G, Papadopoulos A, Chatzinikolaou A, Saouli Z, Karalazou P, Kaiafa G, . Increased values of mean platelet volume and platelet size deviation width may provide a safe positive diagnosis of idiopathic thrombocytopenic purpura. Acta Haematol. 2008;119:173–7.
- Numbenjapon T, Mahapo N, Pornvipavee R, Sriswasdi C, Mongkonsritragoon W, Leelasiri A, . A prospective evaluation of normal mean platelet volume in discriminating hyperdestructive thrombocytopenia from hypoproductive thrombocytopenia. Int J Lab Hematol. 2008;30:408–14.
- Lee WS, Kim TY. Mean platelet volume and platelet distribution width are useful in the differential diagnosis of aplastic anemia and idiopathic thrombocytopenic purpura. Clin Chem Lab Med. 2010; 48:1675–6.
- ten Berg MJ, Huisman A, van den Bemt PM, den Breeijen H, Egberts TC, van Solinge WW. Discriminative value of platelet size indices for the identification of the mechanism of chemotherapy- induced thrombocytopenia. Biomarkers. 2011;16:51–7.
- Chandra H, Chandra S, Rawat A, Verma SK. Role of mean platelet volume as discriminating guide for bone marrow disease in patients with thrombocytopenia. Int J Lab Hematol. 2010;32:498–505.
- Tomita E, Akatsuka JI, Kokubun Y. Differential diagnosis of various thrombocytopenias in childhood by analysis of platelet volume. Pediatr Res. 1980;14:133–7.
- Niethammer AG, Forman EN. Use of the platelet histogram maximum in evaluating thrombocytopenia. Am J Hematol. 1999;60:19–23.
- Noris P, Klersy C, Zecca M, Arcaini L, Pecci A, Melazzini F, . Platelet size distinguishes between inherited macrothrombocytopenias and immune thrombocytopenia. J Thromb Haemost. 2009; 7:2131–6.
- Naina HV, Harris S. Platelet and red blood cell indices in Harris platelet syndrome. Platelets. 2010;21:303–6.
- Chandra H, Chandra S, Rawat A, Verma SK. Megaloblastic pancytopenia vis-a-vis non-megaloblastic pancytopenia: is mean platelet volume useful discriminating indicator. Int J Lab Hematol. 2011; 33:409–13.
- Parker LS. Thrombocytopenia caused by immunologic platelet destruction. In: Lee R, Forrester J, Lukens J, Paraskevas F, Greer J, Rodgers G, editors. Wintrobe's clinical haematology. Baltimore, MD: Williams & Wilkins; 1999. p. 1589.
- Mannucci PM, Sharp AA. Platelet volume and shape in relation to aggregation and adhesion. Br J Haematol. 1967;13:604–17.
- Hirsh J, Glynn MF, Mustard JF. The effect of platelet age on platelet adherence to collagen. J Clin Invest. 1968;47:466–73.
- Shulman NR, Watkins SP Jr, Itscoitz SB, Students AB. Evidence that the spleen retains the youngest and hemostatically most effective platelets. Trans Assoc Am Physicians. 1968;81:302–13.
- Karpatkin S. Heterogeneity of human platelets. I. Metabolic and kinetic evidence suggestive of young and old platelets. J Clin Invest. 1969;48: 1073–82.
- Karpatkin S. Heterogeneity of human platelets. II. Functional evidence suggestive of young and old platelets. J Clin Invest. 1969;48: 1083–87.
- Harker LA, Slichter SJ. The bleeding time as a screening test for evaluation of platelet function. N Engl J Med. 1972;287:155–9.
- Kraytman M. Platelet size in thrombocytopenias and thrombocytosis of various origin. Blood. 1973;41:587–98.
- Karpatkin S. Heterogeneity of human platelets. VI. Correlation of platelet function with platelet volume. Blood. 1978;51:307–16.
- Haver VM, Gear AR. Functional fractionation of platelets. J Lab Clin Med. 1981;97:187–204.
