Abstract
Introduction. Levels of nitric oxide metabolites are elevated in the cervical fluid of women with high-risk human papillomavirus (hrHPV). To elucidate the origin of this elevation we studied the cervical expression and localization of endothelial and inducible nitric oxide synthases (eNOS, iNOS) in women. Material and methods. Expression of eNOS and iNOS was studied by Western blotting in the uterine cervixes of 86 women with (n = 41) and without (n = 45) hrHPV infection. The localization of eNOS and iNOS in cervical cells was studied by immunohistochemistry in 32 randomly selected women. Results. Expression of eNOS and iNOS (in mean [95% CI] density units relative to actin) was higher in women with hrHPV versus those without (eNOS: 33.8 [22.5–45.1] versus 20.2 [6.1–34.3], P = 0.007; iNOS: 12.0 [7.1–16.9]) versus 5.6 [2.0–9.2], P = 0.003). Smoking reduced 64% eNOS (P = 0.001) and 68% iNOS (P = 0.008) in women with hrHPV. Endothelial NOS was localized in the vascular endothelium, while iNOS was present in basal squamous epithelial cells. Low-grade histological lesions were accompanied by elevated expression of both eNOS and iNOS. Conclusions. High-risk HPV-associated elevation in cervical fluid nitric oxide metabolites results from both eNOS and iNOS stimulation. However, smoking seems to suppress this stimulation in hrHPV-infected women.
Key messages
Infection with hrHPV induces expression of eNOS and iNOS in human uterine cervix.
Smoking may reduce expression of eNOS and iNOS in the uterine cervix in hrHPV-infected women.
Introduction
The presence of persistent high-risk human papillomavirus (hrHPV) is an important precursor of cervical cancer, but co-factors are needed for carcinogenesis (Citation1). Nitric oxide (NO) regulates various physiological and pathophysiological conditions (Citation2), and thus NO may work as one such co-factor, since HPV-infected women have increased levels of NO metabolites in their cervical fluid (Citation3–5). Moreover, elevated levels of NO metabolites in the cervical fluid may predict persistent hrHPV (Citation5). It is known that various cervical cells express both endothelial (e) (Citation2,Citation6) and inducible (i) (Citation6–8) NO synthase (NOS). However, only iNOS activity has been shown to be associated with HPV (Citation7,Citation8). Therefore, using Western blot analyses, we studied the quantitative expression of eNOS and iNOS in women with and without hrHPV. Furthermore, we used immunohistochemistry to detect the cells in which these proteins are expressed.
Material and methods
With the permission of the local Ethics Committee, between November 2007 and May 2008 we investigated 86 women, of whom 41 were referred to colposcopy according to national guidelines (Citation9) and 45 were studied in association with gynecological surgical procedures (endometrial polyps, n = 1; ovarian cysts, n = 2; diagnostic laparoscopy, n = 4; sterilization, n = 10; hysterectomy, n = 28). From those 41 women referred to colposcopy 24 women had had repetitive low-grade cytological abnormalities within the previous 2 years, and 17 women had had high-grade cytological abnormalities within the previous 3 months. From women who underwent surgical procedures Pap smears, taken from 1 month to 2 years earlier, were normal in 42 women but showed low-grade cytological changes in 3 women. All women were informed of the study protocol and signed a consent document. Pregnant women and those under 18 years of age were excluded from the study.
Chlamydia trachomatis, Trichomonas, and Candida were not routinely screened for, but diagnostic samples were taken if indicated on clinical grounds (n = 3); no woman included was judged to harbor these infections. All women were tested for the presence of 13 hrHPV types (types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68) by means of the AMPLICOR HPV test (Roche Molecular Systems, Inc., Branchburg, New Jersey, USA) according to the instructions of the manufacturer.
