Abstract
Background. Chronic obstructive pulmonary disease (COPD) is associated with several extrapulmonary effects that contribute to the severity of the disease. Vitamin D is suggested to play a role in COPD and its related extrapulmonary effects. Aims. To determine the prevalence of vitamin D deficiency and its relation with bone density, muscle strength, and exercise capacity in patients with COPD. Methods. Our cross-sectional study included patients with moderate to very severe COPD. We collected data on lung function, body composition, bone density, quadriceps muscle strength, 6-minute walking distance, and plasma 25-hydroxyvitamin D (25(OH)D) concentration. Vitamin D deficiency was defined as plasma 25(OH)D concentration below 50 nmol/L. Results. In total, 151 COPD patients were included; 87 patients (58%) had vitamin D deficiency. Plasma 25(OH)D concentration was positively associated with bone density (P = 0.005) and 6-minute walking distance (P < 0.001) after adjustment for potential confounders. Plasma 25(OH)D concentration was not associated with quadriceps muscle strength. Conclusions. The majority of COPD patients had vitamin D deficiency. Plasma 25(OH)D concentration was positively associated with bone density and exercise capacity. Intervention studies are necessary to determine whether vitamin D supplementation is of benefit in the prevention or treatment of osteoporosis and poor exercise capacity in patients with COPD.
Key messages
Vitamin D deficiency is very common in patients with COPD.
Plasma 25(OH)D concentration is independently associated with bone mineral density and exercise capacity in patients with COPD.
Intervention studies are necessary to determine whether vitamin D supplementation might be of benefit in the prevention or treatment of osteoporosis and poor exercise capacity in patients with COPD.
Introduction
Chronic obstructive pulmonary disease (COPD) is primarily characterized by the presence of air-flow limitation that is not fully reversible (Citation1). However, COPD is also characterized by extrapulmonary effects that may contribute to the severity of the disease (Citation2). Osteoporosis, impaired skeletal muscle strength, and poor exercise capacity can be considered as extrapulmonary effects of COPD, as these are more frequently observed in patients with COPD than in age-matched controls (Citation3–5).
Osteoporosis is characterized by low bone mineral density or micro-architectural changes resulting in impaired bone strength and hence increased fracture risk (Citation6).
Bone strength is influenced by the rate of bone remodelling and the balance between bone formation and resorption. Several factors, like systemic inflammation, use of oral and inhaled corticosteroids, and vitamin D deficiency, have been suggested to interact with pathways of bone remodelling in patients with COPD (Citation7).
In healthy individuals the role of vitamin D in bone health is well established (Citation8). Vitamin D is important in maintaining calcium homeostasis and subsequently mineralization of bone. Indeed, in a randomized controlled trial with subjects aged 65–85 years oral vitamin D supplementation reduced the number of fractures (Citation9).
Another COPD-related systemic effect is skeletal muscle dysfunction (Citation4). Skeletal muscle dysfunction is an independent predictor of mortality in patients with COPD (Citation10). It is characterized by two related phenomena: net loss of muscle mass and malfunctioning of the muscle (Citation11). Indeed, in patients with COPD reduced skeletal muscle mass has been related with impaired skeletal muscle strength. However, many more patho-physiological findings, such as physical inactivity, use of corticosteroids, abnormal protein turnover, systemic inflammation, vitamin D deficiency, and angiotensin-converting enzyme and vitamin D receptor genotypes, have been related with skeletal muscle dysfunction.
Vitamin D is suggested to affect muscle strength and function by several processes, including calcium homeostasis, cell proliferation, cell differentiation, fibre size, prevention of fatty degeneration, protection against insulin resistance, and arachidonic acid mobilization (Citation12). Indeed, vitamin D receptors were identified in human skeletal muscle tissue (Citation13), and a meta-analysis demonstrated that vitamin D supplementation reduced the risk of falling among older individuals (Citation14).
