Abstract
A meta-analysis of possible antihypertensive effects of small doses of bioactive tripeptides isoleucine-proline-proline and valine-proline-proline in commercial milk products or tablets was carried out. A random effects model was used on 19 randomized, placebo-controlled clinical intervention trials (published 1996–October 2010) consisting of about 1500 prehypertensive or mildly hypertensive subjects.The overall blood pressure lowering for systolic blood pressure was –4.0 mmHg (95% CI –5.9 to –2.1 mmHg, P < 0.001) and for diastolic blood pressure –1.9 mmHg (95% CI –3.1 to –0.8 mmHg, P < 0.001). However, a positive effect was not reported in all the studies. The results suggest that rather small daily doses of the lactotripeptides in different functional food products may offer a valuable option as a non-pharmacological treatment of prehypertension or mild hypertension as part of life-style advice.
Key messages
Nineteen randomized clinical intervention trials with small daily doses (2.0–10.2 mg) of milk casein-derived tripeptides showed an overall lowering of systolic (4.0 mmHg) and diastolic (1.9 mmHg) blood pressure in mildly hypertensive subjects in a random effects meta-analysis.
This nutritional treatment may offer a valuable, non-pharmacological option for controlling slightly elevated blood pressure.
Introduction
The effects of milk protein-derived peptides isoleucine-proline-proline (IPP) and valine-proline-proline (VPP) on blood pressure have been reported in more than 20 randomized, placebo-controlled clinical studies. Since the first published data on inhibition of experimental renovascular hypertension in dogs by IPP containing nonapeptide 40 years ago (Citation1), dozens of experimental studies have shown antihypertensive and/or vascular effects of these peptides often administered in milk-based products in animal models of human hypertension (for review see (Citation2)). The main suggested mechanism is angiotensin-converting enzyme (ACE) 1 inhibition at the tissue level (Citation3–6). A recent study also reported inhibition of ACE expression by IPP/VPP in an animal model of hypertension (Citation7). A meta-analysis of nine randomized, placebo-controlled clinical studies on the effects of IPP and VPP on blood pressure was published in 2008 (Citation8). Since its publication, several new studies have been conducted and were included in the recent meta-analysis (Citation9). Daily peptide doses in the studies range widely from 2 to 52 mg/d and were consumed for 4 to 21 weeks in fermented milk products, juice, or tablets. Both meta-analyses showed a significant decrease in both systolic and diastolic blood pressure after consumption of the peptides. Effects were seen both in prehypertensive and stage 1 hypertensive subjects. A third meta-analysis (Citation10) confirmed these observations.
Although most clinical studies show an antihypertensive effect for IPP and VPP, a few studies from the Netherlands (Citation11–13) have reported conflicting results. Several possible explanations have been proposed for the discrepancy: size (weight) of study subjects, differences in habitual diet, inclusion criteria, selection of the control product, length of intervention period, and different methods used in blood pressure measurement.
All previous meta-analyses include studies with a high peptide intake (> 10 mg/day) (Citation8–10), which are not pertinent for functional food products due to the challenges they pose on the sensory properties of products and price. Therefore our aim was to conduct an analysis of studies with peptide doses applicable to commercially available functional food products, i.e. reporting effects of a daily dose of ≤ 10 mg IPP and VPP.
Materials and methods
Medline and PubMed were searched for clinical studies reporting the effects of IPP and VPP on blood pressure in prehypertensive and stage 1 hypertensive subjects, published 1996– October 2010. The search was performed using the terms: lactotripeptide* OR bioactive peptide* OR tripeptide* OR isoleucine-proline- proline OR IPP OR valine-proline-proline OR VPP OR fermented milk AND blood pressure OR hypertens*.
In addition, reference lists from published articles were gone through and a manual Internet search was conducted. When limited by publication type (clinical trial, randomized controlled trial) and English language, 39 articles were found. In addition, eight clinical trials were identified with a hand search.
The following eligibility criteria were used when selecting the studies: 1) Only randomized, placebo-controlled intervention studies were included; 2) Subjects should be defined as having high-normal blood pressure (prehypertension) or hypertension; 3) The effects of IPP and VPP or food products containing them on blood pressure should be reported; 4) The intervention should last at least 4 weeks; and 5) The daily dose of peptides should be ≤ 10 mg.