Cervical biopsy samples (1–4 for diagnosis and/or 2 for research purposes) were collected at colposcopy at sites stained positively with acetic acid, or before a surgical procedure, from the squamocolumnar junction at 6 and 12 o'clock. One research biopsy sample was snap-frozen in liquid nitrogen and stored at –80°C for subsequent Western blotting, and the other one was fixed in 10% formalin, dehydrated, and embedded in paraffin for immunohistochemistry. Histology was studied in all women attending colposcopy (n = 41), but from women who underwent surgical procedures (n = 45) it was assessed only in women with hysterectomy (n = 28). Histological findings (n = 69) were classified as normal, CIN 1, or ≥ CIN 2.
Western blot analysis
Western blot analysis was carried out to assess the expression of eNOS and iNOS proteins in the cervical tissue samples. The frozen biopsy samples were crushed and homogenized with Precellys 24 (Bertin Technologies, Villeurbanne, France) in buffer containing 50 mM Tris-HCl, pH 8.0, 150 mM sodium chloride, 1.0% Igepal CA-630 (NP-40), 0.5% sodium deoxycholate, and 0.1% sodium dodecyl sulfate (Sigma-Aldrich, St Louis, MO, USA). The samples were clarified by centrifugation and stored at –140°C until used. Total protein concentrations were quantified by using Bio-Rad Protein Assays (Bio-Rad Laboratories, Hercules, CA, USA). Samples containing 25 µg of protein were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis at 150 V for 1 h (3%–8% Tris-acetate SDS-PAGE gel; Invitrogen, Carlsbad, CA, USA), and the proteins were transferred to a polyvinylidene fluoride membrane (PVDF), pore size 0.45 µm, 15 V for 35 min, by semi-dry blotting. The membranes were blocked in 3% bovine serum albumin (BSA) (Sigma) at room temperature for 1 h. Primary antibody incubations (mouse monoclonal eNOS/NOS Type III, 1:2500 and iNOS/NOS Type II, 1:10000; BD Transduction Laboratories, Franklin Lakes, NJ, USA) were carried out at +4°C overnight. Polyclonal rabbit anti-mouse immunoglobulins coupled to horseradish peroxidase (DAKO, Glostrup, Denmark) were used as secondary antibodies. Specific signals were detected by means of an enhanced chemiluminescence system (ECL Plus; Amersham GE Healthcare, Little Chalfont, England) according to the manufacturer's instructions and visualized by exposure to ECL hyperfilm (Amersham). Signal intensity was quantified by densitometry (Syngene; Synoptics Limited, Cambridge, UK).
Human umbilical vein endothelial cell (HUVEC) lysates (Biomedicum Helsinki, Finland) and lysates of interferon-γ/LPS-treated mouse macrophages (Transduction Laboratories) were used as positive controls for eNOS and iNOS, respectively (Citation10–13). Actin (C-2, 1:750; Santa Cruz Biotechnology, Santa Cruz, CA, USA) was used as a protein-loading control.
A total of 62 samples (72%; 32 with hrHPV and 30 without) had enough protein (25 µg) for Western blot analysis.
Immunohistochemistry
Thirty-two samples were immunostained to localize eNOS and iNOS proteins in the cervical cells. The samples were randomly selected from subgroups of women with and without hrHPV and with different histological findings.
Briefly, paraffin sections (5 µm) were deparaffinized and pretreated by heating in a microwave oven in 0.01 M citric acid buffer (pH 6.0) for antigen retrieval. Next, immunohistochemistry was carried out with the help of a LabVision autostainer (LV-1; Thermo Fisher Scientific, Inc., Fremont, CA, USA) and a PowerVision+TM Poly-HRP IHC Detection System (Leica Biosystems Newcastle Ltd, UK) according to the manufacturers’ instructions. The sections were sequentially incubated with 3% hydrogen peroxide, pre-blocking solution, primary antibodies (polyclonal rabbit antibodies for eNOS and iNOS; Thermo Fisher Scientific; 1:400, RT 60 min), post-blocking solution, and poly-HRP IgG polymer for post-blocking. Tris-buffered saline (TBS) with Tween, or aqua, was used for washing. Antigens were localized by using diaminobenzidine tetrahydrochloride (DAB) plus substrate. The sections were counterstained with Mayer's hematoxylin solution (Merck KGaA, Darmstadt, Germany), rinsed with aqua, and manually mounted.