- Bath PM, Butterworth RJ. Platelet size: measurement, physiology and vascular disease. Blood Coagul Fibrinolysis. 1996;7:157–61.
- Martin JF, Bath PM. Platelets and megakaryocytes in vascular disease. In: Herman AG, editor. Antithrombotics: pathophysiological rationale for pharmacological inventions. Boston, MA: Kluwer Academic Publishers; 2001. p. 49–62.
- Rand ML, Dean JA. Platelet function in autoimmune (idiopathic) thrombocytopenic purpura. Acta Paediatr Suppl. 1998;424:57–60.
- Eldor A, Avitzour M, Or R, Hanna R, Penchas S. Prediction of haemorrhagic diathesis in thrombocytopenia by mean platelet volume. Br Med J (Clin Res Ed). 1982;285:397–400.
- Klimiuk PS, Holt PJ, Clague RB, Herod E, Dyer PA. Predictive value of mean platelet volume in gold induced thrombocytopenia. Ann Rheum Dis. 1987;46:38–41.
- Makay B, Turkyilmaz Z, Duman M, Unsal E. Mean platelet volume in Henoch-Schonlein purpura: relationship to gastrointestinal bleeding. Clin Rheumatol. 2009;28:1225–8.
- Van der Lelie J, Von dem Borne AK. Platelet volume analysis for differential diagnosis of thrombocytosis. J Clin Pathol. 1986;39: 129–33.
- Baynes RD, Lamparelli RD, Bezwoda WR, Gear AJ, Chetty N, Atkinson P. Platelet parameters. Part II. Platelet volume-number relationships in various normal and disease states. S Afr Med J. 1988;73:39–43.
- Osselaer JC, Jamart J, Scheiff JM. Platelet distribution width for differential diagnosis of thrombocytosis. Clin Chem. 1997;43 (Pt 1):1072–6.
- Syed NN, Usman M, Khurshid M. Thrombocytosis: age dependent aetiology and analysis of platelet indices for differential diagnosis. Indian J Pathol Microbiol. 2007;50:628–33.
- Sehayek E, Ben-Yosef N, Modan M, Chetrit A, Meytes D. Platelet parameters and aggregation in essential and reactive thrombocytosis. Am J Clin Pathol. 1988;90:431–6.
- Small BM, Bettigole RE. Diagnosis of myeloproliferative disease by analysis of the platelet volume distribution. Am J Clin Pathol. 1981;76:685–91.
- Babu E, Basu D. Platelet large cell ratio in the differential diagnosis of abnormal platelet counts. Indian J Pathol Microbiol. 2004; 47:202–5.
- Slavka G, Perkmann T, Haslacher H, Greisenegger S, Marsik C, Wagner OF, . Mean platelet volume may represent a predictive parameter for overall vascular mortality and ischemic heart disease. Arterioscler Thromb Vasc Biol. 2011;31:1215–8.
- Muscari A, De Pascalis S, Cenni A, Ludovico C, Castaldini N, Antonelli S, . Determinants of mean platelet volume (MPV) in an elderly population: relevance of body fat, blood glucose and ischaemic electrocardiographic changes. Thromb Haemost. 2008;99: 1079–84.
- Hekimsoy Z, Payzin B, Ornek T, Kandogan G. Mean platelet volume in type 2 diabetic patients. J Diabetes Complications. 2004;18: 173–6.
- Coban E, Bostan F, Ozdogan M. The mean platelet volume in subjects with impaired fasting glucose. Platelets. 2006;17:67–9.
- Varol E, Akcay S, Ozaydin M, Erdogan D, Dogan A, Altinbas A. Mean platelet volume is associated with insulin resistance in non-obese, non-diabetic patients with coronary artery disease. J Cardiol. 2010; 56:154–8.
- Nadar SK, Blann AD, Kamath S, Beevers DG, Lip GY. Platelet indexes in relation to target organ damage in high-risk hypertensive patients: a substudy of the Anglo-Scandinavian Cardiac Outcomes Trial (ASCOT). J Am Coll Cardiol. 2004;44:415–22.
