Abstract
Nearly half of all heart failure (HF) patients have diastolic HF (DHF) or clinical HF with normal or near-normal left ventricular ejection fraction (LVEF). Although the terminology has not been clearly defined, it is increasingly being referred to as HF with preserved ejection fraction (HFPEF). The prevalence of HFPEF increases with age, especially among older women. Identifying HFPEF is important because the etiology, pathogenesis, prognosis, and optimal management may differ from that for systolic HF (SHF) or HF with reduced ejection fraction. The clinical presentation of HF is similar for both SHF and HFPEF. As in SHF, HFPEF is a clinical diagnosis. Once a clinical diagnosis of HF has been made, the presence of HFPEF can be established by confirming a normal or near-normal LVEF, often by an echocardiogram. HFPEF is often associated with a history of hypertension, concentric left ventricular hypertrophy, vascular stiffness, and left ventricular diastolic dysfunction. As in SHF, HFPEF is also associated with poor outcomes. While therapies with angiotensin-converting enzyme inhibitors and beta-blockers improve outcomes in SHF, there is currently no such evidence of their benefits in older HFPEF patients. In this review recent advances in the diagnosis and management of HFPEF in older adults are discussed.
Key messages
Clinical diagnosis of diastolic heart failure or heart failure with preserved ejection fraction in older patients may be difficult.
Identifying diastolic heart failure is important because the pathogenesis, prognosis, and optimal management can differ from systolic heart failure.
Optimal medical therapy has not been clarified, but integrated approach to the management of this condition may be helpful.
Introduction
Is terminology of DHF is appropriate?
Heart failure (HF) with normal left ventricular ejection fraction (LVEF) has been called diastolic heart failure (DHF), HF with preserved or normal systolic function, and HF with preserved ejection fraction (HFPEF). The terminology should help to understand the syndrome, reflect the underlying pathophysiological mechanisms, and help in investigating modalities and developing therapeutic models. Since DHF patients have indices suggestive of systolic dysfunction at rest or exercise, HF with preserved systolic function may not be the appropriate terminology used for this syndrome (Citation1,Citation2). Since the systolic HF (SHF) and DHF terms have been well established and are familiar to clinicians, the use of DHF seems to be more appropriate, but recently HFPEF is more commonly used in the literature, so use of these two terms are acceptable until further evidence is available.
Definition of chronic HF in older adults
Older adults have been defined as persons ≥65 years of age. According to the 2008 European Society of Cardiology (ESC) guidelines, ‘Heart failure is a syndrome in which the patients should have the following symptoms of HF: typically shortness of breath at rest or during exertion, and/or fatigue; signs of fluid retention such as pulmonary congestion or ankle swelling; and objective evidence of an abnormality of the structure or function of the heart at rest’ (Citation3). The definition from the American College of Cardiology (ACC)/American Heart Association (AHA) guidelines states: ‘Heart failure is a complex clinical syndrome that can result from any structural or functional cardiac disorders that impairs the ability of the ventricles to fill with or eject blood’ (Citation4).
Epidemiology
As the population ages, there is an increasing prevalence of HF and associated morbidity, as well as an increase in hospitalization, rehospitalization, and health care costs (Citation5,Citation6). The lifetime risk for developing HF at 40 years is 20% for both men and women (Citation7), and at 55 years the risk is approximately 33% in men and 28.5% in women (Citation8).
Diastolic dysfunction refers to an abnormality of left ventricular (LV) compliance, filling and/or relaxation, regardless of whether the LVEF is preserved (Citation9). The development of diastolic dysfunction in older adults may be independent of left ventricular mass, heart rate, contractility, or systemic blood pressure (Citation10). DHF is a syndrome with a predominant abnormality of diastolic dysfunction (abnormal LV filling and elevated filling pressures) with absent or mild abnormalities in hemodynamic pump function (preserved or near-normal ejection fraction) and HF symptoms and signs. According to the Framingham criteria, HFPEF is defined as LVEF >45% and the absence of mitral disease, pericardial disease, or non-cardiac causes of dyspnea, edema, and fatigue (Citation11). Findings from large HF registries suggest that baseline characteristics and outcomes in HF patients with LVEF between 40% and 55% are more similar to those >55% than those <40%. Therefore, an LVEF of >40% is increasingly considered a preserved LVEF in the context of HF (Citation12).
The prevalence of HFPEF is around 4%–6% in men and 8%–10% in women for individuals 80 years and over (Citation13). The prevalence in the community is from 1.1% to 5.5% of the general population (Citation14). The prevalence of HFPEF in different epidemiological studies in the world shows an increasing trend, and HFPEF is seen in 50%–55% of the HF population (Citation14,Citation15). Aronow et al. in their study showed the overall prevalence of diastolic heart failure in older African-Americans, Hispanics, and whites is around 51%, using a LVEF cut-off of 50% or more (Citation16). These DHF patients are more likely to be women and have a history of hypertension and multiple co-morbidities (Citation15).
Ethnicity may contribute to differences in risk and outcomes in DHF. In a small cohort study of South-Asians and whites, with a mean age of 68 (±10 years), South-Asians had more often preserved LVEF than did whites (38% versus 23%, P =0.002). An ASCOT (Anglo-Scandinavian Cardiac Outcomes Trial) substudy showed diastolic function was significantly worse in hypertensive subjects of African-American origin than white Europeans (Citation17). In a study on whites and African-American subjects, which included older adult subjects with DHF, the 5-year mortality risk was significantly higher in the African-American cohort when compared to whites (HR 1.34, 95% CI 1.13–1.60, P <0.01) and was most prominent with non-ischemic aetiology (Citation18). Compared with whites, South-Asian ethnicity was associated with lower all-cause mortality (OR 0.71, 95% CI 0.53–0.95, P <0.01) (Citation19). It is possible there are subtle differences in the mechanisms of disease, drug metabolism, and treatment responses to therapy, as well as discrepancies in health care delivery to these ethnic groups. However, the treatment decisions will be better made by genetic profiling when it is widely available rather than taking only ethnicity into consideration (Citation20).
Search strategy
We searched using electronic databases (MEDLINE (1966–May 2011), EMBASE and SCOPUS (1965–May 2011), DARE (1966–May 2011)). Additionally, abstracts from national and international cardiovascular meetings were searched. Where necessary, the relevant authors were contacted to obtain further data. The main data search terms were diastolic heart failure, heart failure with normal or preserved ejection fraction, and DHF/ HFPEF diagnosis and management. Articles not in English were excluded.
Pathophysiology
The mystery of how HF can occur if the ejection fraction is normal
The mystery of DHF stems from the misperception that the LVEF is a valid surrogate for cardiac output (CO). Normal LVEF does not imply normal cardiac output. Low cardiac output is the main abnormality seen in both SHF and DHF (Citation21). Gandhi et al. showed a mean LVEF 20% higher (proportionally approximately 45% higher) in the patients with DHF than in the patients with SHF (Citation22). Nevertheless, stroke volumes differed by at most only 4 mL (7%). CO was actually lower in the DHF patients, more a result of lower heart rate than lower stroke volume (Citation22).
Abnormal left ventricular filling and elevated filling pressures
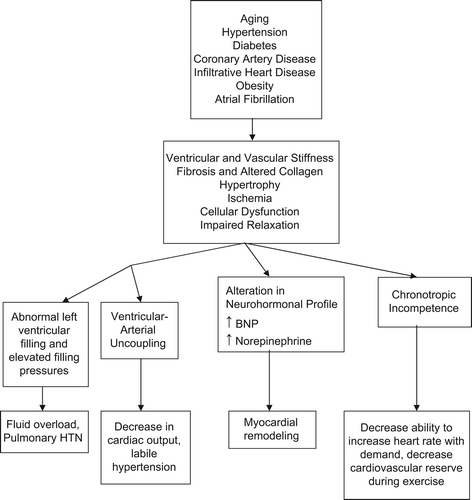
HF in the older adults is due to a combination of age-related changes to the cardiovascular system and a higher prevalence of cardiovascular disease conditions. The mechanisms of DHF are shown in . As one ages, there is increased collagen deposition with a reduction in the amount of elastin which can lead to increased stiffness of the heart and blood vessels. Additionally, the myocardium can become stiff because of pathological processes like fibrosis, infiltration, or hypertrophy (Citation23). The pathophysiological significance of myocardial stiffness is seen in its decreased ability to fill the ventricles during diastole. Altered relaxation and stiffness of the ventricles are also affected by delayed calcium uptake by the myocyte sarcoplasmic reticulum and calcium efflux from the myocyte (Citation24). This can occur in response to pressure overload or increase in ventricular afterload. A slowing of the normal diastolic relaxation of the ventricle can in turn increase the left ventricular and left atrial pressures (Citation25). Endomyocardial biopsies from DHF showed structural and functional differences in cardiomyocytes compared to patients with abnormal systolic function. Biopsies revealed higher myofibrillar density and higher cardiomyocyte diameter in patients with HFPEF when compared to heart failure with reduced LVEF (Citation26). DHF is characterized by a stiff non-compliant LV operating on a steeper diastolic pressure-volume relationship compared with the normal ventricle. This leads to decreased LV end-diastolic volumes and a compensatory rise in LV filling pressure to maintain cardiac output. Therefore end-diastolic wall stress is increased, and systolic wall stress as well as ejection fraction (EF) remains normal (Citation9). Mechanical dyssynchrony can occur without electrical dyssynchrony. Because of the stiffness, there is also decreased relaxation and filling of blood, so there is less blood pumped out of the heart, resulting in backup of fluid in the lungs which leads to symptoms of HF (Citation27). The normal LVEF of DHF is then taken to imply that low CO is not pathophysiologically operative, and the congestive phenomena are attributed to the stiff LV (Citation21).
Neurohormonal influences in HF
HFPEF patients display neurohormonal profiles similar to HF with reduced LVEF, with elevated circulating neurohormones, Brain Natriureteric Peptide (BNP) and norepinephrine (Citation23). Even though different types of hemodynamic factors and circulatory factors play a role in HFPEF, the basic pathophysiologic processes leading to decreased cardiac output in systolic heart failure are also seen in HFPEF. Both SHF and DHF result in a decrease in stroke volume (SV) and lead to activation of peripheral and central baroreflexes and chemoreflexes that are capable of eliciting marked increases in the sympathetic nervous system (Citation23). While there are commonalities in the neurohormonal responses to decreased stroke volume, the neurohormone-mediated events, such as myocardial remodeling, that follow have been most clearly described for individuals with SHF (Citation28,Citation29).
