Abstract
Introduction. It is known that blood levels of natriuretic peptides associate with cognitive disorder among the middle-aged. We aimed to test whether this association is valid in an older population aged 75 years or older. Methods. A total of 601 older subjects aged 75 or older participated in the study. A subgroup of 137 with a diagnosed cognitive disorder were tested for natriuretic peptides (ANP, NT-proANP, and BNP), and compared with age-matched controls (n = 464). The control group was followed-up for 5 years, and the association of the baseline BNP with the occurrence of cognitive impairment was studied. Results. In the youngest age tertile (75–78 y), BNP was significantly associated with a diagnosed cognitive disorder when other factors with a known effect on natriuretic peptides were taken into account. In the oldest tertile (83–96 y), higher BNP values suggested the absence of cognitive dysfunction. ANP and NT-proANP did not associate with the presence of cognitive impairment. Among the control group, BNP predicted a cognitive disorder at follow-up, but only in the youngest tertile. Conclusions. The previously found link between a high BNP concentration and cognitive disorder in older people is only valid among those aged less than 79 years.
Key messages
The previously described association between natriuretic peptides and cognitive disorder alters with age.
BNP predicts cognitive disorder only in those aged less than 79 years.
Introduction
Traditional cardiovascular risk factors—such as hypertension high blood cholesterol level, obesity, smoking, and diabetes—in middle age have been associated with an increased risk of a cognitive disorder later in life (Citation1). However, there is a remarkable diversity in the impact of these risk factors on either existing or future cognitive dysfunction if the cardiovascular risk assessment is performed in an older population (Citation1,Citation2). This is an incentive to find new cardiovascular markers with a more consistent risk stratification capacity for cognitive decline.
Natriuretic peptides are hormones secreted from heart chambers in response to increased mechanical load and wall stretch (Citation3). In a cross-sectional study, elevated levels of the N-terminal fragment of proANP (NT-proANP), used as a functionally inactive surrogate for atrial natriuretic peptide (ANP), have been associated with Alzheimer's disease in relatively young patients (Citation4). In our recent study we showed that while traditional cardiovascular risk markers failed to predict cognitive decline in an older general population, B-type natriuretic peptide (BNP) was independently associated with a newly diagnosed cognitive disorder during follow-up (Citation5).
The primary objective of the present study was to evaluate whether the prognostic capacity of natriuretic peptides regarding cognitive disorder depends on age, as is the case regarding most cardiovascular risk factors. In addition, a systematic assessment of the levels of different natriuretic peptides in the main types of cognitive disorders has been lacking. Therefore, our secondary aim was to analyze the association of three natriuretic peptides (ANP, NT-proANP, and BNP) with three classes of cognitive disorders: Alzheimer's disease (AD), vascular dementia (VaD), and dementia with Lewy bodies (DLB).
Methods and materials
Study population
This study is a part of the larger population-based, multidisciplinary Kuopio 75 + health study. The target population was a stratified random sample (n = 700) of all residents of the city of Kuopio in eastern Finland who were aged 75 years or more on 1 January 1998 (n = 4518).
The cohort included 700 participants. Five persons could not be contacted, 79 refused to take part in the study, and 15 died before the examination. The remaining 601 participants attended a structured clinical examination and an interview conducted by a geriatrician and a trained nurse during the year 1998. As a part of the diagnostic process, brain imaging either by computer tomography (CT) or magnetic resonance imaging (MRI) was carried out for all participants with a suspicion of a cognitive disorder. A cognitive disorder was diagnosed by an experienced neurogeriatrician (R.S.) as Alzheimer's disease, vascular dementia, dementia with Lewy bodies, or dementia due to other medical conditions. The type of the dementia was determined in consensus meetings. A total of 64 patients were diagnosed with AD, 32 with VaD, 30 with DLB, and 11 with dementia due to other medical conditions. A more detailed description of the definitions is found elsewhere (Citation5).
The prognostic power of BNP on cognitive disorder at follow-up (of 5 years) was analyzed for participants with no cognitive disorder at baseline (n = 464). Of these, 303 attended the follow-up visit at 5 years. Of the 161 participants missing, 133 had expired during the study period, and 28 either refused to continue the study or could not be contacted. At the follow-up visit, the diagnostic procedure for cognitive dysfunction was repeated as described earlier.