Sections of umbilical cord were used as positive controls for both eNOS and iNOS (Citation14). Negative control measures included replacing primary antibody with rabbit IgG (Negative Control for Rabbit IgG Ab-1; Thermo Fisher Scientific) and slides incubated without primary antibody.
The degree of staining throughout the whole epithelium was semi-quantitatively assessed by two readers (H.S.-P., R.B.) who were blind to the identity of the slides. The intensity of immunostaining was graded as follows: – = no visible staining; (+) = very faint staining; + = weak staining; + + = moderate staining; +++ = intense staining.
Statistical methods
Categorical data were analyzed by means of chi-square or Fisher's exact tests. The NOS data, given in numbers representing arbitrary units relative to actin, × 102, and expressed as mean ± SE with a 95% confidence interval (95% CI), were not normally distributed and were thus tested by means of the Mann–Whitney U test, or by means of the Kruskal–Wallis test with Bonferroni correction. Correlations were assessed by calculating Spearman's correlation coefficient (r). The tests were carried out by using PASW 18.0 for Windows, Chicago, IL. Probability values of < 0.05 were considered statistically significant.
Results
Of the 86 women, 41 (48%) showed the presence of hrHPV, whereas 45 (52%) did not. The women with hrHPV were younger (P < 0.001), more often used oral contraception (P = 0.04), and more often had abnormal cervical histology (P < 0.001) than the women without hrHPV ().
Table I. Clinical characteristics of the women studied.
In Western blot analyses eNOS was detectable in 60/62 (97%) women and iNOS in 46/62 (74%) women. No difference was observed in detection of eNOS between women with and without hrHPV (32/32 [100%] versus 28/30 [94%], P = 0.2), whereas iNOS was more often detectable in women with hrHPV than in women without it (28/32 [88%] versus 18/30 [60%], P = 0.01).
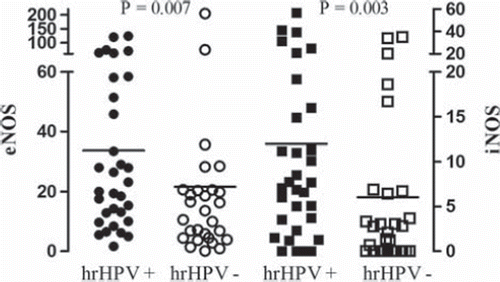
In women with hrHPV, the expression of both eNOS and iNOS was higher than in women without hrHPV (eNOS: 33.8 ± 5.6 [95% CI 22.5–45.1] versus 20.2 ± 6.8 [95% CI 6.1–34.3], P = 0.007; iNOS: 12.0 ± 2.4 [95% CI 7.1–16.9] versus 5.6 ± 1.8 [95% CI 2.0–9.2], P = 0.003) ( and ).
Figure 1. A representative illustration of eNOS and iNOS protein expression (Western blotting) in cervical samples from women with (+) and without (–) hrHPV. Tissue homogenates (containing 25 µg of total protein) were loaded, separated by gel electrophoresis, and immunoblotted with specific monoclonal antibodies to eNOS and iNOS. Signal intensity was quantified by densitometry and normalized to that of β-actin (loading control).
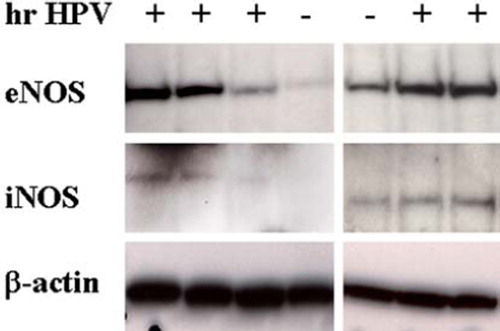
Figure 2. Protein levels of endothelial and inducible nitric oxide synthase (eNOS, iNOS) determined by Western blotting in women with (+) and without (–) high-risk human papillomavirus (hrHPV). The NOS scales (left, eNOS; right, iNOS) represent density in arbitrary units relative to actin. The horizontal lines show the means of the values.