Decreased chronotropic reserve and ventricular vascular coupling
Other mechanisms that contribute to the pathology include ventricular-vascular coupling and chronotropic incompetence (Citation30). Ventricular-vascular coupling is the ratio of arterial to ventricular elastance. Inappropriate ventricular-vascular coupling can exacerbate diastolic dysfunction (Citation31). Ventricular-vascular coupling is defined as the interaction of the heart with the systemic vasculature, and uncoupling is seen in DHF (Citation32). Abnormalities in arterial mechanics like arterial stiffness also play a role in HFPEF. Chronotropic incompetence refers to the inability of the heart to increase its rate with activity or increased demand. The increase in heart rate is the main contributor to a person's ability to perform a sustained aerobic exercise (Citation33). Phan et al. have shown that chronotropic incompetence can be seen in patients with HFPEF during maximal exercise (Citation34). In this study the prevalence of chronotropic incompetence was 35% in HFPEF subjects (Citation34).
There is also an association of mild abnormalities of the systolic function in HFPEF, which can be observed by measuring the longitudinal axis shortening velocity through tissue Doppler echocardiography. Subendocardial and subepicardial myocardial fibers are arranged towards the longitudinal axis of the heart base which corresponds to the atrioventricular ring. Longitudinal ventricular contraction can be measured by the movement of the atrioventricular ring through tissue Doppler echocardiography (Citation35,Citation36). Studies have shown that HFPEF is not an isolated disorder of diastolic dysfunction and showed abnormalities of both LV systolic and diastolic function (Citation37,Citation38). In HFPEF, diastolic and systolic reserve are decreased with exercise, as are chronotropic and vascular reserve (Citation39).
Causes of HFPEF
The common predisposing and precipitating causes are shown in . For patients who present with HF, it is important to identify co-morbidities which are potentially reversible or modifiable. The most common causes of HFPEF are hypertension with left ventricular hypertrophy, aortic stenosis with normal LVEF, infiltrative diseases like amyloidosis, and idiopathic cardiomyopathy (Citation40). Transthyretin (TTR) amyloidosis (ATTR) is an autosomal dominant disease and is associated with senile systemic amyloidosis and familial amyloid cardiomyopathy. ATTR cardiomyopathy can occur at any age, but the typical age of onset is above 60 years, especially in African-American patients with the V122I mutation. It can cause diastolic dysfunction and can lead to symptomatic HF due to restrictive cardiomyopathy (Citation41). Age-related misfolding of TTR leads to deposition of TTR amyloid in the heart and vessels and can lead to senile or wild-type TTR amyloidosis and is seen predominantly in older men. ATTR is non-hereditary and can lead to severe diastolic dysfunction (Citation42). The prevalence of diabetes, hypertension, obesity, renal failure, atrial fibrillation, and anemia is higher in HFPEF than in HF with reduced EF (Citation43). The prognosis is influenced by multisystem involvement like pulmonary hypertension, renal failure, anemia, vascular dysfunction, and atrial fibrillation (Citation12).
Table I. Predisposing and precipitating factors for DHF.
Clinical features—atypical presentations in older adults
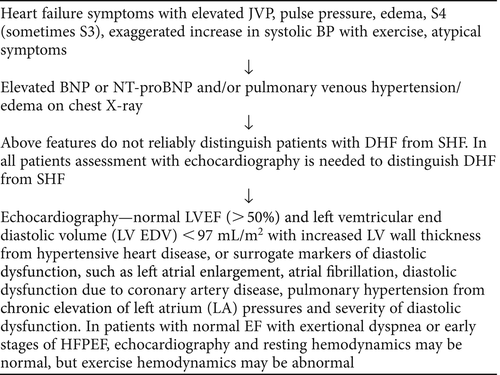
The common symptoms include dyspnea, chest pain, and palpitations. To stage HF, the New York Heart Association (NYHA) classification has been used based on clinical symptoms, especially breathlessness symptoms. Dyspnea has a sensitivity of 66% and specificity of 52%, whereas orthopnea and paroxysmal nocturnal dyspnea have specificities of 85% and 76%, respectively, but have low sensitivities. Musculoskeletal problems also prevent the elderly from reaching a level of activity at which breathlessness may be precipitated. Similarly signs such as jugular venous pressure (JVP) and presence of third heart sound have specificities of 97% and 95%, respectively, but have low sensitivities. Peripheral edema and crepitations in the lungs are commonly seen in HF, but are not specific. These are seen in acute HF, but may be absent in chronic HF, as the interstitial fluid is removed by increased lymphatic drainage, and the left atrial pressure can be elevated without evidence of pulmonary edema due to the adaptive response of the pulmonary lymphatic vessels, which help to remove fluid from pulmonary tissue (Citation44).
Symptoms of HF may be due to unrecognized ischemia and paroxysmal atrial fibrillation (AF). Prevalence of AF has been estimated at 30% in patients with new onset of DHF (Citation45). In a study by Fung et al. (Citation46) HFPEF patients with AF had worse functional class and quality of life, lower 6-min walking distance, and larger left atrial diameter than those without AF (Citation46). A study by Tsang et al. showed that HFPEF is an independent predictor of AF in older adults (Citation47). A study on HFPEF patients with a median follow-up of 38 months showed that a 15% incidence of hospital admission is due to unstable angina (Citation48). Non-cardiac symptoms of HF may include anorexia, nausea, bloating, fatigue, weakness, oliguria, nocturia, and cerebral symptoms like anxiety, memory impairment, and confusion. In the older adult, HFPEF can present atypically with confusion, falls, anxiety, dizziness, or syncope (Citation49). In addition, 50% of older HF patients have cognitive impairment, so history is less reliable and may play a role with both a delay in treatment and non-compliance with management (Citation50).
Typical signs are raised JVP, presence of extra heart sounds (third and fourth heart sounds), tachycardia, crepitations on lung examination, hepatomegaly, and peripheral edema. Although the specificities of clinical signs like JVP and heart sounds are high, in clinical practice the diagnosis of HF is sometimes difficult in those who are obese and in patients who have lung disease (Citation51,Citation52). In addition, elevated JVP is also not frequently seen in chronic HF, unless the right atrial pressure exceeds a critical value (Citation53,Citation54). A retrospective study in frail elderly HF patients has shown that the specificities of clinical signs, chest X-rays, and abnormal electrocardiograms (ECG) were 50%, 20%, and 9% (Citation55).
A recent study with the elderly from Netherlands showed that classic signs of HF (elevated JVP, tachypnea, tachycardia, crepitations in the lungs, leg edema, hepatomegaly, pleural effusion) were absent in one-third of study subjects; whereas loss of appetite, lower body mass index (BMI), and absence of wheezing were features seen in older adults with slow onset of HF (OR 0.75, 95% CI 0.69–0.82; C statistic was 0.75) (Citation56). Early detection of HF is clinically difficult in some older adults, because of atypical symptoms, absence of typical signs, and due to the presence of co-morbid conditions like chronic obstructive pulmonary disease, cognitive impairment, venous insufficiency, and obesity (Citation57).
A study by Foranow et al. showed that uncontrolled hypertension is more commonly seen in HFPEF when compared with SHF, and the increase in JVP and lung crepitations are less commonly seen when compared to SHF (Citation12). Zile et al. showed that the typical symptoms and signs of HF are less commonly seen in DHF when compared to SHF () (Citation58).
Table II. Prevalence of specific symptoms and signs in systolic versus diastolic HF.
Investigations
Acoustic cardiography
Acoustic cardiography is a validated, rapid, and non-invasive technology to measure heart sounds as well as timing relationships to ECG. Data on heart sounds and 12-lead ECG can be obtained simultaneously (Citation59). This technique helps to evaluate diastolic function by the presence of the fourth heart sound, which is shown to be associated with LV stiffness, and systolic function by the presence of third heart sound (S3) (Citation60). It is used in the optimization of the cardiac synchronization therapy devices, which has been used in HF management (Citation59).
Electrocardiogram
Electrocardiogram cannot diagnose HF. ECG has good negative predictive value for heart failure with reduced ejection fraction (HFREF), but poor sensitivity. An abnormal ECG has little predictive value for HF (Citation61). Abnormal QT dispersion can identify HF patients who are at high risk for arrhythmic events (Citation62). Short QT peak correlated (QTpc) <360 ms and AF are independently associated with increased risk of rehospitalizations or death in HF patients (Citation63). Left bundle branch block (LBBB), but not right bundle branch block (RBBB), is an independent predictor of death in chronic HF (Citation64). Some therapies for acute HF, especially intravenous positive inotropic drugs (such as dobutamine) and B-type natriuretic peptide (nestritide), have significant proarrhythmic properties (Citation65–67).
Chest X-ray
Chest radiographs may show evidence of pulmonary venous congestion or pleural effusions. The most common features are dilatation of the superior pulmonary veins (the antler appearance), increased interstitial density of central lung markings (butterfly or batwing appearance), thickened septa and lymphatics (Kerley B lines), alveolar edema, interlobar fluid (pseudotumor), and pleural fluid (Citation68). Cardiomegaly will be seen in HF with decreased systolic function and also in patients with DHF (Citation58). Pulmonary edema features are also seen in transient non-sustained ventricular tachycardia induced by hypoxic obstructive sleep apnea, new onset of atrial fibrillation in hypertensive heart disease, as well as in acute viral myocarditis (Citation69).
Echocardiogram and cardiovascular magnetic resonance (CMR)
After a clinical diagnosis of HF has been made, LVEF should be used to determine SHF or DHF. ACC/AHA guidelines as well as ESC guidelines state that transthoracic Doppler echocardiography is the key investigation in the diagnosis of HF (Citation70,Citation71). It helps to find out whether the patient has preserved or reduced LVEF, structural or functional abnormalities in the LV, as well as valvular and right ventricular abnormalities. To classify the degree of LV diastolic dysfunction, echo Doppler uses LV filling patterns and tissue Doppler imaging of the mitral annulus, and this works best in symptomatic patients with advanced disease (Citation72). When interpreting echocardiography, these types of atypical presentations should be given consideration. The echocardiographic measures which help to diagnose DHF and its severity are mitral flow velocity, mitral annular motion, pulmonary venous flow, atrial pressure, left ventricular relaxation and compliance, and propagation velocity. Pulsed-Doppler-derived transmitral flow (TMF) has been widely used for evaluating LV diastolic function, and it is graded based on the early to late diastolic inflow velocities. Grade I is abnormal relaxation pattern, grade II is pseudonormal pattern, grade III is restrictive pattern, and grade IV is irreversible restrictive pattern (Citation73). Under normal conditions, peak early diastolic mitral annular velocity (e’) occurs simultaneously with the peak early mitral inflow velocity (E). With normal diastolic function, LV must be able to fill without an elevated left atrial pressure. An abnormal or impaired relaxation pattern indicates a diastolic dysfunction without marked elevated left atrial pressures. Echo features may be helpful to assess the relative contribution of LV stiffness/delayed relaxation which reduces E wave versus elevated LV filling pressure which increases E wave; this is what leads to pseudonormalization. The presence of pseudonormalized and restrictive filling patterns, and observed elevated E/e’, indicates the presence of diastolic dysfunction and elevated left atrial pressures (Citation74). Pulmonary venous flow (PVF) pattern is also used for evaluation of LV diastolic dysfunction, especially to differentiate a normal from a pseudonormal TMF pattern. Analysis of TMF and PVF patterns are useful for diagnosing the uncompensated state of HF. Grade III and grade IV diastolic dysfunctions are called restrictive filling dynamics. These are both severe forms of diastolic dysfunction, and patients tend to have advanced HF symptoms (Citation73). Two specific variables that were found to be abnormal in patients with DHF, but not in SHF, are the increased LV mass/volume ratio and exercise pulse pressure (Citation23).