Written informed consent was obtained from the study participants or their relatives as stipulated in the Declaration of Helsinki. The study was approved by the ethics committee of the Hospital District of Northern Savo and the Kuopio University Hospital.
Laboratory analyses
Basic blood count, creatinine, cholesterol profile, and fasting blood glucose were measured after a 12-hour fast with no interruption in the medication of the subjects. Glomerular filtration rate (GFR) was assessed using creatinine, age, and body weight according to the Cockcroft–Gault formula. The blood samples for the natriuretic peptide analysis were drawn after the patient had been in a supine position for 30 min at 8 a.m. as described in detail previously (Citation5).
Statistical analyses
General characteristics were calculated for participants divided into four groups based on the cognitive disorder diagnosis. For continuous variables, the differences between the groups were tested with one-way analysis of variance (ANOVA). The categorical variables were compared using the chi-square test. In addition, levels of natriuretic peptides are presented in three age tertiles for each cognitive disorder group. A multivariable logistic regression analysis was performed to determine the independent impact that each natriuretic peptide yielded on having a cognitive disorder. The following covariates were used: sex, age, systolic arterial pressure, a diagnosis of heart failure, and use of angiotensin-converting enzyme inhibitors or diuretics. In addition, a predefined interaction term between age and natriuretic peptide was included.
The independent predictive value of natriuretic peptides on future cognitive disorder in three age groups (tertiles 75–78 years, 79–82, and ≥ 83) was studied using a logistic regression model. Natriuretic peptide levels were highly skewed and therefore logarithmically transformed in analysis. An additional multivariable logistic regression model in each age group was constructed for the variables with a significant association to future cognitive disorder in our previous study (Citation5). Statistical differences were considered significant at P < 0.05. The data were analyzed using PASW release 17.0 for Windows (SPSS Inc., Chicago, IL, USA).
Results
General characteristics of the participants by cognitive disorder are presented in .
Table I. General characteristics of the participants by cognitive disorder.
General characteristics
The participants in the groups with a diagnosed cognitive disorder were slightly older than those with no diagnosed cognitive disorder. Females formed the majority in all four groups. The participants in the groups with a diagnosed cognitive disorder had lower systolic and diastolic blood pressure as well as lower cholesterol levels when compared to those not suffering from a cognitive disorder. BMI showed a tendency towards being lower in those with a diagnosed cognitive disorder, especially in the case of DLB. Participants with a diagnosed cognitive disorder had a relatively low median Mini-Mental State Examination (MMSE) score (of 14–15) in all diagnostic groups. A previous history of stroke, heart failure, or other cardiovascular disease was much more common in the VaD group than in the other groups.
Natriuretic peptides and cognitive disorder
BNP levels increased with age in participants with normal cognition (P < 0.001), but not among those with a cognitive disorder (P = 0.892) (). The result remained unchanged after excluding the participants who died within 12 months of the beginning of the study (P < 0.001 and P = 0.839). The levels of natriuretic peptides were similar in the four groups (). BNP and age, however, had a highly significant (P = 0.004) interaction in the logistic regression model where the presence of a cognitive disorder was the dependent factor. The study population was divided into age group tertiles for further analysis: 75–78 years (n = 235), 79–82 years (n = 175), and 83 years or older (n = 191). In the youngest tertile, there was a significant positive association between BNP levels and diagnosed cognitive disorder in the adjusted logistic regression model (). In the middle tertile, there was no connection between BNP and cognitive disorder, while the oldest tertile presented an opposite trend: BNP levels were higher among those with no diagnosed cognitive disorder (P = 0.05) in the regression model. ANP and NT-proANP showed no association with the presence of cognitive disorder when tested in the whole study population or the different age tertiles in a similar fashion.
Figure 1. Levels of BNP (with SD error bars) for groups with and without dementia by age tertile. The P values were calculated with analysis of variance.
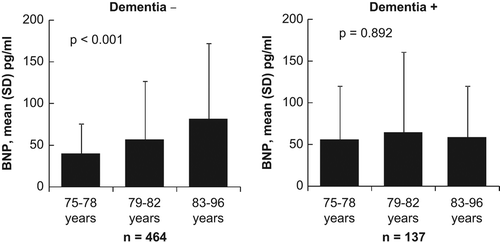
Table II. Blood levels of natriuretic peptides by cognitive disorder.