The levels of eNOS and iNOS proteins were positively correlated (r = 0.6, P < 0.001). In subgroups divided according to hrHPV status significant correlations between protein levels of eNOS and iNOS were also seen (with hrHPV: r = 0.5, P = 0.002; without hrHPV: r = 0.7, P < 0.001).
Smoking was associated with decreased levels of eNOS and iNOS proteins among hrHPV-infected women (P = 0.001, P = 0.008) (). In non-smokers, elevated levels of eNOS and iNOS were associated with hrHPV (P < 0.001, P < 0.001) ().
Table II. Expression of endothelial and inducible nitric oxide synthase (eNOS, iNOS) in smokers and non-smokers according to high-risk human papillomavirus (hrHPV) status. The values represent density in arbitrary units relative to actin (mean × 102 ± SE (95% CI)) as assessed by Western blotting.
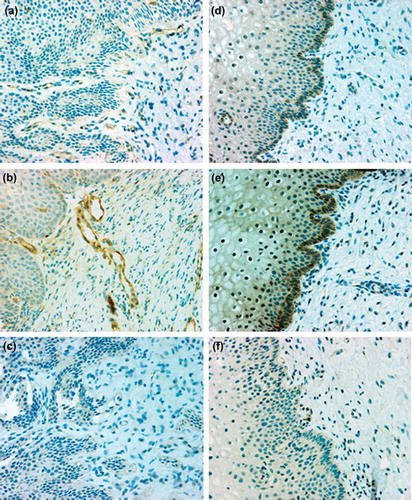
In immunohistochemistry the positive control slides showed localization of eNOS in vascular endothelium and iNOS in smooth muscle and some inflammatory cells, whereas no immunostaining for either eNOS or iNOS was seen in the negative controls. In cervical biopsy samples eNOS was consistently localized in the vascular endothelium (), but the staining intensity did not differ between women with and without hrHPV. Inducible NOS was consistently localized in basal squamous epithelial cells (), and its staining intensity was higher (P = 0.04) in women with hrHPV than in those without. No differences were observed in iNOS staining intensity between different histological types. In hrHPV-infected women iNOS was also detected in parabasal (5/32) and suprabasal (3/32) squamous epithelial cells. Two women with and two without hrHPV showed iNOS in squamous cells with immature metaplasia. Inflammatory cells were detected in 11/32 samples (9 with hrHPV, 2 without), and in only 3 hrHPV-positive samples were a few iNOS-positive inflammatory cells detected.
Figure 3. Immunohistochemical localization of endothelial nitric oxide synthase (eNOS) (left panels, a–c) and inducible nitric oxide synthase (iNOS) (right panels, d–f) in cervical specimens from women without (a, d) and with (b, e) high-risk human papillomavirus. Endothelial NOS was localized to the vascular endothelium and iNOS mainly to the basal layer of epithelial cells. Specimens incubated with irrelevant antibody (IgG) showed no immunoreactivity (c, f). Hematoxylin counterstain, × 200 magnification.
Independent of hrHPV status, in Western blot analyses the women with CIN 1 showed higher protein levels of eNOS and iNOS compared with those in women with normal histology (P = 0.002, P = 0.01, respectively) (). Smoking was not a factor in this regard.
Table III. Expression of endothelial and inducible nitric oxide synthase (eNOS, iNOS) in women with different histological findings. The levels represent density in arbitrary units relative to actin (mean × 102 ± SE (95% CI)) as assessed by Western blotting. Comparisons are made to women with normal histology.
Discussion
We report here that hrHPV infection is accompanied by elevated expression of eNOS and iNOS in cervical cells. Our data on iNOS induction are in line with previous data (Citation6,Citation7), but to the best of our knowledge eNOS expression in relation to hrHPV is a novel finding.