From each study, the following data were collected: 1) Authors, publication date, country; 2) Study population (sample size, age, gender); 3) Study design (blinding, randomization, treatment (dose, active and placebo product), length of intervention, method of blood pressure measurement); and 4) Results (change in blood pressure peptide versus placebo).
Results for home, office, or ambulatory blood pressure measurements were used in the analysis. In case multiple methods were used, the results for the outcome determined as primary were used. The end-point was defined as the end of the intervention (parallel designs) or the end of the first period (cross-over studies). If the primary outcome was not determined, the results for which complete data were presented were used. In case sufficient data were not available for any measurement, the author was contacted to obtain the required data. Not all authors contacted responded. Thus, measurements for which there were enough data to be used in the meta-analysis were included in the analysis.
In studies with multiple doses of peptides, the doses within the range 2–10 mg/day were included. In the study of Engberink et al. (Citation11), with three groups fed the same daily dose of peptides produced with different methods, the same placebo was used for all groups. One study, which investigated the synergistic effects of peptides and minerals (added potassium) on blood pressure, was excluded.
The effect of intervention was not estimated identically in the studies. Therefore the treatment effect is either mean difference in blood pressure change or mean difference in blood pressure values between the active and the control (placebo) groups/periods at the end of the intervention. In case neither one was reported in the article, the author was contacted and was requested to supply the data.
For cross-over trials, only the data from the first period were used in order to get more homogeneous studies with respect to the study design, to avoid carry-over effects, and to omit disturbances from withdrawals of the study persons, which often invalidate long-term intervention trials. A random effects model (StataCorp, College Station, TX, USA) was used for the analysis of the treatment effect (weighted mean difference) in systolic blood pressure and diastolic blood pressure. The random effect meta-analysis assumes that effects, which are estimated, are not identical in different studies but follow some distribution. The centre of this distribution describes the average of the effects and its width the degree of heterogeneity. In addition, Egger regression and its corresponding test for publication bias were performed.
Results
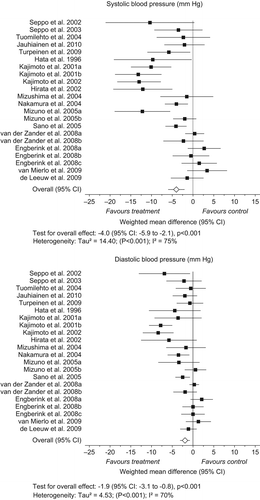
Nineteen randomized, placebo-controlled trials with 21 arms, which fulfilled the eligibility criteria, were found (). Sixteen studies were conducted in a parallel study design, and three were cross-over studies. Altogether 1532 prehypertensive or hypertensive, mostly middle-aged subjects were included in the studies.
Table I. Study characteristics.
Seventeen studies were double-blind, and two were single-blind. The interventions lasted for 4–21 weeks. The daily peptide dose ranged from 2.0 to 10.2 mg (IPP + VPP) and was incorporated into milk products, juice, spread (in one study), or tablets (in three studies). Nine of the published studies were conducted in Japan, five in Finland, four in the Netherlands, and one in Scotland.
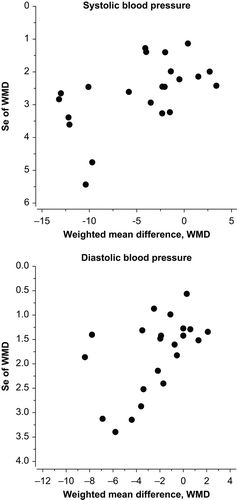
Most studies conducted in Japan and Finland show a clear decrease in blood pressure (peptides versus control), whereas a few done in the Netherlands and Scotland have shown no significant effect. Independently of that, the overall effect of IPP or VPP on systolic blood pressure was –4.0 mmHg (95% CI –5.9 to –2.1 mmHg, P < 0.001), with highly significant heterogeneity between studies (P < 0.001). The overall effect on diastolic blood pressure was –1.9 mmHg (95% CI –3.1 to –0.8 mmHg, P < 0.001) (), with highly significant heterogeneity between studies (P < 0.001). Funnel plots of systolic blood pressure (P = 0.035) and diastolic blood pressure (P = 0.039) () indicated signs of publication bias.