Table III. Cross-sectional multivariable logistic regression analysis presenting SD-adjusted odds ratios of natriuretic peptides for their association with a cognitive disorder in each age tertile.
Elevated BNP is known to increase mortality. This has the potential to distort the present results by eliminating the patients with high BNP from the older age groups. We dealt with the issue by excluding patients who died within the first 12 months after the first visit and repeating the adjusted logistic regression analysis regarding the association between BNP and cognitive disorder. The link remained on the same level with hazard ratios of 1.54 in the youngest tertile (confidence interval (95% CI) 1.03–2.29, P = 0.035), 1.13 in the middle tertile (95% CI 0.72–1.75, P = 0.607), and 0.78 in the oldest tertile (95% CI 0.59–1.08, P = 0.133).
We further analyzed the impact of BNP on cognitive disorder during the follow-up. A total of 60 participants were diagnosed with cognitive disorder at the follow-up visit. The association was present only in the youngest age group (RR 1.83, 95% CI 1.06–3.16, P = 0.027), while in the middle group (79–82 years) there was a trend towards higher BNP among those with a cognitive disorder during the follow-up (RR 1.44, 95% CI 0.85–2.43, P = 0.17). In the oldest group (83 years and older), the association was no longer present (RR 1.11, CI 0.60–2.06, P = 0.75). In multivariable regression analysis including other significantly associated variables (length of education, age, hypertension), the results were parallel (the youngest group: RR 1.89, CI 1.07–3.34, P = 0.028; the middle group: RR 1.81, CI 0.97–3.39, P = 0.062; the oldest group: RR 0.983, CI 0.48–2.01, P = 0.966). The prognostic power of NT-proANP and ANP in similar settings was clearly inferior to BNP (data not shown).
Discussion
The main finding of the present study was that the association of BNP with cognitive disorder changes with aging. In the youngest age tertile (75–78 years), elevated BNP was connected to impaired cognition, while the oldest participants (83–96 years) had lower BNP in conjunction with cognitive disorder. In parallel, in the population free of cognitive disorder at baseline, the previously reported association between BNP and cognitive disorder was present at follow-up only in the age group 75–78 years.
Previous studies
BNP has been reported to have a cross-sectional association with cognitive dysfunction in heart failure patients aged 65.5–70 years (n = 56–60) (Citation6,Citation7). In one study with an even smaller group of patients with cognitive disorder (n = 42) but no cardiovascular disease, BNP levels were linked to cognitive function, whereas the levels of ANP were not (Citation8). In another study, BNP was elevated among patients with subcortical VaD (mean age 69 years, n = 15) but not among age-matched controls (mean age 69 years, n = 19) or patients with AD (mean age 71 years, n = 19); the finding suggested the largest vascular burden in the subcortical VaD group (Citation9). Increased levels of NT-proANP have been found in patients with AD (mean age 73.9 years, n = 94) when compared to healthy controls (mean age 65.5 years, n = 53) (Citation4). In that study, higher mean age and blood pressure in the AD group may explain the difference in the levels of natriuretic peptides (Citation10).
The predictive value of natriuretic peptides on cognitive decline has been addressed only in few prospective studies. In our own investigation, BNP was an independent predictor of future cognitive disorder among older people when cardiovascular risk factors were taken into account (Citation5). The present analysis revealed that the significant predictive power of BNP on cognitive disorder prevailed in the youngest age group, 75–78 years, while the association was absent in older groups. Recently, it was demonstrated that NT-proANP predicted the progression of mild cognitive impairment into AD in 134 persons below the age of 72 years (Citation11). However, NT-proANP concentrations did not predict the conversion of mild cognitive impairment to AD in patients older than 72 years.
The present investigation
The predictive power of traditional cardiovascular risk factors alters with age (Citation1), and, similarly, the impact of blood levels of BNP appears to change when it comes to indicating cognitive dysfunction in a cross-sectional setting or as a prognostic tool for cognitive disorder among non-demented individuals. In the present age group of 75–78 years, BNP was significantly connected to a diagnosed cognitive disorder. This trend was reversed in the oldest age group, among whom high BNP levels were linked to a lower prevalence of cognitive disorders. This age-related change in the association between BNP and cognitive disorder seemed to be explained by the elevation of BNP among non-demented individuals () as the BNP concentration among the patients with cognitive disorder remained at the same level regardless of the age group.