It is known that hrHPV infects basal squamous epithelial cells (Citation15,Citation16). However, hrHPV-induced eNOS expression was not seen in these cells but in the vascular endothelium. It is possible that hrHPV induces epithelial cells (Citation17) and/or macrophages (Citation8,Citation18,Citation19) to produce angiogenic mediators, such as vascular endothelial growth factor (Citation20–22), which thereafter could induce increased expression of eNOS in vascular endothelium. In contrast, iNOS was located in the basal squamous epithelial cells, and thus it was probably induced directly by hrHPV. It is also possible that iNOS production was triggered by hrHPV-related cytokines, such as interleukins and interferon-γ (Citation23,Citation24); our data do not allow us to differentiate between these explanations, and both of them may be valid. Moreover, it is known that hrHPV-infected basal cells migrate toward the surface of epithelium (Citation15,Citation16), and this may explain why parabasal and suprabasal epithelial cells also stained for iNOS. Interestingly, inflammatory cells were seen only in some biopsy samples, and a few of them showed iNOS expression. This is in contrast to the results of earlier studies in which iNOS has been detected abundantly in inflammatory cells of hrHPV-associated cervical lesions (Citation7,Citation8). The explanation for this discrepancy may be the phase of hrHPV infection—inflammatory cells may not be present in the early stages of infection (Citation15,Citation16). Nevertheless, because of the low number of inflammatory cells it is unlikely that non-specific inflammation, rather than hrHPV, was solely responsible for eNOS and iNOS activation.
Smoking was associated with decreased expression of eNOS and iNOS in hrHPV-infected women. We could not find any previous data on cervical NOSs and smoking, but it is known that exhaled NO levels are decreased in smokers as compared with non-smokers (Citation25). Moreover, when exposed to tobacco extract, pulmonary arterial endothelial cells, mast cells, and airway epithelial cells show decreased eNOS and iNOS mRNA and protein levels (Citation26–28). It is possible that tobacco components also affect the expression of cervical NOSs, since nicotine and cotinine, for example, become concentrated in cervical mucus (Citation29). The clinical meaning of this finding remains open, but since it is known that smoking is associated with HPV persistence (Citation30), it is possible that stimulated eNOS and iNOS proteins in non-smokers were related to immune defense. Moreover, it is known that the possible eNOS and iNOS-mediated carcinogenic effects depend on concentration; high levels suppress, while low ones promote cancer (Citation31). Therefore, smoking associated with low eNOS and iNOS expression could favor cervical carcinogenesis. However, from our study population it is impossible to evaluate eNOS and iNOS carcinogenic effects, since none of the women studied had cancer.
We can only speculate on the significance of hrHPV-induced eNOS and iNOS expression in cervical lesions. In addition, we do not know whether eNOS and iNOS have separate functions or if they interact with each other. However, our data provide some evidence that in cervical lesions both eNOS and iNOS are stimulated, although we cannot deduce if this reflects only the presence of hrHPV, or if eNOS and iNOS contribute to progression of the lesion. In one study iNOS was associated with high-grade cervical lesions (Citation7), whereas the present study and another one (Citation8) showed elevated iNOS to be associated with low-grade lesions. This stimulation in low-grade lesions could be a sign of active host immunity, since such lesions most often regress spontaneously (Citation32). Further prospective studies are needed to give definitive answers in connection with the roles of eNOS and iNOS in hrHPV infection and associated cell lesions.
A limitation of our study that we should mention is that we assessed the presence of only 13 hrHPV types, and we could not distinguish different viral genotypes. However, these hrHPV types are the most pathogenic, and they can all cause cervical cancer (Citation33). Nevertheless, we admit that a test differentiating viral genotypes would have been more valuable. We also acknowledge that a larger population of women, especially with high-grade lesions, could have been more informative. We consider that strengths of our study are our methods to assess both eNOS and iNOS concomitantly, and the localization of these proteins in different cells.
In conclusion, cervical hrHPV infection induces expression of eNOS and iNOS in the human uterine cervix, in either the vascular endothelium or basal squamous epithelial cells. However, smoking seems to suppress both eNOS and iNOS expression in hrHPV-infected women. Prospective studies are needed to clarify the clinical significance of these findings.