Nowadays, it is suggested that vitamin D plays an important role in COPD and its related systemic effects (Citation15). Recent data showed that the prevalence of vitamin D deficiency was higher in patients with COPD than in smoking controls (Citation16). Additionally, in patients with advanced pulmonary diseases waiting for lung transplantation vitamin D deficiency was associated with reduced femur neck T-scores (Citation17) and a lower 6-minute walking distance (Citation18). Moreover, in patients with COPD FokI and BsmI polymorphisms of the vitamin D receptor gene were associated with quadriceps muscle strength (Citation19).
Based on these findings we hypothesized that vitamin D might play a role in COPD-related osteoporosis and skeletal muscle dysfunction. The aims of our cross-sectional study were to examine the prevalence of vitamin D deficiency and its relation with bone mineral density, quadriceps muscle strength, and functional exercise capacity in patients with COPD.
Subjects and methods
Subjects
Data were extracted from the records of 168 clinically stable patients with COPD who were evaluated at CIRO + , Centre of Expertise for Chronic Organ Failure, in Horn (the Netherlands) between June and September 2009. The inclusion criteria were: Caucasian race, aged 40 years or older, and moderate to very severe COPD according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines (Citation1). In total, 151 patients fulfilled the inclusion criteria. Seventeen patients were excluded due to the following: 1 patient was 37 years old, 4 patients had an FEV1/FVC (forced expiratory volume in 1 second/forced vital capacity) ratio above 0.70, 7 patients had mild COPD, and in 5 patients no blood samples were collected. Because of the use of de-identified and pre-existing data, our retrospective study is institutional review board exempt.
Measurements
Before entering pulmonary rehabilitation, all patients were screened for disease-related physiological problems. Medical Research Council (MRC) dyspnoea scale was used to assess dyspnoea perception. Daily vitamin D intake was assessed using a validated cross-check dietary history method and calculated using the Dutch Food Composition Database.
Pulmonary function measurements were performed with standardized equipment (Masterlab®, Jaeger, Germany) according to the American Thoracic Society (ATS)/European Respiratory Society (ERS) guidelines. Forced expiratory volume in 1 second (FEV1) and forced vital capacity (FVC) were measured. The diffusing capacity of the lung (DLCO) was determined by the single breath carbon monoxide gas transfer method and expressed as percentage predicted of reference values.
Total body weight and height were measured to calculate body mass index (BMI). Fat-free mass (FFM) was measured by using dual-energy X-ray absorptiometry (Lunar Prodigy system, GE Healthcare, Madison, USA). Furthermore, FFM index (FFMI) was calculated as FFM divided by height2.
Bone mineral density was measured at the hip and the lumbar spine (L1-L4) by dual-energy X-ray absorptiometry. Diagnosis of osteoporosis was based on the lowest T-score of these locations and defined according to the World Health Organization (osteoporosis: T-score ≤ –2.5; osteopenia: T-score between –1.0 and –2.5; and normal bone tissue: T-score ≥ –1.0) (Citation6).
Isometric quadriceps maximum voluntary contraction strength (QMVC) was measured using a Biodex dynamometer (Biodex Medical Corporation, Shirley, NY). The best of three efforts was used. The 6-minute walking distance (6MWD), including a practice walk, was determined to assess functional exercise capacity.
Plasma 25-hydroxyvitamin D (25(OH)D) concentration was assayed by radioimmunoassay (Immunodiagnostic Systems, Boldon, UK) at the department of Clinical Chemistry of Maastricht University Medical Centre + , the Netherlands. The serum was extracted with acetonitrile in alkaline conditions to precipitate serum proteins. Next, the radioimmunoassay was performed using sheep anti-25(OH)D and 125I 25(OH)D. Precipitation of the formed antigen-antibody complex was obtained by adding anti-sheep IgG coupled to cellulose.
Data on the optimal plasma 25(OH)D concentration for bone and muscle health are conflicting. A Dutch population-based study determined the threshold plasma 25(OH)D concentration with regard to parathyroid hormone, bone turnover markers, bone mineral density, and physical performance in a subpopulation of the Longitudinal Aging Study Amsterdam (Citation20). They showed a threshold of about 40 nmol/L for osteocalcin and deoxypyridinoline/creatinine, 50 nmol/L for bone mineral density, and 60 nmol/L for physical performance. In our study plasma 25(OH)D concentrations below 50 nmol/L (20 ng/mL) were classified as deficient (Citation16,Citation21,Citation22).