The elevation of natriuretic peptides with aging has been previously reported in several studies (Citation10,Citation12,Citation13). Renal dysfunction and left ventricular dysfunction as well as other cardiovascular diseases all increase the levels of natriuretic peptides (Citation3). These conditions have been suggested as the reason for the elevation in natriuretic peptides among older people (Citation12,Citation13). However, the effect of age on BNP levels is not completely explained by a known co-morbidity, advocating the involvement of a mechanism not yet defined (Citation10). The present results suggest that the process leading to cognitive impairment may also decrease the levels of BNP in a way that is not directly linked to previously recognized factors modulating BNP.
Natriuretic peptides
In the present study, BNP was superior in its connection to cognitive disorder when compared to ANP or NT-proANP. The related reasons are speculative. The biological effects of ANP and BNP are mediated by a common receptor, NPR-A (Citation14), rendering the effects qualitatively similar. They are both natural antagonists of the renin-angiotensin-aldosterone system and serve as regulators of salt and fluid balance. In addition, they inhibit the sympathetic nervous system as well as the pathophysiological mechanisms leading to ventricular and vascular hypertrophy and remodeling. The plasma levels of all these natriuretic peptides are equally affected by age and sex (Citation15–17).
In the healthy state, ANP and NT-proANP are secreted mainly from the atria of the heart, whereas BNP is secreted from both atria and ventricles (Citation18). In the context of heart failure, BNP has outperformed ANP as a marker for cardiac abnormalities and mortality (Citation15,Citation19). Generally, BNP has also been slightly superior to NT-proANP as a diagnostic and prognostic marker (Citation20–22), although there are contradictory findings (Citation15,Citation22,Citation23). The differences in clearance between the natriuretic peptides, and possibly the more direct association of the secretion of BNP with changes in cardiac gene expression as opposed to ANP (Citation18), might better reflect the burden on the cerebral vasculature and explain the superior performance of BNP in the present study.
Study limitations, strengths, and conclusions
The number of participants diagnosed with VaD or DLB was low, which decreased the power to detect a significant difference between the cognitive disorder groups. However, the absolute values for natriuretic peptides were relatively close to each other, suggesting that potential differences would not be clinically important. A second limitation was that our study was carried out in an exclusively Caucasian population, limiting the generalizability. The strengths of our study include the population-based approach, the extensive characterization of the study population, the careful diagnostics and classification of cognitive disorders, and the small dropout rate during follow-up. The total number of the present participants with diagnosed cognitive impairment is superior to earlier studies on natriuretic peptides and cognitive dysfunction.
In conclusion, the previously found link between a high BNP concentration and cognitive dysfunction is age-dependent. In the oldest segment of the general population, elevated BNP levels are associated with a lower prevalence of cognitive disorders.
Declaration of interest: The authors report no conflicts of interest. This work was supported by Hilja and Onni Tuovinen Founda- tion and the P ij t-H me Regional Fund of the Finnish Cultural Foundation.
References
- Kerola T, Kettunen R, Nieminen T. The complex interplay of cardiovascular system and cognition: How to predict dementia in the elderly? Int J Cardiol. 2011;150:123–9.
- Kloppenborg RP, van den Berg E, Kappelle LJ, Biessels GJ. Diabetes and other vascular risk factors for dementia: which factor matters most? A systematic review. Eur J Pharmacol. 2008;585:97–108.
- Vuolteenaho O, Ala-Kopsala M, Ruskoaho H. BNP as a biomarker in heart disease. Adv Clin Chem. 2005;40:1–36.
- Buerger K, Ernst A, Ewers M, Uspenskaya O, Omerovic M, Morgenthaler NG, . Blood-based microcirculation markers in Alzheimer's disease-diagnostic value of midregional pro-atrial natriuretic peptide/C-terminal endothelin-1 precursor fragment ratio. Biol Psychiatry. 2009;65:979–84.
- Kerola T, Nieminen T, Hartikainen S, Sulkava R, Vuolteenaho O, Kettunen R. B-type natriuretic peptide as a predictor of declining cognitive function and dementia—a cohort study of an elderly general population with a 5-year follow-up. Ann Med. 2010;42:207–15.