Discussion
Our meta-analysis consisting of 19 clinical studies with over 1500 subjects showed that food products containing IPP and VPP at low 2–10 mg daily doses found in commercial products significantly lower both systolic and diastolic blood pressure to a degree which is regarded clinically relevant and epidemiologically significant (Citation30,Citation31). The result is thus in line with the recent meta-analyses of Xu et al. (Citation8), Cicero et al. (Citation9), and Pripp (Citation10).
The meta-analysis of Xu et al. (Citation8) included 9 studies (12 trials) with 585 subjects who consumed daily 2.6 to 52.5 mg of IPP and VPP for 4–12 weeks. All studies included mostly middle-aged subjects of both genders. A significant decrease both in systolic (–4.8 mmHg, 95% CI –3.7 to –6.0 mmHg) and diastolic blood pressure (–2.2 mmHg, 95% CI –1.3 to –3.1 mmHg) was observed with peptide treatment when compared to placebo in prehypertensive and hypertensive subjects. The hypotensive effects increased with higher baseline blood pressure and longer duration of the intervention. However, no clear dose-response was found.
Cicero et al. (Citation9) conducted a meta-analysis of 18 randomized, placebo-controlled trials with altogether 1691 subjects. The length of interventions ranged from 4 to 21 weeks, with a mean duration of 6.8 weeks. Peptide doses of 2 to 52 mg/day were consumed in fermented milk products, juice, or tablets. The pooled effect of peptides was a reduction of –3.7 mmHg (95% CI –6.7 to –1.8 mmHg) in systolic blood pressure and –2.0 mmHg (95% CI –3.9 to –0.6 mmHg) in diastolic blood pressure. Exclusion of any one of the studies did not significantly affect the results. However, ethnicity of the subjects was a significant source of heterogeneity: the effects of lactotripeptides were more evident in Asian subjects than in Caucasians.
Pripp (Citation10) included 15 clinical trials with 826 subjects in the meta-analysis, of which 13 studies concerned milk-derived peptides. Casein-based lactotripeptides IPP and VPP were investigated only in nine of them. However, the stratified meta-analysis of the IPP and VPP trials showed a very similar result as the analysis of all studies, i.e. a –4.6 mmHg and a –2.2 mmHg decrease in systolic and diastolic blood pressure, respectively.
Contrary to the present meta-analysis, all previous meta-analyses conducted (Citation8–10) have included studies with high peptide doses (> 10 mg/day). Yet, the meta-analysis of Cicero et al. (Citation9) suggested no dose-dependency, indicating that no additional benefit on blood pressure is gained with higher doses. High doses are also not relevant for commercial food products due to the challenges they pose on the sensory properties of the product as well as price. Therefore, the present meta-analysis was conducted with doses relevant for functional foods. A few low-dose studies not included in the previous meta-analyses were also included. Significant heterogeneity between studies was identified, also within European studies (Finnish and Dutch). Nevertheless, country-specific analyses were not done, since they would result in small data sets with very limited power. The aim of this meta-analysis was to get an overview of the effects of low doses of peptides, applicable to functional food products.
Several possible explanations have been proposed for the discrepancy between studies conducted in the Netherlands and elsewhere, e.g. size (weight) of study subjects and differences in habitual diet, which might influence both the absorption of the peptides and their action at the target level. Other confounding factors would be the selection of the control product, different milk drinks with e.g. varying electrolyte contents, and different methods used in blood pressure measurement (). Furthermore, in some Dutch studies (Citation11,Citation27) blood pressure markedly decreased already during the run-in period, and thus at the beginning of the intervention period some of the subjects no longer fulfilled the inclusion criteria of being clearly hypertensive. The length of the intervention period might also be of importance. In an European trial where the treatment lasted only four weeks no antihypertensive effects were reported (Citation12). This agrees with many of the clinical and experimental studies where no clear effect in a month was seen (for review, see (Citation2)). An important observation was made by Seppo et al. (Citation19): when the peptide treatment was finished, and blood pressure was followed for four weeks, the blood pressure values returned to the pre-treatment level. This kind of follow-up period has not generally been included in the present intervention studies. An interesting theoretical question is the diversity of the production processes of the peptides, e.g. different bacterial or purified enzymes from different origin. Using modern computerized modelling of the tripeptides, four different spatial configurations and positions of the prolines (trans/cis) were revealed (Jarkko Valjakka, University of Tampere, personal communication). This naturally influences their penetration to and occupation of the possible target enzyme (e.g. ACE). The configurations of the peptides in the study products have not been analysed. Thus, the significance of this on the conflicting results is unknown.