Some echo Doppler studies showed reduced systolic function, especially long-axis systolic dysfunction with HFPEF and a decline in systolic velocities of basal myocardial and mitral valve annular motion (Citation75–77,Citation36). Moreover, tissue Doppler imaging (TDI) and diastolic wall strain (DWS) are being considered as emerging supplementary tools for diagnosing and assessing the severity of diastolic dysfunction. The DWS index is inversely correlated with diastolic stiffness and the outcome of the patients with HFPEF (Citation78). End-systolic volume (ESV) is a simple parameter that can be measured easily in clinical practice using echocardiography. The end-systolic volume index (ESVI) is a simple marker of LV remodeling and can be measured by ESV indexed to body surface area. In the study by McManus et al. ESVI predicted a 4.3-fold increase in HF hospitalization in patients with normal EF (Citation79).
Cardiovascular magnetic resonance (CMR) provides a comprehensive assessment of cardiac structures, size, and function (Citation80). CMR can evaluate the diastolic function parameters like LV relaxation and stiffness abnormalities, mitral valve inflow, pulmonary veins inflow, as well as diastolic torsion rate better than echocardiogram. CMR has advantages over echocardiography, as it can get images in any desired planes with an unrestricted field of view. Therefore CMR measurement on volume, mass, and flow assessment have been shown to be accurate and reproducible. Gadolinium enhancement with MR imaging can give information about the extent of myocardial fibrosis, which influences LV stiffness (Citation81,Citation82).
Blood biomarkers
Recently new biomarkers are available for diagnosing HF. A multimarker panel approach may be useful to differentiate the cause of dyspnea, risk stratification, and treatment. In the future, more research is needed especially in older adults (Citation83).
Brain natriuretic peptide (BNP)
BNP comes mainly from the cardiac ventricles and is released in response to cardiac wall stretching. The plasma levels of BNP are raised in HF, HFPEF, and heart failure with left ventricular systolic dysfunction (HFLVSD) (Citation84). However, it is a non-specific test as levels may rise with other cardiac conditions like ischemia and arrhythmias as well as non-cardiac conditions like pulmonary emboli and renal failure (Citation85). A randomized controlled study showed that increased plasma levels of N-terminal pro-BNP (NT-proBNP) were highly predictive of events in patients with DHF (Citation86). In the Breathing Not Properly Study, BNP levels at the time of emergency admission were significantly elevated in HFPEF, when compared to dyspnea due to other causes of HF (413 versus 34 pg/mL, P <0.001), but significantly lower than in patients with SHF (413 versus 821 pg/mL, P <0.001). When compared with patients without HF, a BNP level of 100 pg/mL had a sensitivity of 86%, a negative predictive value of 96%, and an accuracy of 75% for detecting diastolic dysfunction (Citation84).
Growth differentiation factor-15
Growth differentiation factor-15 (GDF-15) is a member of the beta transforming growth factor (TGF) family. It is elevated in HFPEF and is as good as NT-proBNP in diagnosis. Combining both markers may improve diagnostic accuracy in DHF (Citation87).
Tissue inhibitors of matrix metalloproteinases-1 (TIMP-1)
In hypertensive patients and in patients with aortic stenosis the measurement of TIMP-1 has been proposed as a marker of myocardial stiffness or the development of HFPEF (Citation88). There is decreased matrix degradation in the myocardial tissue because of down-regulation of matrix metalloproteinases (MMPs) and up-regulation of TIMPs (Citation89).
Pentraxin 3
Pentraxin 3 is a new inflammatory marker and a member of pentraxin superfamily including C-reactive protein (CRP). Pentraxin 3 was found to be an independent marker seen in the presence of left ventricular diastolic dysfunction (LVDD) and HFPEF (Citation90). It can be a marker to indicate new ischemia in addition to troponin (Citation91). Therefore elevated pentraxin 3 may indicate new ischemia and inflammation in LVDD and HFPEF (Citation91).
Diagnostic difficulties in older adults
The diagnosis of HFPEF is generally based on the signs and symptoms of HF in the setting of having normal LVEF (45%) on echocardiography with evidence of abnormal LV relaxation or diastolic stiffness (Citation71). Differentiation between SHF and DHF cannot be made on the basis of the history, physical examination, electrocardiogram, or chest X-ray alone (). In contrast most older patients are chronically ill and have relatively mild symptoms (Citation92). Older adults with HFPEF may also lack the typical signs and symptoms of HF, therefore they should be assessed for atypical presentation, raised BNP, and echocardiographic evidence of normal or near-normal LVEF (). The new diagnostic algorithm according to the 2007 European Working Group includes: 1) symptoms and signs of HF, 2) LVEF ≥50% in a non-dilated LV, and 3) evidence of elevated LV filling pressures (Citation93). Early-stage HFPEF may have normal echocardiography with normal resting hemodynamics, but the diagnosis can be made with exercise hemodynamics (Citation94).
Prognosis
A prospective study by Aronow et al. showed that the mortality rate over 5 years was significantly higher both in men and women with abnormal compared to normal LVEF (P =0.0001) (Citation95). In older adults living in the community, the mortality risk from congestive heart failure is lower in persons with normal systolic function than in those with decreased systolic function (Citation96). Clinical predictors of poor mortality are age (Citation97), ischemic HF (Citation98), and anemia coexisting with HF (Citation99). The DIG (Digitalis Investigation Group) study also points out that female gender has a significant independent association with improved survival but has no association with all-cause, cardiovascular, or HF hospitalizations (Citation100). Echocardiographic measures have been associated with increased morbidity and mortality (Citation72). A recent study by Nguyen et al. showed that echocardiographic measures of diastolic function were predictive of all-cause mortality; especially propagation velocity (Vp) was independently associated with total mortality (Citation101). The TMF pattern is utilized to predict the prognosis of patients with HF: reversible restrictive filling has a high probability of improvement of HF, whereas those with an irreversible restrictive filling pattern have high mortality (Citation102). The extent of myocardial systolic or contractile dysfunction in HFPEF was associated with increased mortality. Systolic dysfunction is a marker of more severe disease (Citation77). Asymptomatic diastolic dysfunction, especially moderate to severe, has been associated with cardiac and all-cause mortality (Citation103,Citation104).
Integrated approach to the management of DHF in the elderly
Management can be based on clinical features, pathophysiology, echo staging, addressing underlying co-morbidities known to cause or exacerbate HF, and considerations of goals of care. General measures like attention to diet and good life-style, weight monitoring, patient education, and close medical follow-up should be done on all patients.
Acute DHF
HFPEF can be acute as seen as the first effect of acute ischemia on ventricular function. Severe acute HF can manifest as pulmonary edema and cardiogenic shock. Non-adherence to diet and medications (especially prescribed antihypertensive therapy), acute decompensation of renal failure, renovascular disease like renal artery stenosis, and unrecognized idiopathic self-terminating ventricular tachycardia can cause flash pulmonary edema in these patients (Citation105). The management is similar to SHF with the use of oxygen, diuretics, morphine, and nitrates to relieve venous congestion and pulmonary edema (Citation106). Tachycardia is poorly tolerated in DHF, and the use of calcium channel blockers or beta-blockers may be indicated for rate control, especially in the presence of AF (Citation11).
Chronic DHF
At present, the treatment is based on management of symptoms and risk reduction by different medications (). Currently, the evidence is not robust for pharmacologic management of HFPEF subjects concerning improving HF-related events or mortality.
Table III. Drug trials in DHF/HFPEF.
Diuretics
In patients who have pulmonary congestion and peripheral edema, thiazide and loop diuretics are the essential component of therapy (Citation107) for symptom control. The Hong Kong DHF randomized study compared diuretics (hydrochlorothiazide, furosemide, dyazide) alone in combination with angiotensin-converting enzyme (ACE) inhibitor (ramipril) or angiotensin receptor blocker (ARB) (irbesartan). Diuretics significantly improved symptoms and quality of life (QOL) (P <0.01). Little further benefit on symptoms and QOL was seen with the addition of ramipril or irbesartan, but marginally improved LV systolic and diastolic longitudinal function and decreased NT-proBNF levels were seen (P <0.05 for all) (Citation108).
A randomized study by Roongsritong et al. with spironolactone, an aldosterone antagonist, showed a significant improvement in diastolic performance at 4 months as assessed by mitral Doppler inflow deceleration time (P =0.035) and E/A ratio (P =0.025) and may also have a beneficial effect on the serum marker of cardiac fibrosis procollagen type 1 carboxy-terminal peptide (PICP level), when compared to baseline value (289.2+59.5 versus 239.3+57.7; P <0.05) (Citation109). HYVET (HYpertension in the Very Elderly Trial), a randomized international trial, showed that antihypertensive treatment with a sustained release formulation of the diuretic indapamide with or without the addition of perindopril significantly reduced HF in patients 80 years of age or older (HF reduction by 64% (HR 0.36, CI 95% 0.17–0.48, P <0.001)) (Citation110). TOPCAT, a multicenter, international, randomized double-blind, placebo-controlled trial of the aldosterone antagonist spironolactone on HFPEF, is on-going and is expected to complete in 2013 (Citation111).
Electrolyte imbalances such as hypokalemia and hypomagnesemia associated with the use of diuretics should be also treated. Monitoring for these imbalances is particularly important if the patient is also receiving digoxin. Serum potassium levels should be maintained between 4 and 5 mg/dL (Citation112).