- Coban E, Yazicioglu G, Berkant Avci A, Akcit F. The mean platelet volume in patients with essential and white coat hypertension. Platelets. 2005;16:435–8.
- Boos CJ, Beevers GD, Lip GY. Assessment of platelet activation indices using the ADVIATM 120 amongst ‘high-risk’ patients with hypertension. Ann Med. 2007;39:72–8.
- Varol E, Akcay S, Icli A, Yucel H, Ozkan E, Erdogan D, . Mean platelet volume in patients with prehypertension and hypertension. Clin Hemorheol Microcirc. 2010;45:67–72.
- Coban E, Afacan B. The effect of rosuvastatin treatment on the mean platelet volume in patients with uncontrolled primary dyslipidemia with hypolipidemic diet treatment. Platelets. 2008;19:111–4.
- Demirtunc R, Duman D, Basar M. Effects of doxazosin and amlodipine on mean platelet volume and serum serotonin level in patients with metabolic syndrome: a randomised, controlled study. Clin Drug Investig. 2007;27:435–41.
- Kario K, Matsuo T, Nakao K. Cigarette smoking increases the mean platelet volume in elderly patients with risk factors for atherosclerosis. Clin Lab Haematol. 1992;14:281–7.
- Papanas N, Symeonidis G, Maltezos E, Mavridis G, Karavageli E, Vosnakidis T, . Mean platelet volume in patients with type 2 diabetes mellitus. Platelets. 2004;15:475–8.
- Demirtunc R, Duman D, Basar M, Bilgi M, Teomete M, Garip T. The relationship between glycemic control and platelet activity in type 2 diabetes mellitus. J Diabetes Complications. 2009;23:89–94.
- Jindal S, Gupta S, Gupta R, Kakkar A, Singh HV, Gupta K, . Platelet indices in diabetes mellitus: indicators of diabetic microvascular complications. Hematology. 2011;16:86–9.
- Inanc T, Kaya MG, Yarlioglues M, Ardic I, Ozdogru I, Dogan A, . The mean platelet volume in patients with non-dipper hypertension compared to dippers and normotensives. Blood Press. 2010; 19:81–5.
- Yarlioglues M, Kaya MG, Ardic I, Dogdu O, Kasapkara HA, Gunturk E, . Relationship between mean platelet volume levels and subclinical target organ damage in newly diagnosed hypertensive patients. Blood Press. 2011;20:92–7.
- Gupta RK, Kjeldsen SE, Motley E, Weder AB, Zweifler AJ, Julius S. Platelet function during antihypertensive treatment with quinapril, a novel angiotensin converting enzyme inhibitor. J Cardiovasc Pharmacol. 1991;17:13–19.
- Pathansali R, Smith NM, Bath PM. Prothrombotic megakaryocyte and platelet changes in hypertension are reversed following treatment: a pilot study. Platelets. 2001;12:144–9.
- Bath P, Algert C, Chapman N, Neal B; PROGRESS Collaborative Group. Association of mean platelet volume with risk of stroke among 3134 individuals with history of cerebrovascular disease. Stroke. 2004;35:622–6.
- Yazici M, Kaya A, Kaya Y, Albayrak S, Cinemre H, Ozhan H. Lifestyle modification decreases the mean platelet volume in prehypertensive patients. Platelets. 2009;20:58–63.
- Ozhan H, Aydin M, Yazici M, Yazgan O, Basar C, Gungor A, . Mean platelet volume in patients with non-alcoholic fatty liver disease. Platelets. 2010;21:29–32.
- Shin WY, Jung DH, Shim JY, Lee HR. The association between non-alcoholic hepatic steatosis and mean platelet volume in an obese Korean population. Platelets. 2011;22:442–6.
- Lattimore JD, Celermajer DS, Wilcox I. Obstructive sleep apnea and cardiovascular disease. J Am Coll Cardiol. 2003;41:1429–37.
- Varol E, Ozturk O, Gonca T, Has M, Ozaydin M, Erdogan D, . Mean platelet volume is increased in patients with severe obstructive sleep apnea. Scand J Clin Lab Invest. 2010;70:49–502.