Statistical analysis
All statistical analyses were performed using Statistical Package for Social Sciences version 17.0 (SPSS, Inc., Chicago, IL, USA). Two-sided P values < 0.05 were considered statistically significant. All data were first assessed for normal distribution.
Discrete variables were compared by the chi-square test and presented as percentages. Moreover, continuous variables were presented as mean ± standard deviation (SD) and were compared by the standardized Student t test.
Determinants of bone mineral density, quadriceps muscle strength, and functional exercise capacity were assessed by standard multiple regression analyses (enter procedure). Covariates were based on the literature. Covariates used to assess determinants of the lowest T-score were gender, age, BMI, FEV1, 25(OH)D, 6MWD, and corticosteroid use (Citation3,Citation6,Citation7,Citation23). Additionally, covariates used to assess determinants of quadriceps muscle strength and functional exercise capacity were gender, age, height, FFMI, FEV1, 25(OH)D, MRC, and 6MWD or QMVC (Citation4,Citation24). These covariates were included into the final multivariate regression analyses when significant at P < 0.10 in the univariate regression analyses. Multicollinearity was assessed by Pearson correlation, tolerance, and variance inflation factor (VIF). We found no multicollinearity between variables (Pearson correlation < 0.70, tolerance > 0.2, and VIF < 10).
Results
Characteristics and vitamin D status
In total, 151 patients with moderate to very severe COPD entering pulmonary rehabilitation met the inclusion criteria. All data were collected before the patients started pulmonary rehabilitation. Thirty-four (22.5%) patients had had rehabilitation previously.
shows the patients’ characteristics. Vitamin D deficiency was found in 87 patients (58%). No differences were found in gender, age, vitamin D intake, use of oral or inhaled corticosteroids, arterial blood gases, BMI, FFMI, QMVC, and bone mineral density between patients with and without vitamin D deficiency. Vitamin D-deficient patients had a significantly lower FEV1 and FVC and performed worse on the 6MWD compared to patients with normal plasma 25(OH)D concentrations.
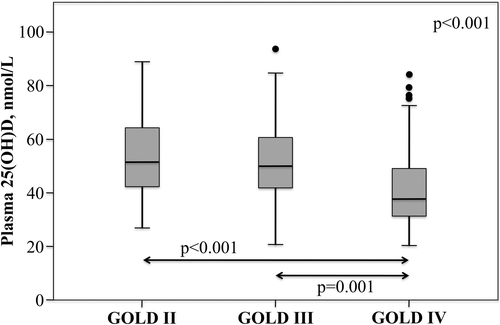
The prevalence of vitamin D deficiency was significantly different between the GOLD stages (P = 0.001). Vitamin D deficiency was found in 43% of GOLD stage II patients, 50% of GOLD stage III patients, and 76% of GOLD stage IV patients. shows the plasma 25(OH)D concentrations among the GOLD stages.
Table I. Patients’ characteristics.
Determinants of bone mineral density and skeletal muscle function
and show the univariate and multivariate analyses for bone mineral density. Gender, 6MWD, and the use of corticosteroids were not associated with bone mineral density. In the multivariate analysis, age remained negatively associated with the lowest T-score, and BMI and 25(OH)D remained positively associated with the lowest T-score. This model explained 22% of the variability of bone mineral density.
Table II. Univariate regression analyses for bone mineral density (T score).
Table III. Multivariate regression analyses for bone mineral density (T score).
and show the univariate and multivariate regression analyses for quadriceps muscle strength and functional exercise capacity. In the multivariate analysis for muscle strength, age remained negatively associated with QMVC, and height, FFMI, and 6MWD remained positively associated with QMVC. This model explained 58% of the variability of muscle strength. In addition, in the multivariate analysis for functional exercise capacity, 25(OH)D and QMVC remained positively associated with 6MWD. This model explained 37% of the variability of functional exercise capacity.
Table IV. Univariate regression analyses for quadriceps muscle strength and 6MWD.
Table V. Multivariate regression analyses for quadriceps muscle strength and 6MWD.