Declaration of interest: This work was supported by the Clinical Research Fund of Helsinki University Central Hospital (grant number TYH2008261), National Graduate School of Clinical Investigation, the Research Foundation of Instrumentarium Corporation, the Research Foundation of Orion Corporation, the Finnish Cultural Foundation, the Finnish Medical Foundation, the Finnish Society against Sexually Transmitted Diseases, the Finnish-Norwegian Medical Research Foundation, the Maud Kuistila Memorial Foundation, the Finnish Gynaecological Society, and the Society of Gynaecological Surgery in Finland.
The authors declare that there are no conflicts of interest. Päivi Rahkola-Soisalo and Hanna Savolainen-Peltonen contributed equally.
References
- Bosch FX, Lorincz A, Munoz N, Meijer CJ, Shah KV. The causal relation between human papillomavirus and cervical cancer. J Clin Pathol. 2002;55:244–65.
- Feletou M, Köhler R, Vanhoutte PM. Nitric oxide: orchestrator of endothelium-dependent responses. Ann Med. 2011 Sep 7. [Epub ahead of print].
- Rahkola P, Mikkola TS, Nieminen P, Ylikorkala O, Vaisanen-Tommiska M. Abnormal cervical cytology is associated with increased nitric oxide release in the uterine cervix. Acta Obstet Gynecol Scand. 2009;5:1–5.
- Rahkola P, Mikkola TS, Ylikorkala O, Vaisanen-Tommiska M. Association between high risk papillomavirus DNA and nitric oxide release in the human uterine cervix. Gynecol Oncol. 2009;114:323–6.
- Rahkola P, Vaisanen-Tommiska M, Tuomikoski P, Ylikorkala O, Mikkola TS. Cervical nitric oxide release and persistence of high-risk human papillomavirus in women. Int J Cancer. 2011;128:2933–7.
- Tornblom SA, Maul H, Klimaviciute A, Garfield RE, Bystrom B, Malmstrom A, . mRNA expression and localization of bNOS, eNOS and iNOS in human cervix at preterm and term labour. Reprod Biol Endocrinol. 2005;3:33–41.
- Hiraku Y, Tabata T, Ma N, Murata M, Ding X, Kawanishi S. Nitrative and oxidative DNA damage in cervical intraepithelial neoplasia associated with human papilloma virus infection. Cancer Sci. 2007;98:964–72.
- Mazibrada J, Ritta M, Mondini M, De Andrea M, Azzimonti B, Borgogna C, . Interaction between inflammation and angiogenesis during different stages of cervical carcinogenesis. Gynecol Oncol. 2008;108:112–20.
- Nieminen P, Ahtila A, Butzow R, Heikkilä E, Hiltunen-Back E, Mäenpää J, . Update on current care guidelines. Diagnosis, treatment and follow-up of cytological changes in the cervix, vagina and vulva. Duodecim. 2010;126:1965–6.
- Lyons CR, Orloff GJ, Cunningham JM. Molecular cloning and functional expression of an inducible nitric oxide synthase from a murine macrophage cell line. J Biol Chem. 1992;267:6370–4.
- de Assis MC, Plotkowski MC, Fierro IM, Barja-Fidalgo C, de Freitas MS. Expression of inducible nitric oxide synthase in human umbilical vein endothelial cells during primary culture. Nitric Oxide. 2002;7:254–61.
- Geng YJ, Almqvist M, Hansson GK. cDNA cloning and expression of inducible nitric oxide synthase from rat vascular smooth muscle cells. Biochim Biophys Acta. 1994;1218:421–4.
- Lamas S, Marsden PA, Li GK, Tempst P, Michel T. Endothelial nitric oxide synthase: molecular cloning and characterization of a distinct constitutive enzyme isoform. Proc Natl Acad Sci U S A. 1992;89:6348–52.
- Vaisanen-Tommiska, Butzow R, Ylikorkala O, Mikkola TS. Mifepristone-induced nitric oxide release and expression of nitric oxide synthases in the human cervix during early pregnancy. Hum Reprod. 2006;21:2180–4.
- zur Hausen H. Papillomaviruses and cancer: from basic studies to clinical application. Nat Rev Cancer. 2002;2:342–50.