- Varol E, Ozturk O, Yucel H, Gonca T, Has M, Dogan A, . The effects of continuous positive airway pressure therapy on mean platelet volume in patients with obstructive sleep apnea. Platelets. 2011; 22:552–6.
- Berger JS, Eraso LH, Xie D, Sha D, Mohler ER 3rd. Mean platelet volume and prevalence of peripheral artery disease, the National Health and Nutrition Examination Survey, 1999–2004. Atherosclerosis. 2010;213:58–91.
- Wang RT, Li Y, Zhu XY, Zhang YN. Increased mean platelet volume is associated with arterial stiffness. Platelets. 2011;22:447–51.
- Jung DH, Lee HR, Lee YJ, Kim JK, Park BJ, Shim JY. The association between coronary artery calcification and mean platelet volume in the general population. Platelets. 2011;22:567–71.
- Haberl R, Becker A, Leber A, Knez A, Becker C, Lang C, . Correlation of coronary calcification and angiographically documented stenoses in patients with suspected coronary artery disease: results of 1,764 patients. J Am Coll Cardiol. 2001;37:451–7.
- Budoff MJ, Gul KM. Expert review on coronary calcium. Vasc Health Risk Manag. 2008;4:315–24.
- De Luca G, Santagostino M, Secco GG, Cassetti E, Giuliani L, Franchi E, . Mean platelet volume and the extent of coronary artery disease: results from a large prospective study. Atherosclerosis. 2009; 206:292–7.
- Kilciler G, Genc H, Tapan S, Ors F, Kara M, Karadurmus N, . Mean platelet volume and its relationship with carotid atherosclerosis in subjects with non-alcoholic fatty liver disease. Ups J Med Sci. 2010;115:253–9.
- Endler G, Klimesch A, Sunder-Plassmann H, Schillinger M, Exner M, Mannhalter C, . Mean platelet volume is an independent risk factor for myocardial infarction but not for coronary artery disease. Br J Haematol. 2002;117:399–404.
- De Luca G, Venegoni L, Iorio S, Secco GG, Cassetti E, Verdoia M, . Platelet distribution width and the extent of coronary artery disease: results from a large prospective study. Platelets. 2010;21:508–14.
- Choi CU, Seo HS, Kim YK, Na JO, Lim HE, Kim JW, . Can mean platelet volume predict coronary vasospasm? Platelets. 2011; 22:173–8.
- Chu H, Chen WL, Huang CC, Chang HY, Kuo HY, Gau CM, . Diagnostic performance of mean platelet volume for patients with acute coronary syndrome visiting an emergency department with acute chest pain: the Chinese scenario. Emerg Med J. 2011;28: 569–74.
- Yilmaz MB, Cihan G, Guray Y, Guray U, Kisacik HL, Sasmaz H, . Role of mean platelet volume in triagging acute coronary syndromes. J Thromb Thrombolysis. 2008;26:49–54.
- Chu SG, Becker RC, Berger PB, Bhatt DL, Eikelboom JW, Konkle B, . Mean platelet volume as a predictor of cardiovascular risk: a systematic review and meta-analysis. J Thromb Haemost. 2010;8: 148–56.
- Klovaite J, Benn M, Yazdanyar S, Nordestgaard BG. High platelet volume and increased risk of myocardial infarction: 39,531 participants from the general population. J Thromb Haemost. 2011;9:49–56.
- Cameron HA, Phillips R, Ibbotson RM, Carson PH. Platelet size in myocardial infarction. Br Med J (Clin Res Ed). 1983;287:449–51.
- Pizzulli L, Yang A, Martin JF, Luderitz B. Changes in platelet size and count in unstable angina compared to stable angina or non-cardiac chest pain. Eur Heart J. 1998;19:80–4.
- Martin JF, Bath PM, Burr ML. Influence of platelet size on outcome after myocardial infarction. Lancet. 1991;338:1409–11.