Table II. Methods for blood pressure measurement.
Minerals present in milk (calcium, potassium) may contribute to the antihypertensive effect of milk products. However, in most studies, the placebo and tripeptide products contained similar amounts of minerals, and the effect of minerals would therefore be included in the placebo effect. According to a recent meta-analysis, a mean daily 1.2 g calcium supplementation decreases systolic blood pressure by 1.86 mmHg and diastolic blood pressure by 0.99 mmHg (Citation34). Respectively, a 3 g/day potassium supplementation decreased systolic and diastolic blood pressure by 3.1 and 2.0 mmHg in a meta-analysis by Whelton et al. 1997 (Citation35), whereas no significant reduction in blood pressure was detected in the meta-analysis of Dickinson et al. 2009 (Citation36). Thus, changes in mineral intake could have a small effect on the results in a few of the studies, but this does not explain the overall result.
Concerning the mechanism of the antihypertensive effect of the tripeptides, inhibition of angiotensin-converting enzyme (ACE) type 1 has been widely accepted (for review, see (Citation2)). Obviously the inhibition at tissue level (vascular wall) is more important than the inhibition of circulating ACE, and its development is slow in experimental animals due to retarded accumulation into the vasculature (Citation32).
Inhibition of ACE1, but not ACE2 (Citation5), does not alone seem to explain the beneficial vascular effects (vasodilatation, endothelial function) of IPP and VPP seen in animal experiments and in clinical studies for review, see (Citation2). These possible mechanisms include nitric oxide (e.g. inhibition of arginase) and endothelium-derived hyperpolarization factor (EDHF)-related mechanisms (for review, see (Citation2)) which might be involved in the antihypertensive effect of the tripeptides. A recent study (Citation7) also shows slightly reduced ACE expression after IPP/VPP treatment, which may contribute to the overall effect.
In conclusion, when all available data are taken into consideration, the results suggest that casein-derived peptides IPP and VPP have antihypertensive effects in subjects with elevated blood pressure. Thus, they may offer a valuable option as a non-pharmacological, nutritional treatment of prehypertension or mild hypertension as part of life-style recommendations.
Postscript
After the present meta-analysis was conducted, a new study reporting effects of 3 mg IPP/VPP in normotensive and prehypertensive subjects was published (Citation37). The study of Cicero et al. showed no effect for lactotripeptides in normotensive subjects, but a significant reduction in systolic blood pressure (–1.7 mmHg, P < 0.002), diurnal diastolic blood pressure (–1.6 mmHg, P < 0.042), and 24-hour diastolic blood pressure (–5.4 mmHg, P < 0.011) was seen in subjects with high-normal blood pressure.
Acknowledgements
We are grateful to Aino Siltari for her excellent technical help in preparing the manuscript.
Declaration of interest: Anu Turpeinen is an employee of Valio Ltd, Riitta Korpela was an employee of Valio Ltd until August 2010, and Heikki Vapaatalo was a consultant to Valio Ltd until December 2010.
References
- Miller ED Jr, Samuel AI, Haber E, Barger AC. Inhibition of angiotensin conversion in experimental renovascular hypertension. Science. 1972;177:1106–9.
- Jäkälä P, Vapaatalo H. Antihypertensive peptides from milk protein. Pharmaceuticals. 2010;3:251–72.
- Nakamura Y, Yamamoto N, Sakai K, Okubo A, Yamazaki S, Takano T. Purification and characterization of angiotensin I-converting enzyme inhibitors from sour milk. J Dairy Sci. 1995;78:777–83.