Antihypertensives
A prospective case-controlled study by Aronow et al., treating with enalapril plus diuretic therapy, showed significant reduction in LV mass (P < 0.001) and significant increase in treadmill exercise tolerance (P < 0.001) respectively (Citation113). Trials on irbesartan, candesartan, and perindopril did not show any significant mortality benefit (Citation114–116). The CHARM (Candesartan in Heart Failure-Assessment of Reduction in Mortality and Morbidity) preserved trial of ARBs for HFPEF in elderly patients showed no significant reduction of cardiovascular death but showed significant reduction in HF hospitalization with candesartan (Citation115). The PEP-CHF (Perindopril in Elderly People with Chronic Heart Failure) study was a randomized placebo-controlled trial of ACE inhibitor perindopril 4 mg/day with DHF, which showed no significant effect on mortality, but showed significant benefit in unplanned HF hospitalization in one year (P =0.033) as well as change in 6-minute walking distance (P = 0.02) (Citation116). In the Hong Kong DHF study, adding irbesartan to diuretics decreased the LV mass significantly (P < 0.05) (Citation108). Meta-analysis by Klingbeil et al. demonstrated that ACE and ARBs are among the most effective antihypertensive drugs in reducing LV mass (Citation117). Propanolol in patients with HF and EF ≥40 treated with diuretics and ACEIs showed a significant decrease in total mortality and non-fatal myocardial infarction (Citation118). The SENIORS (Study of Effects of Nebivolol Intervention on Outcomes and Rehospitalization in Seniors with Heart Failure) showed that a beta1-blocker with nitric oxide-potentiating vasodilatory effect, nebivolol, improves morbidity in older HF patients with preserved and reduced EF (Citation119). Non-dihydropyridine calcium channel blockers like verapamil and diltiazem, through their negative inotropic and chronotropic properties, may help to relax and improve diastolic dysfunction (Citation120). A small cross-over study over a period of 4 weeks showed that verapamil significantly improved patients’ exercise capacity by 33% compared to baseline (13.9 versus 10.7 minutes at baseline, P < 0.05) and peak ventricular filling rate by 30% (2.29 versus 1.85 End Diastolic Volume (EDV)/s at baseline, P < 0.05) (Citation120). HFPEF patients are sensitive to overdiuresis and aggressive vasodilator therapy because of the exaggerated increase in systolic blood pressure after small increases in left ventricular end diastolic volume, as well as a marked increase in systolic blood pressure after a further increase in arterial elastance in the presence of a high-end systolic elastance (Citation121). The treatment should also target the underlying pathological disease like hypertension and coronary artery disease. The VALIDD (VALsartan In Diastolic Dysfunction) study showed that, in patients with hypertension and diastolic dysfunction, blood pressure reduction was similar with valsartan-based regimen or a regimen which does not include inhibitors of the renin-angiotensin-aldosterone system (Citation122). A recent meta-analysis of randomized controlled trials with different antihypertensive medication showed that treatment improved exercise tolerance and cardiac function but not mortality (Citation123). In another recent study Banach et al., in their post-hoc analysis of the DIG (Digitalis Investigation Group) trial during 5 years of follow-up, showed the impact of baseline systolic blood pressure in patients with mild to moderate chronic SHF and DHF. Baseline SBP ≤120 mmHg was associated with increased HF mortality (HR 1.30, 95% CI 1.08–1.57, P=0.006) and HF hospitalization (HR 1.21, 95% CI 1.07–1.36, P = 0.002), which was independent of other baseline characteristics (Citation124).
Digoxin
Digoxin was reported to yield symptomatic improvement and decreased hospitalizations without mortality benefits in the DIG study in patients with DHF (Citation125). Digoxin reduced HF hospitalization but increased unstable angina hospitalization, almost cancelling out each other (Citation125).
Statins
Fukuda et al., in a retrospective case-control study that followed up 132 subjects for a period of 21±12 months, were first to show a survival benefit with statins (RR 0.22, 95% CI 0.07–0.64, P = 0.006) (Citation126). Another study with statins has also shown mortality reduction in patients with DHF (Citation127). The study by Tehrani et al. did not show a benefit in reducing the hospitalization rate (7.1±6.3 versus 7.8±7.7, P = 0.52) (Citation128). At this point, statin therapy has the potential to reduce mortality. Yet more evidence is needed about its role in reducing the morbidity and mortality in DHF patients (Citation129).
D-ribose
D-ribose improves left atrial contribution to ventricular filling and improves diastolic functional parameters. Studies have produced serial echocardiographic findings showing smaller left atrial dimensions and shortened E wave deceleration in a D-ribose group when compared to the control group (Citation130,Citation131).
Specific management in the elderly based on pathophysiology and echocardiographic findings
Abnormal left ventricular filling and elevated left ventricular filling pressure
Use of diuretics.
Control of heart rate by using beta-blockers. In older patients, nebivolol and bisoprolol may be better choices, since carvedilol can cause orthostatic hypotension due to alpha-blocking effect, and metoprolol, which is a lipophilic drug, can cause side-effects such as insomnia, dreams, and nightmares (Citation132).
Role of afterload reduction therapy is unknown in this setting.
Pulmonary hypertension can be treated with ARBs.
Ventricular arterial uncoupling
In DHF at early stages diastolic dyssynchrony occurs dominantly, and in advanced stage systolic dyssynchrony, where LVEF is not preserved, and the role of biventricular pacing should be considered. However, in DHF/HFPEF, the target of the treatment should be focused on reducing LV mass and reducing the afterload.
Use of beta-blocker to reduce LV wall stress. Some evidence seems to suggest that that the greatest regression of LV mass and hypertrophy may occur when weight loss is combined with beta-adrenergic blocker therapy in patients with obese diastolic dysfunction hypertension in comparison to other hypertensive agents (Citation118).
Reducing LV mass can help with improving ventricular arterial coupling. The use of ACEI/ARB to regress LV mass has been shown in many studies (Citation107).
Non-dihydropyridine calcium channel blockers (Citation119).
Alteration of neurohormonal activation and improvement of ventricular remodeling
Use of ACEI to prevent neurohormonal activation and prevent ventricular remodeling.
Use of aldosterone antagonist to prevent myocardial fibrosis causing ventricular remodeling.
Treatment of chronotropic incompetence
In DHF, patients need co-ordinated atrial contraction because of the stiff LV causing increased LV filling pressure. Interestingly most of the patients with DHF have dilated LA which might be the cause or the effect of AF, compromising atrial contribution to the heart during diastole (Citation133).
Control heart rate by using beta-blocker or non-dihydropyridine calcium channel blocker.
Use of rhythm-restoring agent (amiodarone) for restoration of sinus rhythm to obtain effective atrial contribution.
Isotonic exercise conditioning (Citation134,Citation135).
Treatment of underlying ischemia by anti-ischemic medications and consider revascularization in drug-resistant cases.
Device therapy in DHF or HFPEF
Cardiac resynchronization therapy (CRT) is achieved by simultaneously pacing the right and left ventricle, and it has been observed to help in reducing adverse LV remodeling and to improve myocardial efficiency in diastolic dysfunction (Citation136).
Chronic baroreflex activation: baroreflex activation therapy (BAT) in HFPEF using an implanted device has been shown to cause regression of left ventricular hypertrophy, normalization of autonomic imbalance, and inhibition of renin-angiotensin system (Citation137).
Gene therapy in DHF
Angiotensin II type 1 receptor gene polymorphisms are seen in DHF (Citation138). In HF pressure load, circulating neurohormones, oxidative stress, and cytokines activate a complex network of signaling pathways which alter gene expression and induce myocyte hypertrophy and dysfunction (Citation139). In failing hearts, SERCA 2a deficiency drives the systolic and diastolic dysfunction because of abnormal calcium handling. Replacement therapy of this enzyme via gene transfer can correct the contractile and relaxation deficits (Citation140,Citation141).
Device therapy and gene therapy is not widely used at present but may become common potential therapies in the future.
Role of vitamin D in DHF
Excess parathormone (PTH) levels associated with low vitamin D levels may play a role in cardiovascular disease by leading to cardiomyocyte hypertrophy and interstitial fibrosis of the heart (Citation142). Vitamin D also plays a role in cardiomyocyte relaxation and may abrogate the hypercontractility associated with DHF (Citation143,Citation144). Animal studies have found that vitamin D suppresses cardiac hypertrophy (Citation145). Epidemiological studies have shown low vitamin D levels are associated with hypertension, left ventricular hypertrophy, and diastolic dysfunction (Citation146). At present, it is unknown if vitamin D supplementation improves function or survival in HF.
Sleep apnea and DHF
Sleep-related breathing disorders with repetitive episodes of hypoxia may adversely affect heart function (Citation147). These breathing disorders are associated with excessive arousals resulting in sympathetic stimulation (Citation148). A prospective elderly study in HFPEF showed disordered breathing problems seen in 70% of subjects. Obstructive sleep apnea was seen in 40%, and central sleep apnea was seen in 30% of subjects (Citation149). Adaptive seroventilation effectively reduces Cheyne–Stokes respiration in HFPEF and improves cardiac function and symptoms (Citation150).
Anemia and HFPEF
Anemia is commonly seen in HF patients, both in HFPEF as well as reduced EF. In a small study of 137 patients with HFPEF, prevalence of anemia was 52% (Citation100). Anemia in these patients is due to chronic kidney disease with low erythropoietin production and elevated cytokines like tumor necrosis factor alpha and interleukin-6 (Citation151). The interaction between HF, kidney disease, and anemia is called ‘cardio-renal anemia syndrome’ (Citation152,Citation153). Epidemiological and retrospective studies in DHF/HFPEF showed anemia is associated with increased mortality (Citation154–156). SENIORS (Study of the Effects of Nebivolol Interventions on Outcomes and Rehospitalization in Seniors with heart failure) showed that both HFPEF and reduced EF anemic subjects had 32% higher risk of death from any cause and also higher risk for hospitalization, with anemia as an independent risk factor (Citation157). A randomized controlled trial showed darbepoetin given subcutaneously and iron improved right and left ventricular systolic and diastolic function in anemic patients with HF which led to improved cardiac performance and exercise capacity (Citation158).
Obesity paradox in HFPEF
Increased BMI was associated with an increased risk of HF (Citation159). Recently HFPEF has been associated with increased serum leptin levels. Leptin gene and leptin receptor gene polymorphism increases 3-fold the risk of developing HFPEF (Citation160). Higher BMI has been paradoxically linked to a decreased risk of outcomes when compared to BMI in the normal range (termed ‘the obesity paradox’), both in HFPEF and HF with reduced EF. In a study by Kapoor et al., in subjects with HFPEF, a U-shaped relationship was seen between BMI and survival (Citation161). Similar results were also seen in the recent I-PRESERVE (Irbesartan in Heart Failure with Preserved Ejection Fraction) trial (Citation162).