- Feola M, Rosso GL, Peano M, Agostini M, Aspromonte N, Carena G, . Correlation between cognitive impairment and prognostic parameters in patients with congestive heart failure. Arch Med Res. 2007;38:234–9.
- Gunstad J, Poppas A, Smeal S, Paul RH, Tate DF, Jefferson AL, . Relation of brain natriuretic peptide levels to cognitive dysfunction in adults > 55 years of age with cardiovascular disease. American J Cardiol. 2006;98:538–40.
- Naito J, Naka Y, Watanabe H. Clinical impression of brain natriuretic peptide levels in demented patients without cardiovascular disease. Geriatr Gerontol Int. 2009;9:242–5.
- Kondziella D, Gothlin M, Fu M, Zetterberg H, Wallin A. B-type natriuretic peptide plasma levels are elevated in subcortical vascular dementia. Neuroreport. 2009;20:825–7.
- Redfield MM, Rodeheffer RJ, Jacobsen SJ, Mahoney DW, Bailey KR, Burnett JC Jr. Plasma brain natriuretic peptide concentration: impact of age and gender. J Am Coll Cardiol. 2002;40:976–82.
- Buerger K, Uspenskaya O, Hartmann O, Hansson O, Minthon L, Blennow K, . Prediction of Alzheimer's disease using midregional proadrenomedullin and midregional proatrial natriuretic peptide: a retrospective analysis of 134 patients with mild cognitive impairment. J Clin Psychiatry. 2011;72:556–63.
- Luchner A, Burnett JC Jr, Jougasaki M, Hense HW, Heid IM, Muders F, . Evaluation of brain natriuretic peptide as marker of left ventricular dysfunction and hypertrophy in the population. J Hypertens. 2000;18:1121–8.
- Sayama H, Nakamura Y, Saito N, Kinoshita M. Why is the concentration of plasma brain natriuretic peptide in elderly inpatients greater than normal? Coron Artery Dis. 1999;10:537–40.
- Potter LR, Yoder AR, Flora DR, Antos LK, Dickey DM. Natriuretic peptides: their structures, receptors, physiologic functions and therapeutic applications. Handb Exp Pharmacol. 2009:341–66.
- Alehagen U, Svensson E, Dahlstrom U. Natriuretic peptide biomarkers as information indicators in elderly patients with possible heart failure followed over six years: a head-to-head comparison of four cardiac natriuretic peptides. J Card Fail. 2007;13:452–61.
- Bhandari SS, Davies JE, Struck J, Ng LL. Influence of confounding factors on plasma mid-regional pro-adrenomedullin and mid-regional pro-A-type natriuretic peptide concentrations in healthy individuals. Biomarkers. 2011;16:281–7.
- Loke I, Squire IB, Davies JE, Ng LL. Reference ranges for natriuretic peptides for diagnostic use are dependent on age, gender and heart rate. Eur J Heart Fail. 2003;5:599–606.
- Ruskoaho H. Cardiac hormones as diagnostic tools in heart failure. Endocr Rev. 2003;24:341–56.
- Abassi Z, Karram T, Ellaham S, Winaver J, Hoffman A. Implications of the natriuretic peptide system in the pathogenesis of heart failure: diagnostic and therapeutic importance. Pharmacol Ther. 2004;102: 223–41.
- Vasan RS, Benjamin EJ, Larson MG, Leip EP, Wang TJ, Wilson PW, . Plasma natriuretic peptides for community screening for left ventricular hypertrophy and systolic dysfunction: the Framingham heart study. JAMA. 2002;288:1252–9.
- McDonagh TA, Robb SD, Murdoch DR, Morton JJ, Ford I, Morrison CE, . Biochemical detection of left-ventricular systolic dysfunction. Lancet. 1998;351:9–13.
- Yamamoto K, Burnett JC Jr, Jougasaki M, Nishimura RA, Bailey KR, Saito Y, . Superiority of brain natriuretic peptide as a hormonal marker of ventricular systolic and diastolic dysfunction and ventricular hypertrophy. Hypertension. 1996;28:988–94.
- Ala-Kopsala M, Ruskoaho H, Leppaluoto J, Seres L, Skoumal R, Toth M, . Single assay for amino-terminal fragments of cardiac A- and B-type natriuretic peptides. Clin Chem. 2005;51:708–18.