- Huczek Z, Kochman J, Filipiak KJ, Horszczaruk GJ, Grabowski M, Piatkowski R, . Mean platelet volume on admission predicts impaired reperfusion and long-term mortality in acute myocardial infarction treated with primary percutaneous coronary intervention. J Am Coll Cardiol. 2005;46:284–90.
- Estevez-Loureiro R, Salgado-Fernandez J, Marzoa-Rivas R, Barge-Caballero E, Perez-Perez A, Noriega-Concepcion V, . Mean platelet volume predicts patency of the infarct-related artery before mechanical reperfusion and short-term mortality in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Thromb Res. 2009;124:536–40.
- Maden O, Kacmaz F, Selcuk H, Selcuk MT, Aksu T, Tufekcioglu O, . Relationship of admission hematological indexes with myocardial reperfusion abnormalities in acute ST segment elevation myocardial infarction patients treated with primary percutaneous coronary interventions. Can J Cardiol. 2009;25:e164–8.
- Pereg D, Berlin T, Mosseri M. Mean platelet volume on admission correlates with impaired response to thrombolysis in patients with ST-elevation myocardial infarction. Platelets. 2010;21:117–21.
- Taglieri N, Saia F, Rapezzi C, Marrozzini C, Bacchi Reggiani ML, Palmerini T, . Prognostic significance of mean platelet volume on admission in an unselected cohort of patients with non ST-segment elevation acute coronary syndrome. Thromb Haemost. 2011;106: 132–40.
- Lopez-Cuenca AA, Tello-Montoliu A, Roldan V, Perez-Berbel P, Valdes M, Marin F. Prognostic value of mean platelet volume in patients with non-ST-elevation acute coronary syndrome. Angiology. 2011 Jul 6. [Epub ahead of print].
- Bessman JD, Williams LJ, Gilmer PR Jr. Mean platelet volume. The inverse relation of platelet size and count in normal subjects, and an artifact of other particles. Am J Clin Pathol. 1981;76:289–93.
- Lamparelli RD, Baynes RD, Atkinson P, Bezwoda WR, Mendelow BV. Platelet parameters. Part I. Platelet counts and mean platelet volume in normal and pregnant subjects. S Afr Med J. 1988;73:36–9.
- Azab B, Torbey E, Singh J, Akerman M, Khoueiry G, McGinn JT, . Mean platelet volume/platelet count ratio as a predictor of long-term mortality after non-ST-elevation myocardial infarction. Platelets. 2011;22:557–66.
- Goncalves SC, Labinaz M, Le May M, Glover C, Froeschl M, Marquis JF, . Usefulness of mean platelet volume as a biomarker for long-term outcomes after percutaneous coronary intervention. Am J Cardiol. 2011;107:204–9.
- Duygu H, Turkoglu C, Kirilmaz B, Turk U. Effect of mean platelet volume on postintervention coronary blood flow in patients with chronic stable angina pectoris. J Invasive Cardiol. 2008;20:120–4.
- Huczek Z, Filipiak KJ, Kochman J, Michalak M, Roik M, Piatkowski R, . Baseline platelet size is increased in patients with acute coronary syndromes developing early stent thrombosis and predicts future residual platelet reactivity. A case-control study. Thromb Res. 2010; 125:406–12.
- Yang A, Pizzulli L, Luderitz B. Mean platelet volume as marker of restenosis after percutaneous transluminal coronary angioplasty in patients with stable and unstable angina pectoris. Thromb Res. 2006;117:371–7.
- Kandis H, Ozhan H, Ordu S, Erden I, Caglar O, Basar C, . The prognostic value of mean platelet volume in decompensated heart failure. Emerg Med J. 2011;28:575–8.
- Yilmaz MB, Ozeke O, Akin Y, Guray U, Biyikoglu SF, Kisacik HL, . Platelet aggregation in left ventricular thrombus formation after acute anterior myocardial infarction: mean platelet volume. Int J Cardiol. 2003;90:123–5.