Prevention of DHF
Treatment of hypertension, diabetes, and CAD, especially in the early stages, preferably at the stage of high risk of development of HF in the absence of symptoms, is effective. Hypertension increases the risk of developing HF 2–3-fold, and it precedes HF in over 90% of individuals (Citation163). Several clinical trials have shown that adequate control of blood pressure reduces the risk of HF by approximately 50% (Citation164). Framingham Heart Study data showed that a 20 mmHg increase in systolic blood pressure was linked to a 56% increase in the risk of developing HF (Citation165). HYVET (HYpertension in the Very Elderly Trial) showed the best impact of controlling hypertension on DHF: during a median follow-up of 1.8 years, there was a dramatic 64% reduction in the rate of HF and a 21% reduction in deaths from any cause (Citation11). Therefore, health care professionals should control both systolic and diastolic high blood pressures (Citation109). In both preclinical and clinical HF subjects, the goal when treating hypertension should be less than 140/90 mmHg (Citation166). The best way to prevent HF lies in targeted and sustained reduction of blood pressure by adherence to prescribed medications and preventing renal injury. Further research is needed to discover whether treating prehypertension may prevent HF (Citation167). Hypertension should be aggressively treated to prevent LV diastolic stiffness, LV concentric hypertrophy, and arterial stiffness especially by ACEIs or ARBs, or calcium channel blockers as well, as these agents improve dynamic relaxation in these patients (Citation168). The studies mentioned below have shown that the improvement in diastolic function may be independent of the blood pressure-lowering effects. Brilla et al. showed a reduction in collagen volume fraction, in hydroxyproline concentration, and improvement in diastolic function in hypertensive patients treated with lisinopril compared with a thiazide (Citation169). The aldosterone antagonist, canrenone, was shown to reduce diastolic function, without any reduction in blood pressure (Citation170).
Insulin resistance mediates several of the risk factors for developing HF. Under normal circumstances, the heart derives the majority of its energy from the beta-oxidation of fatty acids (Citation171). The failing heart has limited energy reserves and becomes increasingly dependent on glucose as its preferred substrate for ATP (Citation172). Myocardial insulin resistance may limit the ability of the failing heart to effectively take up glucose and oxidize it for ATP (Citation173). Treating myocardial insulin resistance may be a new therapeutic approach to prevent HF.
Exercise- or pacing-induced acute and chronic ischemia impairs LV relaxation and reduces diastolic compliance. The acute induction or worsening of diastolic dysfunction by ischemia raises left atrial and therefore pulmonary venous pressure. This explains why many patients with coronary heart disease have respiratory symptoms with their anginal pain, including wheezing, an inability to take a deep breath, shortness of breath, and overt pulmonary edema. These respiratory symptoms can occur in the absence of anginal pain and are often referred to as ‘anginal equivalents’. During episodes of angina pectoris, myocardial stiffness is increased (Citation174,Citation175). Ranolazine, a partial fatty acid oxidation inhibitor, showed a beneficial mechanism of improvement in regional coronary blood flow in areas of myocardial ischemia (Citation176). Ranolazine has been shown to improve LV diastolic dysfunction and the severity of angina in patients with ischemic heart disease (Citation177). Trimetazidine, a partial free fatty acid oxidation inhibitor acts by shifting the energy substrate from fatty acid metabolism toward glucose metabolism and supplying better fuel for myocardial efficiency and can be an adjunctive treatment in patients with HF (Citation178). Consideration should be given in selected patients for revascularization.
Pre-load dependence with concentric left ventricular hypertrophy (LVH) leads to inadequate diastolic filling time. These patients tolerate tachycardia poorly. So prevention of tachycardia and maintenance of sinus rhythm is important. Beta-blockers and/or calcium channel blockers should be considered to keep the heart rate <80/min. In patients with AF, ventricular rate should be adequately controlled and, if possible, converted to sinus rhythm.
Diastolic dysfunction and its progression to DHF
The causes leading to DHF are inappropriate tachycardia, impaired systolic relaxation, and decreased compliance. Diastolic dysfunction represents a mechanical malfunction of the relaxation of the LV chamber that is primarily diagnosed by transthoracic echocardiography and usually does not present early clinically as HF. The abnormal relaxation is usually separated in different degrees, based on the severity of reduction in passive compliance and active myocardial relaxation (Citation179). In a recent study, extremely impaired dynamic Starling mechanism in HFPEF may reflect advanced ventricular and arterial stiffness in HFPEF, possibly contributing to reduced forward output and pulmonary congestion (Citation180).
Among the different factors leading to HF, systemic hypertension is often considered as one of the leading clinical conditions. Diastolic dysfunction might represent an important pathophysiological intermediate between hypertension and HF (Citation122). Aldosterone is often considered to cause myocardial fibrosis, LV hypertrophy, and dysfunction, therefore the use of an aldosterone antagonist in hypertensive patients with suspected DHF improves myocardial function by reducing LV wall thickness and left atrial area and increasing myocardial wall strain in diastole (Citation181).
Diastolic dysfunction and its progression to SHF
Currently, only few data have been reported on diastolic dysfunction and its progression to SHF. Both systolic and diastolic dysfunction can be seen in patients with HFPEF (Citation182–184,Citation30). A recent study showed impaired global and regional LV contractility in HFPEF (Citation76). The high prevalence of moderate and severe diastolic dysfunction in patients with HF and reduced EF supports the importance of diastolic dysfunction in both forms of HF (Citation185).
Spectrum of dyssynchrony in heart failure
Dyssynchrony was assessed by the maximal time difference of peak systolic longitudinal velocity (systolic dyssynchrony) or early filling velocity among different regions in the LV (diastolic dyssynchrony). A study by Wang et al. in 120 HF narrow-QRS complex patients (half with ‘diastolic HF’, defined as EF ≥50%, mean pulmonary capillary wedge pressure >12 mmHg, and/or time constant of LV relaxation >48 ms) found systolic dyssynchrony in 30% to 40% and diastolic dyssynchrony in 60% of both HF groups (Citation30). In one study of HFPEF patients, diastolic dyssynchrony and systolic dyssynchrony were observed in 40% of patients but were coincident in only 15% of these individuals (Citation183). CRT has been shown to improve abnormal left ventricular performance due to systolic and diastolic dyssynchrony (Citation186). After CRT, diastolic dyssynchrony improved in patients with SHF (Citation187).
Conclusions
Approximately 50% of older HF patients will have DHF or HFPEF. Accurate diagnosis is the first step in the management of HFPEF in older adults. However, early recognition of DHF is difficult as the typical presenting symptoms of breathlessness on exertion, fatigue, and edema are non-specific, especially in older adults, who suffer from multiple morbidities. An LVEF is not essential in the diagnosis of HF and should not be estimated before making a clinical diagnosis of HF. Once a clinical diagnosis of HF has been established, LVEF should be estimated, preferably using an echocardiogram. An LVEF of greater than 40% is often considered preserved. Even though DHF or HFPEF is a distinct clinical entity, systolic and diastolic dysfunction is seen in both SHF and DHF, suggesting it may be different phenotypes of the same disease spectrum. Because the clinical presentation of HFPEF is similar to SHF, fluid volume management in HFPEF is also similar to that in SHF. Currently there is no evidence that neurohormonal blockade with ACE inhibitors, ARBs, beta-blockers, or aldosterone antagonists may improve outcomes in these patients. However, these drugs are often used to manage etiologic factors or co-morbidities such as hypertension, coronary artery disease, and diabetes. Most device therapies in HF are directed toward SHF, and their role in HFPEF remains unclear. Future basic and clinical studies are needed to better understand this complex syndrome so that proper therapeutic approaches can be developed and tested in properly designed clinical trials.
Declaration of interest: The authors report no conflicts of interest.
References
- Baicu CF, Zile MR, Aurigemma GP, Gaasch WH. Left ventricular systolic performance, function, and contractility in patients with diastolic heart failure. Circulation. 2005;111:2306–12.
- Ennezat PV, Lefetz Y, Marechaux S, . Left ventricular abnormal response during dynamic exercise in patients with heart failure and preserved left ventricular ejection fraction at rest. J Card Fail. 2008;14:475–80.
- Dickstein K, Cohen-Solal A, Filippatos G, McMurray JJ, Ponikowski P, Poole-Wilson PA, .; ESC Committee for Practice Guidelines (CPG). ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM). Eur Heart J. 2008;29:2388–442.
- Hunt SA, Abraham WT, Chin MH, . ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: endorsed by the Heart Rhythm Society. Circulation. 2005;112:e154–235.
- Rosamond W, Flegal K, Friday G, . Heart disease and stroke statistics—2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007;115:e69–171.
- Jessup M, Brozena S. Heart failure. N Engl J Med. 2003;348:2007–18.
- Lloyd-Jones DM, Larson MG, Leip EP, . Life-time risk for developing congestive heart failure: the Framingham Study. Circulation. 2002;106:3068–72.
- Bleumink GS, Knetsch AM, Sturkenboom MC, . Quantifying the heart failure epidemic: prevalence, incidence rate, lifetime risk and prognosis of heart failure. The Rotterdam Study. Eur Heart J. 2004;25:1614–9.
- Aurigemma GP, Zile MR, Gaasch WH. Contractile behavior of the left ventricle in diastolic heart failure: with emphasis on regional systolic function. Circulation. 2006;113:286–304.
- Kitzman DW, Sheikh KH, Beere PA, . Age-related alterations of Doppler left ventricular filling indexes in normal subjects are independent of left ventricular mass, heart rate, contractility and loading conditions. J Am Coll Cardiol. 1991;18:1243.
- Hunt SA, Abraham WT, Chin MH, . Focused update incorporated into the ACC/AHA 2005 guidelines for the diagnosis and management of heart failure in adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation. 2009;119: e391–479.
- Fonarow GC, Stough WG, Abraham WT, . Characteristics, treatments and outcomes of patients with preserved systolic function hospitalized for heart failure. A report from the Optimize HF registry. J Am Coll Cardiol. 2007;50:768–77.
- Ceia F, Fonseca C, Mota T, . Prevalence of chronic heart failure in Southwestern Europe: the EPICA study. Eur J Heart Fail. 2002;4:531–9.
- Owan TE, Hodge DO, Davie AP, . Epidemiology of chronic heart failure. Prog Cardiovasc Dis. 2005;47:320–32.
- Hogg K, Swedberg K, McMurray J. Heart failure with preserved left ventricular systolic function: epidemiology, clinical characteristics, and prognosis. J Am Coll Cardiol. 2004;43:317–27.
- Aronow WS, Ahn C, Kronzon I. Comparison of incidence of congestive heart failure in older African-Americans, Hispanics, and whites. Am J Cardiol. 1999;84:611–2.