- Masuda O, Nakamura Y, Takano T. Antihypertensive peptides are present in aorta after oral administration of sour milk containing these peptides in spontaneously hypertensive rats. J Nutr. 1996;126:3063–8.
- Luhtala S, Vaajanen A, Oksala O, Valjakka J, Vapaatalo H. Activities of angiotensin-converting enzymes ACE1 and ACE2 and inhibition by bioactive peptides in porcine tissues. J Ocul Pharmacol Ther. 2009;25:23–8.
- Leo FD, Panarese S, Gallerani R, Ceci LR. Angiotensin converting enzyme (ACE) inhibitory peptides: production and implementation of functional food. Curr Pharmaceut Des. 2009;15:3622–43.
- Ehlers PI, Kivimäki AS, Turpeinen AM, Korpela R, Vapaatalo H. High blood pressure lowering and vasoprotective effects of milk products in experimental hypertension. Br J Nutr. 2011;106:1353–63.
- Engberink MF, Schouten EG, Kok FJ, van Mierlo LAJ, Brouwer IA, Geleijnse JM. Lactotripeptides show no effect in human blood pressure. Hypertension. 2008;51:399–405.
- van Mierlo LAJ, Koning MMG, van der Zander K, Draijer R. Lactotripeptides do not lower blood pressure in untreated whites: Results from 2 controlled multicenter crossover studies. Am J Clin Nutr. 2009;89:617–23.
- van der Zander K, Bots ML, Bak AAA, Koning MMG, de Leeuw PW. Enzymatically hydrolyzed lactotripeptides do not lower blood pressure in mildly hypertensive subjects. Am J Clin Nutr. 2008;88:1697–702.
- Xu J-Y, Qin L-Q, Wang P-Y, Li W, Chang C. Effects of milk tripeptides on blood pressure: A meta-analysis of randomized controlled trials. Nutrition. 2008;24:933–40.
- Cicero AFG, Gerocarni B, Laghi L, Borghi C. Blood pressure lowering effect of lactotripeptides assumed as functional foods: a meta-analysis of current available clinical trials. J Hum Hypertens. 2010;25:1–12.
- Pripp AH. Effect of peptides derived from food proteins on blood pressure: a meta-analysis of randomized controlled trials. Food Nutr Res. 2008;52. doi: 10.3402/fnr.v25i0.1641.
- Hata Y, Yamamoto M, Ohni M, Nakajima K, Nakamura Y, Takano T. A placebo-controlled study of the effect of sour milk on blood pressure in hypertensive subjects. Am J Clin Nutr. 1996;64:767–71.
- Kajimoto O, Aihara K, Hirata H, Takahashi R, Nakamura Y. Hypotensive effects of tablets containing “lactotripeptides (VPP, IPP)”. J Nutr Food. 2001;4:51–61.
- Kajimoto O, Nakamura Y, Yada H, Moriguchi S, Hirata H, Takahashi T. Hypotensive effects of sour milk in subjects with mild or moderate hypertension. J Jpn Soc Nutr Food Sci. 2001;54:347–54.
- Kajimoto O, Kurosaki T, Mizutani J, Nagisa I, Kaneko K, Aihara K, . Antihypertensive effects of liquid yoghurts containing “lactotripeptides (VPP, IPP)” in mild hypertensive subjects. J Nutr Food. 2002;5: 55–66.
- Hirata H, Nakamura Y, Yada H, Moriguchi S, Kajimoto O, Takahashi T. Clinical effects of new sour milk drink on mild or moderate hypertensive subjects. J New Rem Clin. 2002;51:61–9.
- Seppo L, Kerojoki O, Suomalainen T, Korpela R. The effect of a Lactobacillus helveticus LBK-16H fermented milk on hypertension—a pilot study on humans. Milchwissenschaft. 2002;57:124–7.
- Seppo L, Jauhiainen T, Poussa T, Korpela R. A fermented milk high in bioactive peptides has a blood pressure-lowering effect in hypertensive subjects. Am J Clin Nutr. 2003;77:326–30.
- Mizushima S, Oshige K, Watanabe J, Kimura M, Kadowaki T, Nakamura Y, . Randomized controlled trial of sour milk on blood pressure in borderline hypertensive men. Am J Hypertens. 2004;17: 701–6.