- Yilmaz MB, Akin Y, Biyikoglu SF, Guray U, Kisacik HL, Korkmaz S. Left ventricular thrombosis is associated with increased mean platelet volume in patients with dilated cardiomyopathy and sinus rhythm. Acta Cardiol. 2004;59:41–5.
- Ha SI, Choi DH, Ki YJ, Yang JS, Park G, Chung JW, . Stroke prediction using mean platelet volume in patients with atrial fibrillation. Platelets. 2011;22:408–14.
- Yuce M, Cakici M, Davutoglu V, Ozer O, Sari I, Ercan S, . Relationship between mean platelet volume and atrial thrombus in patients with atrial fibrillation. Blood Coagul Fibrinolysis. 2010;21:722–5.
- Varol E, Ozaydin M, Turker Y, Alaca S. Mean platelet volume, an indicator of platelet activation, is increased in patients with mitral stenosis and sinus rhythm. Scand J Clin Lab Invest. 2009;69:708–12.
- Yavuz B, Ertugrul DT, Yalcin AA, Kucukazman M, Ata N, Dal K. Increased mean platelet volume in rheumatic mitral stenosis: a possible factor for thromboembolic events. J Cardiol. 2009;53:204–7.
- Varol E, Arslan A, Yucel H, Ozaydin M, Erdogan D, Dogan A. Increased mean platelet volume in patients with aortic stenosis. Clin Appl Thromb Hemost. 2010 Aug 3. [Epub ahead of print].
- Mayda-Domac F, Misirli H, Yilmaz M. Prognostic role of mean platelet volume and platelet count in ischemic and hemorrhagic stroke. J Stroke Cerebrovasc Dis. 2010;19:66–72.
- McCabe DJ, Harrison P, Sidhu PS, Brown MM, Machin SJ. Circulating reticulated platelets in the early and late phases after ischaemic stroke and transient ischaemic attack. Br J Haematol. 2004;126:861–9.
- Tohgi H, Suzuki H, Tamura K, Kimura B. Platelet volume, aggregation, and adenosine triphosphate release in cerebral thrombosis. Stroke. 1991;22:17–21.
- Nadar SK, Lip GY, Blann AD. Platelet morphology, soluble P selectin and platelet P-selectin in acute ischaemic stroke. The West Birmingham Stroke Project. Thromb Haemost. 2004;92:1342–8.
- Butterworth RJ, Bath PM. The relationship between mean platelet volume, stroke subtype and clinical outcome. Platelets. 1998;9:359–64.
- Muscari A, Puddu GM, Cenni A, Silvestri MG, Giuzio R, Rosati M, . Mean platelet volume (MPV) increase during acute non-lacunar ischemic strokes. Thromb Res. 2009;123:587–91.
- Pikija S, Cvetko D, Hajduk M, Trkulja V. Higher mean platelet volume determined shortly after the symptom onset in acute ischemic stroke patients is associated with a larger infarct volume on CT brain scans and with worse clinical outcome. Clin Neurol Neurosurg. 2009; 111:568–73.
- Ntaios G, Gurer O, Faouzi M, Aubert C, Michel P. Mean platelet volume in the early phase of acute ischemic stroke is not associated with severity or functional outcome. Cerebrovasc Dis. 2010;29:484–9.
- Braekkan SK, Mathiesen EB, Njolstad I, Wilsgaard T, Stormer J, Hansen JB. Mean platelet volume is a risk factor for venous thromboembolism: the Tromso Study, Tromso, Norway. J Thromb Haemost. 2010;8:157–62.
- Kostrubiec M, Labyk A, Pedowska-Wloszek J, Hrynkiewicz- Szymanska A, Pacho S, Jankowski K, . Mean platelet volume predicts early death in acute pulmonary embolism. Heart. 2010;96:460–5.
- Varol E, Icli A, Uysal BA, Ozaydin M. Platelet indices in patients with acute pulmonary embolism. Scand J Clin Lab Invest. 2011;71:163–7.
- Acikgoz N, Karincaoglu Y, Ermis N, Yagmur J, Atas H, Kurtoglu E, . Increased mean platelet volume in Behcet's disease with thrombotic tendency. Tohoku J Exp Med. 2010;221:119–23.