- Sharp A, Tapp R, Francis DP, . Ethnicity and left ventricular diastolic function in hypertension an ASCOT (Anglo-Scandinavian Cardiac Outcomes Trial) substudy. J Am Coll Cardiol. 2008;52:1015–21.
- East MA, Peterson ED, Shaw LK, . Racial difference in the outcomes of patients with diastolic heart failure. Am Heart J. 2004; 148:151–6.
- Newton JB, Blackledge HM, Squire IB. Ethnicity and variation in prognosis for patients newly hospitalized for heart failure. A matched historical cohort study. Heart. 2003;91:1545–50.
- Nancy CW. The prevention of heart failure in minority communities and discrepancies in healthcare delivery systems. Med Clin N Am. 2004;88:1347–68.
- Andrew P. Diastolic heart failure demystified. Chest. 2003;124: 744–53.
- Gandhi SK, Powers JC, Nomeir AM, . The pathogenesis of acute pulmonary edema associated with hypertension. N Engl J Med. 2001;344:17–22.
- Kitzman DW, Little WC, Brubaker PH, . Pathophysiological characterization of isolated diastolic heart failure in comparison to systolic heart failure JAMA. 2002;288:2144–50.
- Zile MR, Baicu CF, Gaasch WH. Diastolic heart failure-abnormalities in active relaxation and passive stiffness of the left ventricle N Engl J Med. 2004;350:1953–9.
- Oxenham HC, Young AA, Cowan BR, . Age related changes in myocardial relaxation using three dimensional tagged magnetic resonance imaging. J Cardiovasc Magn Reson. 2003;5:421–30.
- van Heerebeek L, Borbely A, Niessen HW, . Myocardial function and structure differ in systolic and diastolic heart failure. Circulation. 2006;113:1966–73.
- Zile MR, Brutsaert DL. New concepts of diastolic dysfunction and diastolic heart failure: Diagnosis, prognosis, and measurement of diastolic function. Circulation. 2002;105:1387–93.
- Weber KT, Brilla CG. Pathological hypertrophy and cardiac interstitium. Fibrosis and renin-angiotensin-aldosterone system. Circulation. 1991;83:1849–65.
- Rapp JA, Gheorghiade M. Role of neurohormonal modulators in heart failure with relatively preserved systolic function. Cardiol Clin. 2008;26:23–40.
- Wang J, Kurrelmeyer KM, Torre-Amione N, Nagueh SF. Systolic and diastolic dyssynchrony in patients with diastolic heart failure and the effect of medical therapy. J Am Coll Cardiol. 2007;49:88–96.
- Lam CS, Roger VL, Rodeheffer R, . Cardiac structure and ventri cular-vascular function in persons with heart failure and preserved ejection fraction: from Olmsted county, Minnesota. Circulation. 2007;115:1982–90.
- Borlaug BA, Kass KA. Ventricular-vascular interaction in heart failure. Heart Fail Clin. 2008;4:23–36.
- Orso F, Baldasseroni S, Maggioni A. Heart rate in coronary syndromes and heart failure. Prog Cardiovasc Dis. 2009;32:38–43.
- Phan TT, Shivu GN, Abozuia K, . Impaired heart rate recovery and chronotropic incompetence in patients with heart failure with preserved ejection fraction. Circ Heart Fail. 2010;3:29–34.
- Borlaug BA, Olson TP, Lam CS, . Global cardiovascular reserve dysfunction in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2010;56:845–54.
- Vinereanu D, Nicolaides E, Tweddel AC, Fraser AG. Pure diastolic dysfunction is associated with long-axis systolic dysfunction: Implications for the diagnosis and classification of heart failure. Eur J Heart Fail. 2005;7:820–8.
- Garcia EH, Perna ER, Farias EF, . Reduced systolic performance by tissue Doppler in patients with preserved and abnormal ejection fraction: New insights in chronic heart failure. Int J Cardiol. 2006;108:181–8.
- Tan YT, Wenzelburger F, Le H, . Exercise echocardiography reveals complex abnormalities of both systolic and diastolic ventricular function involving torsion, untwist and longitudinal motion. J Am Coll Cardiol. 2009;54:36–46.
- Jorge AJL, Silva EN, Fernandes LC, . Evaluation of longitudinal systolic function in heart failure with normal ejection fraction. Arq Bras Cardiol. 2010;94:799–805.
- Chen HH, Lainchbury JG, Senni M, . Diastolic heart failure in the community: underlying cardiovascular diseases and precipitative factors. Circulation. 2000;102:771–80.
- Jacobson DR, Passore RD, Yaghoubian R, . Variant-sequence transthyretin (Isoleucine 122) in late-onset cardiac amyloidosis in Black Americans. N Engl J Med. 1999;356:466–73.
- Falk RH. Diagnosis and management of the cardiac amyloidosis. Circulation. 2005;112:2047–60.
- McMurray JJ, Carson PE, Komajda M, . Heart failure with preserved ejection fraction: clinical characteristics of 4133 patients enrolled in the I-PRESERVE trail. Eur J Heart Fail. 2008;10149–56.
- Szidon JP. Pathophysiology of the congested lung. Cardiol Clin. 1989;7:39–48.
- Chen HH, Luinchbury JG, Senni M, . Diastolic heart failure in the community: Clinical profile, natural history, therapy and impact of proposed diagnostic criteria. J Card Fail. 2002;8:279–87.
- Fung JW, Sanderson JE, Yip GW, . Impact of atrial fibrillation in heart failure with normal ejection fraction: a clinical and echocardiographic study. J Card Fail. 2007;13:649–55.
- Tsang TS, Gersh BJ, Appleton CP, . Left ventricular diastolic dysfunction as a predictor of first diagnosed non valvular atrial fibrillation in 840 elderly men and women. J Am Coll Cardiol. 2002;40:1636–44.
- Ahmed A, Zile MR, Rich MW, . Hospitalizations due to unstable angina pectoris in diastolic and systolic heart failure. Am J Cardiol. 2007;99:460–4.
- Tresch DD. Atypical presentation of cardiovascular disorders in the elderly. Geriatrics. 1987;42:41–6.
- Zuccala G, Cattel C, Manes-Gravina E, . Left ventricular dysfunction: A clue to cognitive impairment in older patients with heart failure. J Neurol Neurosurg Psychiatry. 1997;63:509–12.
- Davie AP, Francis CM, Caruana L,. Assessing diagnosis in heart failure: which features are of any use? QJM. 1997;90:335–9.
- Remes J, Miettinen H, Reunanen A, Pyorala K. Validity of clinical diagnosis of heart failure in primary heart care. Eur Heart J. 1991;12:315–21.
- Ahmed A. DEFEAT-Heart failure: A guide to management of geriatric heart failure by generalist physicians. Minerva Med. 2009;100:39–50.
- Stevenson LW, Perloff JK. The limited reliability of physical signs for estimating hemodynamics in chronic heart failure. JAMA. 1989;261:884–8.
- Lien CTC, Gillespie ND, Struthers AD, McMurdo MET. Heart failure in frail elderly patients: Diagnostic difficulties, co-morbidities, polypharmacy and treatment dilemmas. Eur J Heart Fail. 2002;4:91–8.
- Oudejans I, Mosterd A, Bluemen JA, . Clinical evaluation of geriatric outpatients with suspected heart failure: value of symptoms, signs and additional tests. Eur J Heart Fail. 2011;13:518–27.
- Rich MW. Office management of heart failure in the elderly. Am J Med. 2005;118:342–48.
- Zile MR, Brutsaert DL. New concepts in diastolic dysfunction and diastolic heart failure. Part II: causal mechanisms and treatment. Circulation. 2002;105:1503–8.
- Erne P. Beyond auscultation—acoustic cardiography in the diagnosis and assessment of cardiac disease. Swiss Med Wkly. 2008;138:439–52.
- Dillier R, Zuber M, Arand P, . Assessment of systolic and diastolic function in asymptomatic subjects using ambulatory monitoring with acoustic cardiography. Clin Cardiol. 2011;34:384–8.
- Dargie HJ, McMurray JJ. Diagnosis and management of heart failure. BMJ. 1994;308:321–8.
- Galinier M, Vialette JC, Fourcade J, . QT interval dispersion as a predictor of arrhythmic events in congestive heart failure. Eur Heart J. 1998;19:1054–62.
- Zareba KM, Shenkman HJ, Bibognano JD. Predictive value of admission electrocardiography in patients with heart failure. Congest Heart Fail. 2008;4:173–9.
- Baldasteroni S, Gentile A, Gorini M, . Intraventricular conduction defects in patients with congestive heart failure: left but not right bundle branch block is an independent predictor of prognosis. A report from the Italian network on congestive heart failure (In-CHF database). Ital Heart J. 2003;4:607–13.
- Anderson JL, Askins JC, Gilbert EM, Menlove RL, Lutz JR. Occurrence of ventricular arrhythmias in patients receiving acute and chronic infusions of Milrinone. Am Heart J. 1986;111:466–74.
- Tisdale JE, Patel R, Webb CR, . Electrophysiologic and proarr hythmic effects of intravenous inotropic agents. Prog Cardiovasc Dis. 1995;38:167–180.
- Burger AJ, Elkayam U, Neibaur MT, . Comparison of the occurrence of ventricular arrhythmias in patients with acutely decompensated congestive heart failure receiving dobutamine versus nesiritide therapy. Am J Cardiol. 2001;88:35–9.
- Guidelines for the diagnosis of heart failure. The Task Force of the European Society of Cardiology. Eur Heart J. 1995;16:741–51.
- Mattu A, Martinez JP, Kelly BS. Modern management of cardiogenic pulmonary edema. Emerg Med Clin North Am. 2005;23:1105–25.
- Hunt S, Baker D, Chin M, . ACC/AHA guidelines for the evaluation and management of chronic heart failure in the adult: executive summary report of the American College of cardiology/ American Heart association Task Force on Practice Guidelines. Circulation. 2001; 104:2996.
- Swedberg K, Cleland J, Dargie H, . Guidelines for the diagnosis and treatment of chronic heart failure: executive summary (update 2005). Eur Heart J. 2005;26:1115–40.
- Nagueh SF, Appleton CP, Gillebert TC, . Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiography. 2009;22:107–33.
- Murata K. Predictive significance of evaluation of left ventricular diastolic function in patients with heart failure. Rinsho Byoki. 2010;58:792–8.
- Masutani S, Little WC, Hasegawah, . Restrictive left ventricular filling pattern does not result from increased left atrial pressure alone. Circulation. 2008;117:1550–54.