- Stanley M. Immune responses to human papillomavirus. Vaccine. 2006;30:S16–22.
- Dobbs SP, Hewett PW, Johnson IR, Carmichael J, Murray JC. Angiogenesis is associated with vascular endothelial growth factor expression in cervical intraepithelial neoplasia. Br J Cancer. 1997;76:1410–5.
- Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124:263–6.
- Giraudo E, Inoue M, Hanahan D. An amino-bisphosphonate targets MMP-9-expressing macrophages and angiogenesis to impair cervical carcinogenesis. J Clin Invest. 2004;114:623–33.
- Monk BJ, Willmott LJ, Sumner DA. Anti-angiogenesis agents in metastatic or recurrent cervical cancer. Gynecol Oncol. 2010;116:181–6.
- Kuemmel S, Thomas A, Landt S, Fuger A, Schmid P, Kriner M, . Circulating vascular endothelial growth factors and their soluble receptors in pre-invasive, invasive and recurrent cervical cancer. Anticancer Res. 2009;29:641–5.
- Song SH, Lee JK, Hur JY, Kim I, Saw HS, Park YK. The expression of epidermal growth factor receptor, vascular endothelial growth factor, matrix metalloproteinase-2, and cyclooxygenase-2 in relation to human papilloma viral load and persistence of human papillomavirus after conization with negative margins. Int J Gynecol Cancer. 2006;16:2009–17.
- De Andrea M, Mondini M, Azzimonti B, Dell'oste V, Germano S, Gaudino G, . Alpha- and betapapillomavirus E6/E7 genes differentially modulate pro-inflammatory gene expression. Virus Res. 2007;124:220–5.
- Song SH, Lee JK, Seok OS, Saw HS. The relationship between cytokines and HPV-16, HPV-16 E6, E7, and high-risk HPV viral load in the uterine cervix. Gynecol Oncol. 2007;104:732–8.
- Kharitonov SA, Robbins RA, Yates D, Keatings V, Barnes PJ. Acute and chronic effects of cigarette smoking on exhaled nitric oxide. Am J Respir Crit Care Med. 1995;152:609–12.
- Su Y, Han W, Giraldo C, De Li Y, Block ER. Effect of cigarette smoke extract on nitric oxide synthase in pulmonary artery endothelial cells. Am J Respir Cell Mol Biol. 1998;19:819–25.
- Barbera JA, Peinado VI, Santos S, Ramirez J, Roca J, Rodriguez-Roisin R. Reduced expression of endothelial nitric oxide synthase in pulmonary arteries of smokers. Am J Respir Crit Care Med. 2001;164:709–13.
- Wei XM, Kim HS, Kumar RK, Heywood GJ, Hunt JE, McNeil HP, . Effects of cigarette smoke on degranulation and NO production by mast cells and epithelial cells. Respir Res. 2005;6:108–17.
- Hellberg D, Nilsson S, Haley NJ, Hoffman D, Wynder E. Smoking and cervical intraepithelial neoplasia: nicotine and cotinine in serum and cervical mucus in smokers and nonsmokers. Am J Obstet Gynecol. 1988;158:910–3.
- Matsumoto K, Oki A, Furuta R, Maeda H, Yasugi T, Takatsuka N, .; Japan HPV and Cervical Cancer (JHACC) Study Group. Tobacco smoking and regression of low-grade cervical abnormalities. Cancer Sci. 2010;101:2065–73.
- Ridnour LA, Thomas DD, Donzelli S, Espey MG, Roberts DD, Wink DA, . The biphasic nature of nitric oxide responses in tumor biology. Antioxid Redox Signal. 2006;8:1329–37.
- Wright TC Jr, Cox JT, Massad LS, Carlson J, Twiggs LB, Wilkinson EJ; 2001 ASCCP-sponsored Consensus Workshop. 2001 consensus guidelines for the management of women with cervical intraepithelial neoplasia. J Low Genit Tract Dis. 2003;7:154–67.
- Bosch FX, de Sanjose S. The epidemiology of human papillomavirus infection and cervical cancer. Dis Markers. 2007;23:213–27.