- Sharpe PC, Desai ZR, Morris TC. Increase in mean platelet volume in patients with chronic renal failure treated with erythropoietin. J Clin Pathol. 1994;47:159–61.
- Jagroop IA, Tsiara S, Mikhailidis DP. Mean platelet volume as an indicator of platelet activation: methodological issues. Platelets. 2003;14:335–6.
- Jagroop IA, Mikhailidis DP. Mean platelet volume is an independent risk factor for myocardial infarction but not for coronary artery disease. Br J Haematol. 2003;120:169–70.
- Bozkurt N, Yilmaz E, Biri A, Taner Z, Himmetoglu O. The mean platelet volume in gestational diabetes. J Thromb Thrombolysis. 2006; 22:51–4.
- Erikci AA, Muhcu M, Dundar O, Ozturk A. Could mean platelet volume be a predictive marker for gestational diabetes mellitus? Hematology. 2008;13:46–8.
- Hutt R, Ogunniyi SO, Sullivan MH, Elder MG. Increased platelet volume and aggregation precede the onset of preeclampsia. Obstet Gynecol. 1994;83:146–9.
- Dundar O, Yoruk P, Tutuncu L, Erikci AA, Muhcu M, Ergur AR, . Longitudinal study of platelet size changes in gestation and predictive power of elevated MPV in development of pre-eclampsia. Prenat Diagn. 2008;28:1052–6.
- Ceyhan T, Beyan C, Baser I, Kaptan K, Gungor S, Ifran A. The effect of pre-eclampsia on complete blood count, platelet count and mean platelet volume. Ann Hematol. 2006;85:320–2.
- Gioia S, Piazze J, Anceschi MM, Cerekja A, Alberini A, Giancotti A, . Mean platelet volume: association with adverse neonatal outcome. Platelets. 2007;18:284–8.
- Milovanovic M, Nilsson E, Jaremo P. Relationships between platelets and inflammatory markers in rheumatoid arthritis. Clin Chim Acta. 2004;343:237–40.
- Kayahan H, Akarsu M, Ozcan MA, Demir S, Ates H, Unsal B, . Reticulated platelet levels in patients with ulcerative colitis. Int J Colorectal Dis. 2007;22:1429–35.
- Canpolat F, Akpinar H, Eskioglu F. Mean platelet volume in psoriasis and psoriatic arthritis. Clin Rheumatol. 2010;29:325–8.
- Magen E, Mishal J, Zeldin Y, Feldman V, Kidon M, Schlesinger M, . Increased mean platelet volume and C-reactive protein levels in patients with chronic urticaria with a positive autologous serum skin test. Am J Med Sci. 2010;339:504–8.
- Yazici S, Yazici M, Erer B, Erer B, Calik Y, Bulur S, . The platelet functions in patients with ankylosing spondylitis: anti-TNF-alpha therapy decreases the mean platelet volume and platelet mass. Platelets. 2010;21:126–31.
- Yazici S, Yazici M, Erer B, Erer B, Calik Y, Ozhan H, . The platelet indices in patients with rheumatoid arthritis: mean platelet volume reflects disease activity. Platelets. 2010;21:122–5.
- Kim DA, Kim TY. Controversies over the interpretation of changes of mean platelet volume in rheumatoid arthritis. Platelets. 2011; 22:77–8.
- Kisacik B, Tufan A, Kalyoncu U, Karadag O, Akdogan A, Ozturk MA, . Mean platelet volume (MPV) as an inflammatory marker in ankylosing spondylitis and rheumatoid arthritis. Joint Bone Spine. 2008;75:291–4.
- Gunebakmaz O, Kaya MG, Kaya EG, Ardic I, Yarlioglues M, Dogdu O, . Mean platelet volume predicts embolic complications and prognosis in infective endocarditis. Int J Infect Dis. 2010;14:e982–5.