- Yip G, Wang M, Fung JWH, . Left ventricular long axis function in diastolic heart failure is reduced in both diastole and systole: time for a redefinition? Heart. 2002;87:121–5.
- Yip GWK, Zhang G, Xie JM, . Resting global and regional left ventricular contractility in patients with heart failure and normal ejection fraction: insights from speckle-tracking echocardiography. Heart. 2011;97:287–94.
- Bruch C, Gradaus R, Guinia S, . Doppler tissue analysis of mitral annular velocities: evidence for systolic abnormalities in patients with diastolic heart failure. J Am Soc Echocardiography. 2003;16:1031–6.
- Ohtani T, Yamamoto K, Dunlay SM, . Severity of LV diastolic stiffness as assessed by the diastolic wall strain index is associated with worse outcomes in heart failure with preserved ejection fraction. Circulation. 2010;122:A12731.
- McMannus DD, Shah SI, Fabi MR, . Prognostic value of left ventricular end-systolic volume index as a predictor of heart failure hospitalization in stable coronary artery disease: Data from the heart and soul study. J Am Soc Echocardiogr. 2009;22:190–7.
- Assoumull RG, Penell DJ, Prasad SK. Cardiovascular magnetic resonance in the evaluation of heart failure. Heart. 2007;93:985–92.
- Caudron J, Fares J, Bauer F, . Evaluation of left ventricular diastolic function with cardiac MR imaging. Radiographics. 2011;31: 239–59.
- Kedar J, Tarun P. Evaluation of diastolic dysfunction using cardiac magnetic resonance imaging. European Cardiology. 2010;6:21–5.
- Gori CS, Magrini L, Travalino F. Di Somma S. Role of biomarkers in patients with dyspnea. Eur Rev Med Pharmacol Sci. 2011; 15:229–40.
- Maisel AS, McCord J, Nowak RM, . Bedside B type natriuretic peptide in the emergency diagnosis of heart failure with reduced or preserved ejection fraction. Results from the Breathing Not Properly Multinational Study. J Am Coll Cardiol. 2003;41:2010–7.
- McKie PM, Burnett JC. B-type natriureteric peptide as a biomarker beyond heart failure, speculations and opportunities. Mayo Clin Proc. 2005:80:1029–36.
- Cleland JG, Taylor J, Tendera M. Prognosis in heart failure with a normal ejection fraction. N Engl J Med. 2007;357:829–30.
- Stahrenberg R, Edelman F, Mende M, . The novel biomarker growth differentiation factor 15 in heart failure with normal ejection fraction. Eur J Heart Fail. 2010;12:1309–16.
- Heymans S, Schrien B, Verneersch P, . Increased cardiac expression of tissue inhibitor of metalloproteinase-1 and tissue inhibitor of metalloproteinase-2 is related to cardiac fibrosis and dysfunction in the chronic pressure overloaded human heart. Circulation. 2005;112: 1136–44.
- Gonzalez A, Lopez B, Querejeta R, . Filling pressure and collagen metabolism in hypertensive patients with heart failure and normal ejection fraction. Hypertension. 2010;55:1418–24.
- Matsubara J, Sugiyama S, Nozaki T, . Pentraxin 3 is a new inflammatory marker correlated with left ventricular diastolic dysfunction and heart failure with normal ejection fraction. J Am Coll Cardiol. 2011;57:861–9.
- Kume N, Mitsuoka H, Hayashida K, Tanaka M. Pentraxin 3 as a biomarker for acute coronary syndrome. Comparison with biomarkers for cardiac damage. J Cardiol. 2011;58:38–45.
- Fonseca C, Morais H, Mota T, . The diagnosis of heart failure in primary care: value of symptoms and signs. Eur J Heart Fail. 2004;6:795–800.
- Paulus WJ, Tschope C, Sanderson JE, . How to diagnose diastolic heart failure: a consensus statement for the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Association of the European Society Cardiology. Eur Heart J. 2007;28:2539–50.
- Borlaug BA, Nishimura RA, Sorajja P, . Exercise hemodynamics enhance diagnosis of early heart failure with preserved ejection fraction. Circ Heart Fail. 2010;117:2051–60.
- Aronow WS, Ahn C, Kronzon I. Prognosis of congestive heart failure after prior myocardial infarction in older men and women with abnormal versus normal left ventricular ejection fraction. Am J Cardiol. 2000;85:1382–4.
- Gottdiener JS, McClelland RL, Marshall R, . Outcome of congestive heart failure in elderly persons: influence of left ventricular systolic function. The Cardiovascular Health Study. Ann Intern Med. 2002;137:631–9.
- O’Connor CM, Gattis WA, Shaw L, . Clinical characteristics and long-term outcomes of patients with heart failure and preserved systolic function. Am J Cardiol. 2000;86:863–7.
- McAlister FA, Teo KK, Taher M, . Insights into the contemporary epidemiology and outpatient management of congestive heart failure. Am Heart J. 1999;138:87–94.
- Brucks S, Little WC, Chao T, . Relation of anemia to diastolic heart failure and the effect on outcome. Am J Cardiol. 2004;93:1055–7.
- Ahmed MI, Lainscak M, Mujib M, . Gender-related dissociation in outcomes in chronic heart failure: reduced mortality but similar hospitalization in women. Int J Cardiol. 2011;148:36–42.
- Nguyen PK, Schmittger I, Heidenreich PA. A comparison of echocardiographic measures of diastolic function for predicting all-cause mortality in a predominantly male population. Am Heart J. 2011;161:530–4.
- Borlaug BA, Lam CS, Roger VL, . Contactility and ventricular systolic stiffening in hypertensive heart disease insights into the pathogenesis of heart failure with preserved ejection fraction. J Am Coll Cardiol. 2009;54:410–8.
- Bella JN, Palmieri V, Roman MJ, . Mitral ratio of peak early to late diastolic filling velocity as a predictor of mortality in middle-aged and elderly adults: the Strong Heart Study. Circulation. 2002;105:1928–33.
- Ren X, Ristow B, Na B, . Prevalence and prognosis of asymptomatic left ventricular diastolic dysfunction in ambulatory patients with coronary heart disease. Am J Cardiol. 2007;99:1643–7.
- Kumar R, Gandhi SK, Little WC. Acute heart failure with preserved systolic function. Crit Care Med. 2008;36(suppl):S52–6.
- Aurigemma GP, Gaasch WH. Diastolic heart failure. N Engl J Med. 2004;351:1097–2005.
- Sica DA, Gehr TWB, Frishman WH. Use of diuretics in the treatment of heart failure in the elderly. Clin Geriatr Med. 2007;23:107–21.
- Yip GWK, Wang M, Wang T, . The Hong Kong diastolic heart failure study: a randomized controlled trial of diuretics, irbesartan and ramipril on quality of life, exercise capacity, left ventricular global and regional function in heart failure with a normal ejection. Heart. 2008;94:573–80.
- Roongsritong C, Sutthiwan P, Bradley J, . Sprinolactone improves diastolic function in the elderly. Clin Cardiol. 2005;28:484–7.
- Beckett NS, Peters R, Fletcher AE, .; HYVET study group. Treatment of hypertension in patients 80 years of age or older. N Eng J Med. 2008;358:1887–98.
- Martinez FA. Aldosterone inhibition and cardiovascular protection: more important than it once appeared. Cardiovasc Drug Ther. 2010;24:345–50.
- Shapiro W. Correlative studies of serum digitalis levels and the arrhythmias of digitalis intoxication. Am J Cardiol. 1978;41:852–9.
- Aronow WS, Kronzon I. Effect of enalapril on congestive heart failure treated with diuretics in elderly patients with prior myocardial infarction and normal left ventricular ejection fraction. Am J Cardiol. 1993;71:602–4.
- Massie BM, Carson PE, Mcmurray JJ, . Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med. 2008;359:2456–67.
- Yusuf S, Pfeffer MA, Swedberg K, . Effects of candesartan in patients with chronic heart failure and preserved left ventricular ejection fraction: the CHARM Preserved Trial. Lancet. 2003;362:777–81.
- Cleland JG, Tendera M, Adamus J, . The perindopril in elderly people with chronic heart failure (PEP-CHF) study. Eur Heart J. 2006;27:2338–45.
- Klingbeil AU, Schneider M, Martus P, . A meta-analysis of the effects of treatment on left ventricular mass in essential hypertension. Am J Med. 2003;115:41–6.
- Aronow WS, Ahn C, Kronzon I. Effect of propanolol versus no propranolol on total mortality plus nonfatal myocardial infarction in older patients with prior myocardial infarction, congestive heart failure, and left ventricular ejection fraction >40% treated with diuretics plus angiotensin-converting enzyme inhibitors. Am J Cardiol. 1997;80:207–9.
- Ghio S, Magrini G, Serio A, . Effects of nebivolol in elderly heart failure patients with or without systolic left ventricular dysfunction: results of the Seniors echocardiographic substudy. Eur Heart J. 2006;27:562–8.
- Setaro JF, Zaret BL, Schulman DS, Black HR, Soufer R. Usefulness of verapamil for congestive heart failure associated with abnormal left ventricular diastolic filling and normal left ventricular systolic performance. Am J Cardiol. 1990;66:981–6.
- Frenneaux M, Willims L. Ventricular-arterial and ventricular-ventricular interactions and their relevance to diastolic filling. Prog Cardiovasc Dis. 2007;49:252–62.
- Solomon SD, Janardhanan R, Verma A, . Effect of angiotensin receptor blockade and antihypertensive drugs on diastolic function in patients with hypertension and diastolic dysfunction: a randomized trial. Lancet. 2007;369:2079–87.
- Holland DJ, Kumbhani DJ, Ahmed SH, Marwick TH. Effects of treatment on exercise tolerance, cardiac function, and mortality in heart failure with preserved ejection fraction, a meta-analysis. J Am Coll Cardiol. 2011;57:1676–86.
- Banach M, Bhatia V, Feller MA, . Relation of baseline systolic blood pressure and long-term outcomes in ambulatory patients with chronic mild to moderate heart failure. Am J Cardiol. 2011;107:1208–14.
- Ahmed A, Rich MW, Fleg JL, . Effects of digoxin on morbidity and mortality in diastolic heart failure; the ancillary digitalis investigation group trial. Circulation. 2006;114:266–70.
- Fukuta H, Same DC, Brucks S, Little WC, . Statin therapy may be associated with lower mortality in patients with diastolic heart failure: A preliminary report. Circulation. 2005;112:357–63.
- Shah R, Wang Y, Foody JM. Effects of statin, angiotensin-converting enzyme inhibitors and beta blockers on survival in patients ≥65 years of age with heart failure and preserved left ventricular systolic function. Am J Cardiol. 2008;2:217–22.