- Nakamura Y, Kajimoto O, Kaneko K, Aihara K, Mizutani J, Ikeda N, . Effects of the liquid yogurts containing “lactotripeptide (VPP, IPP)” on high normal blood pressure. J Nutr Food. 2004;7:123–37.
- Tuomilehto J, Lindström J, Hyyrynen J, Korpela R, Karhunen M-L, Mikkola L, . Effect of ingesting sour milk fermented using Lactobacillus helveticus bacteria producing peptides on blood pressure in subjects with mild hypertension. J Hum Hypertens. 2004;18:795–802.
- Mizuno S, Matsuura K, Gotou T, Nishimura S, Kajimoto O, Yabune M, . Antihypertensive effect of casein hydrolysate in a placebo-controlled study in subjects with high-normal blood pressure and mild hypertension. Br J Nutr. 2005;94:84–91.
- Sano J, Ohki K, Higuchi T, Aihara K, Mizuno S, Kajimoto O, . Effect of casein hydrolysate, prepared with protease derived from Aspergillus oruzae on subjects with high-normal blood pressure or mild hypertension. J Med Food. 2005;8:423–30.
- van der Zander K, Jäkel M, Bianco V, Koning MMG. Fermented lactotripeptides-containing milk lowers daytime blood pressure in high normal-to-mild hypertensive subjects. J Hum Hypertens. 2008;22: 804–6.
- de Leeuw PW, van der Zander K, Kroon AA, Rennenberg RMW, Koning MMG. Dose-dependent lowering of blood pressure by dairy peptides in mildly hypertensive subjects. Blood Press. 2009;18:44–50.
- Turpeinen AM, Kumpu M, Rönnback M, Seppo L, Kautiainen H, Jauhiainen T, . Antihypertensive and cholesterol-lowering effects of a spread containing bioactive peptides Ile-Pro-Pro and Val-Pro-Pro and plant sterols. J Funct Foods. 2009;1:260–5.
- Jauhiainen T, Rönnback M, Vapaatalo H, Wuolle K, Kautiainen H, Groop P-H, . Long-term intervention with Lactobacillus helveticus fermented milk reduces augmentation index in hypertensive subjects. Eur J Clin Nutr. 2010;64:424–31.
- Collins R, MacMahon S. Blood pressure, antihypertensive drug treatment and the risks of stroke and coronary heart disease. Br Med Bull. 1994:365:272–98.
- Krousel-Wood MA, Muntner P, He J, Whelton PK. Primary prevention of essential hypertension. Med Clin North Am. 2004:88: 223–38.
- Jauhiainen T, Wuolle K, Vapaatalo H, Kerojoki O, Nurmela K, Lowrie C, . Oral absorption, tissue distribution and excretion of radiolabelled analog of milk-derived antihypertensive peptide, Ile-Pro-Pro in rats. Int Dairy J. 2007;17:1216–23.
- Jauhiainen T, Rönnback M, Vapaatalo H, Wuolle K, Kautiainen H, Korpela R. Lactobacillus helveticus fermented milk reduces arterial stiffness in hypertensive subjects. Int Dairy J. 2007;17:1209–11.
- van Mierlo LAJ, Arends LR, Streppel MT, Zeegers MPA, Kok FJ, Grobbee DE, . Blood pressure response to calcium supplementation: a meta-analysis of randomized controlled trials. J Hum Hypertens. 2006;20:571–80.
- Whelton PK, He J, Cutler JA, Brancati FL, Appel LJ, Follmann D, . Effect of oral potassium on blood pressure. Meta-analysis of randomized controled clinical trials. JAMA. 1997;277:1624–32.
- Dickinson HO, Nicolson D, Campbell F, Beyer FR, Mason J. Potassium supplementation for the management of primary hypertension in adults. Cochrane Database Syst Rev. 2006;3:CD004641.
- Cicero AFG, Rosticci M, Veronesi M, Bacchelli S, Strocchi E, Melegari C, . Hemodynamic effects of lactotripeptides from casein hydrolysate in Mediterranean normotensive subjects and patients with high-normal blood pressure: a randomized, double-blind, crossover clinical trial. J Med Food. 2010;13:1363–8.