- Makay B, Turkyilmaz Z, Unsal E. Mean platelet volume in children with familial Mediterranean fever. Clin Rheumatol. 2009;28:975–8.
- Yuksel O, Helvaci K, Basar O, Koklu S, Caner S, Helvaci N, . An overlooked indicator of disease activity in ulcerative colitis: mean platelet volume. Platelets. 2009;20:277–81.
- Biljak VR, Pancirov D, Cepelak I, Popovic-Grle S, Stjepanovic G, Grubisic TZ. Platelet count, mean platelet volume and smoking status in stable chronic obstructive pulmonary disease. Platelets. 2011;22: 466–70.
- Gasparyan AY, Ayvazyan L, Mikhailidis DP, Kitas GD. Mean platelet volume: a link between thrombosis and inflammation? Curr Pharm Des. 2011;17:47–58.
- Varol E, Uysal BA, Ozaydin M. Platelet indices in patients with pulmonary arterial hypertension. Clin Appl Thromb Hemost. 2011 Mar 14. [Epub ahead of print].
- Yilmaz H, Ertugrul O, Ertugrul B, Ertugrul D. Mean platelet volume in patients with subclinical hypothyroidism. Platelets. 2011;22: 143–7.
- Purnak T, Efe C, Yuksel O, Beyazit Y, Ozaslan E, Altiparmak E. Mean platelet volume could be a promising biomarker to monitor dietary compliance in celiac disease. Ups J Med Sci. 2011;116:208–11.
- Bowles KM, Bloxham DM, Perry DJ, Baglin TP. Discrepancy between impedance and immunofluorescence platelet counting has implications for clinical decision making in patients with idiopathic thrombocytopenia purpura. Br J Haematol. 2006;134:320–2.
- Diquattro M, Gagliano F, Calabro GM, Tommasi M, Scott CS, Mancuso G, . Relationships between platelet counts, platelet volumes and reticulated platelets in patients with ITP: evidence for significant platelet count inaccuracies with conventional instrument methods. Int J Lab Hematol. 2009;31:199–206.
- Patterson K. Platelet parameters generated by automated blood counters. CME Bull Haematol. 1997;1:13–16.
- Dastjerdi MS, Emami T, Najafian A, Amini M. Mean platelet volume measurement, EDTA or citrate? Hematology. 2006;11:317–9.
- Diaz-Ricart M, Brunso L, Pino M, Navalon F, Jou JM, Heras M, . Preanalytical treatment of EDTA-anticoagulated blood to ensure stabilization of the mean platelet volume and component measured with the ADVIA counters. Thromb Res. 2010;126:e30–5.
- Lance MD, van Oerle R, Henskens YM, Marcus MA. Do we need time adjusted mean platelet volume measurements? Lab Hematol. 2010; 16:28–31.
- Demirin H, Ozhan H, Ucgun T, Celer A, Bulur S, Cil H, . Normal range of mean platelet volume in healthy subjects: Insight from a large epidemiologic study. Thromb Res. 2011;128:358–60.
- Briggs C, Harrison P, Machin SJ. Continuing developments with the automated platelet count. Int J Lab Hematol. 2007;29:77–91.
- Vizioli L, Muscari S, Muscari A. The relationship of mean platelet volume with the risk and prognosis of cardiovascular diseases. Int J Clin Pract. 2009;63:1509–15.
- Kuwana M, Kurata Y, Fujimura K, Fujisawa K, Wada H, Nagasawa T, . Preliminary laboratory based diagnostic criteria for immune thrombocytopenic purpura: evaluation by multi-center prospective study. J Thromb Haemost. 2006;4:1936–43.
- Soranzo N, Rendon A, Gieger C, Jones CI, Watkins NA, Menzel S, . A novel variant on chromosome 7q22.3 associated with mean platelet volume, counts, and function. Blood. 2009;113:3831–7.
- Lippi G, Filippozzi L, Salvagno GL, Montagnana M, Franchini M, Guidi GC, . Increased mean platelet volume in patients with acute coronary syndromes. Arch Pathol Lab Med. 2009;133:1441–3.