- Tehrani F, Morrissey R, Phan A, . Statin therapy in patients with diastolic heart failure. Clin Cardiol. 2010;35:e1–5.
- Banach M, Mikhailidis DP, Kjeldsen SE, Rysz J. Time for new indications for statins? Med Sci Monit. 2009;15:MS1–5.
- Omran H, Illien S, MacCarter D, . D-ribose improves diastolic function and quality of life in congestive heart failure patients: a prospective feasibility study. Eur J Heart Fail. 2003;5:615–9.
- MacCarter D, Vijay N, Washam M, . D-ribose aids. Advanced ischemic heart failure patients. Int J Cardiol. 2009;137:79–80.
- Cruickshank JM. Beta blockers and heart failure. Indian Heart J. 2010;62:101–10.
- Banerjee P, Banerjee T, Khand A, . Diastolic heart failure: neglected or misdiagnosed. J Am Coll Cardiol. 2002;39:138–41.
- Brenner DA, Apstein CS, Saupe KW. Exercise training attenuates age-associated diastolic dysfunction in rats. Circulation. 2001; 104:221–6.
- Takemoto KA, Bernstein L, Lopez JF, . Abnormalities of diastolic filling of the left ventricle associated with aging are less pronounced in exercise-trained individuals. Am Heart J. 1992;124:143–8.
- Waggoner AD, Faddis MN, Gleva MJ, . Cardiac resynchronization therapy acutely improves diastolic function. J Am Soc Echocardiogr. 2005;18:216–20.
- Georgegaropoulos D, Little CW, Abraham WT, . Chronic baroreflex activation: A potential therapeutic approach to heart failure with preserved ejection fraction. J Card Fail. 2011;17:167–78.
- Wu CK, Tsai CT, Chang YC, . Genetic polymorphisms of the angiotensin II type 1 receptor gene and diastolic heart failure. J Hypertens. 2009;27:502–7.
- Cappola T. Molecular remodeling in human heart failure. J Am Coll Cardiol. 2008;15:137–8.
- Arai M, Alpert N R, MacLennan D H, . Alterations in sarcoplasmic reticulum gene expression in human heart failure. A possible mechanism for alterations in systolic and diastolic properties of the failing myocardium. Circ Res. 1993;72:463–9.
- Chaudhri B, del Monte F, Harding S, . Gene transfer in cardiac myocytes. Surg Clin North Am. 2004;84:141–9.
- Rostland SG, Drueke TB. Parathyroid hormone, vitamin D and cardiovascular disease in chronic renal failure. Kidney Int. 1999;56:383–92.
- Tishkoff DX, Nibbelink KA, Holmberg KH, . Function vitamin D receptor (VDR) in the t-tubules of cardiac myocytes: VDR knockout cardiomyocyte contractility. Endocrinology. 2008;149:558–64.
- Green II, Robinson DA, Wilson GE, . Calcitriol modulation of cardiac contractile performance via protein kinase C. J Mol Cell Cardiol. 2006;41:350–9.
- Wu I, Garamani M, Cheng T, Gardner DG. 1,25(OH) 2 vitamin D3, and retinoic acid antagonize endothelin-stimulated hypertrophy of neonatal rat cardiac myocytes. J Clin Invest. 1996;97:1577–88.
- Nigwekar SU, Bhan I, Thadhani R. Vitamin D, hypertension, left ventricular hypertrophy, and diastolic dysfunction. Curr Cardiovasc Risk Rep. 2011:1–9.
- Javaheri S, Parker TJ, Lining D, . Sleep apnea in 81 ambulatory male patients with stable heart failure. Types and their prevalence, consequences and presentations. Circulation. 1998;97:2154–9.
- Somers VK, Dyken M, Clary MS, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest. 1995;96:1897–904.
- Bitter T, Prinz CN, Faber L, . Sleep-disordered breathing in heart failure with normal left ventricular ejection fraction. Eur J Heart Fail. 2009;11:602–8.
- Bitter T, Westerheide N, Faber L, . Adaptive seroventilation in diastolic heart failure and cheyne-stokes respiration. Eur Respir J. 2010;36:385–92.
- Anand IS. Heart failure and anemia: mechanisms and pathophysiology. Heart Fail Rev. 2008;13:379–86.
- Silverberg DS, Wexler D, Iaina A, Schwartz D. Anemia, chronic renal disease and chronic heart failure: The Cardio Renal Anemia Syndrome: The need for cooperation between cardiologists and nephrologists. Int Urol Nephrol. 2006;38:295–310.
- Malyszko J, Zbroch E, Malyszko J, . The cardio-renal-anaemia syndrome predicts survival in peritoneally dialyzed patients. Arch Med Sci. 2010;6:539–44.
- Felker GM, Shah LK, Stough WG, . Anemia in patients with heart failure and preserved systolic function. Am Heart J. 2006; 151:45–62.
- Tehrani F, Phan A, Morrisey R, . Prognostic value of anemia in patients with diastolic heart failure. Tex Heart Inst J. 2009;36:220–5.
- Shamagian LG, Roman AV, Garcia-Acuna JM, . Anemia is associated with higher mortality among patients with heart failure with preserved systolic function. Heart. 2006;92:780–4.
- von Haehling S, van Veldhuisen DJ, Roughton M, . Anemia among patients with heart failure and preserved or reduced ejection fraction: Results from the SENIORS study. Eur J Heart Fail. 2011;13:656–63.
- Parissis JT, Kourea K, Panou F, . Effects of darbepoetin alpha on right and left ventricular systolic and diastolic function in anemic patients with chronic heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am Heart J. 2008;155:751.e1–7.
- Kenchaiah S, Evans JC, Levy D, . Obesity and the risk of heart failure. N Engl J Med. 2002;347:305–13.
- Abdel-Aziz TA, Mohamed RH, Pasha HF. Leptin, leptin gene and leptin receptor gene polymorphism in heart failure with preserved ejection fraction. Heart Vessels. 2011 May 17. [Epub ahead of print].
- Kapoor JR, Heinden Reich PA. Obesity and survival in patients with heart failure and preserved systolic function: A U-shaped relationship. Am Heart J. 2010;159:75–80.
- Haass M, Kitzman DW, Anand IS, . Body mass index and adverse cardiovascular outcomes in heart failure with preserved ejection fraction: results from the Irbesartan in heart failure with preserved ejection fraction (I-PRESERVE) trial. Circ Heart Fail. 2011;4:305–13.
- Chobanian AV, Bakris GL, Black HR, . The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003; 289:2560–72.
- Baker DW. Prevention of heart failure. J Card Fail. 2002;8:333–46.
- Haider AW, Larson MC, Franklin SS, Levy D. Systolic blood pressure, diastolic blood pressure and pulse pressure as predictors of risk for congestive heart failure in the Framingham Heart Study. Ann Intern Med. 2003;138:10–16.
- Lee DS, Vasan SR. Goals and guidelines for treating hypertension in a patient with heart failure. Curr Treat Options Cardiovasc Med. 2006;8:334–44.
- Wang Y, Wang QJ. The prevalence of prehypertension and hypertension among US adults according to the new Joint National Committee guidelines: new challenges of the old problem. Arch Intern Med. 2004;163:2126–34.
- Julius S, Nesbitt SD, Egan BM, . Feasibility of treating hypertension with an angiotensin-receptor blocker. N Engl J Med. 2006;354: 1685–97.
- Brilla CG, Funck RC, Rupp H. Lisinopril-mediated regression of myocardial fibrosis in patients with hypertensive heart disease. Circulation. 2000;102:1388–93.
- Grandi AM, Imperiale D, Santillo R, . Aldosterone antagonist improves diastolic function in essential hypertension. Hypertension. 2002;40:647–52.
- Depre C, Rider MH, Hue L. Mechanism of control of heart glycolysis. Eur J Biochem. 1998;258:277–90.
- Morgan HE. Fueling the heart. Circ Res. 2003;92:1276–8.
- Abel ED. Myocardial insulin resistance and cardiac complications of diabetes. Immune Endocr Metabol Disord. 2005;5:219–26.
- Grossman W, Mann JT. Evidence for impaired left ventricular relaxation in man. Eur J Cardiol. 1978;7(suppl):239–45.
- Bourdillon PD, Loreel BH, Mirsky I, . Increased regional myocardial stiffness of the left ventricle during pacing—induced angina in man. Circulation. 1983;67:316–23.
- Stone HP, Chaitman BR, Stocke K, . The anti-ischemic action of Ranolazine in stable ischemic heart disease. J Am Coll Cardiol. 2010;56:934–42.
- Hayashida W, Vaneyll C, Rousseau MF, Pouleur H. Effects of Ranolazine on left ventricular diastolic function in patients with ischemic heart disease. Cardiovasc Drugs Ther. 1994;8:741–7.
- Fragosso G, Palloshi A, Puchett P, . A randomized clinical trial of trimetazidine, a partial free fatty acid oxidation inhibitor, in patients with heart failure. J Am Coll Cardiol. 2006;48:992–8.
- Schwarz ER, Dashti R. The clinical quandary of left and right ventricular diastolic dysfunction and diastolic heart failure. Cardiovas J Afr. 2010;21:212–40.
- Shibata S, Hastings JL, Prasad A, . Congestive heart failure with preserved ejection fraction is associated with severely impaired dynamic Starling mechanism. J Appl Physiol. 2011;110: 964–71.
- Mottram PM, Haluska B, Leano R, . Effect of aldosterone antagonism on myocardial dysfunction in hypertensive patients with diastolic heart failure. Circulation. 2004;110:558–65.
- Clements IP. Spectrum of systolic and diastolic dysfunction in the presence of normal left ventricular ejection fraction. J Cardiac Fail. 2003;9:S24–6.
- Yu CM, Zhang Q, Yip GW, . Diastolic and systolic asynchrony in patients with diastolic heart failure: a common but ignored condition. J Am Coll Cardiol. 2007;49:97–105.
- Wang YC, Hwang JJ, Lai LP, . Coexistence and exercise exacerbation of intra-left ventricular contractile dyssynchrony in hypertensive patients with diastolic heart failure. Am Heart J. 2007;154: 278–84.
- Schuster I, Habib G, Jego C, . Diastolic asynchrony is more frequent than systolic asynchrony in dilated cardiomyopathy and is less improved by cardiac resynchronization therapy. J Am Coll Cardiol. 2005;46:2250–7.
- Schuler BT, Leon AR. Cardiac resynchronization therapy. Curr Cardiol Rep. 2005;7:321–8.
- Shanks M, Bertini M, Delgado V, . Effect of biventricular pacing on diastolic dyssynchrony. J Am Coll Cardiol. 2010;56:1567–75.