Discussion
Our data demonstrated that vitamin D deficiency was present in the majority of patients with COPD entering pulmonary rehabilitation. Additionally, after adjustment for potential confounders, plasma 25(OH)D concentration was positively associated with bone mineral density and functional exercise capacity. However, plasma 25(OH)D concentration was not independently associated with quadriceps muscle strength. Based on these findings we suggest there might be a link between vitamin D and COPD-related osteoporosis and poor exercise capacity.
Although all blood samples were collected in summer, vitamin D deficiency was found in the majority of COPD patients (58%). Vitamin D deficiency was found in 43% of GOLD stage II, 50% of GOLD stage III, and 76% of GOLD stage IV COPD patients. These data are in line with data collected in Belgium during all seasons by Janssens et al. (Citation16). They found vitamin D deficiency in 47% of GOLD stage II, 60% of GOLD stage III, and 77% of GOLD stage IV COPD patients. Moreover, the prevalence of vitamin D deficiency seems to be higher in our COPD cohort compared with the general Dutch population. In the Hoorn Study, a Dutch population-based cohort study, the prevalence of vitamin D deficiency (< 50 nmol/L) was 34% in summer and 51% in winter (Citation25). In the Longitudinal Aging Study Amsterdam the prevalence of vitamin D deficiency (< 50 nmol/L) was 48% (Citation20).
Our data showed that COPD patients with vitamin D deficiency had a significantly lower FEV1 and FVC than COPD patients with normal plasma 25(OH)D concentrations. Additionally, a large study of the general population demonstrated a strong relationship between plasma 25(OH)D concentration and pulmonary function (Citation26). This relationship even remained after adjustment for the degree of leisure time physical activity and vitamin D intake. Moreover, in animal models vitamin D deficiency caused deficits in lung function (Citation27) and the rs7041 variant in the vitamin D-binding gene was associated with the presence of COPD (Citation16).
Although it is suggested that vitamin D influences lung function, the mechanism is still unknown. However, some mechanisms have been suggested. First, vitamin D deficiency might be related to decreased pulmonary function due to thoracic vertebral fractures. Second, vitamin D deficiency has been suggested to result in altered host defence of the lung, with subsequent growth of an abnormal flora that triggers inflammation. Hence, acute exacerbations of COPD are an important cause of hospitalization and lead to a faster decline in lung function. Third, vitamin D is suggested to have an effect on the extracellular matrix homeostasis within the lung, resulting in the development of COPD (Citation28).
Furthermore, plasma 25(OH)D concentration was independently associated with bone mineral density. Førli et al. (Citation17) demonstrated that vitamin D deficiency was associated with reduced femur neck T-scores in underweight patients with end-stage pulmonary diseases waiting for lung transplantation. However, Franco et al. (Citation22) did not confirm this association in a small group (n = 49) of postmenopausal women and men with COPD.
In healthy subjects it is well established that vitamin D is critical for bone health. A low plasma 25(OH)D concentration is associated with decreased intestinal calcium absorption causing compensatory increased parathyroid hormone concentrations. Additionally, parathyroid hormone, which has been identified as a determinant of osteoporosis in patients with COPD (Citation29), activates osteoblasts which stimulate the transformation of pre-osteoclasts into mature osteoclasts. Mature osteoclasts dissolve the mineralized collagen matrix in bone causing osteopenia and osteoporosis (Citation21).
Besides low plasma 25(OH)D concentration, more patho-physiological mechanisms have been described to interact with pathways of bone remodelling in patients with COPD. Our data demonstrated that the prevalence of osteoporosis was still high (30%) in patients with normal plasma 25(OH)D concentrations. This finding suggests that more factors besides plasma 25(OH)D concentration correlate with bone mineral density. Indeed, in line with previous data (Citation3,Citation23), we showed that older age, lower BMI, and lower FEV1 were associated with reduced bone mineral density. However, many more factors, such as systemic inflammation, genetic background, smoking, physical inactivity, use of oral or inhaled corticosteroids, and the presence of emphysema, have been associated with reduced bone mineral density (Citation7).
Nowadays, it is assumed that vitamin D has a role in skeletal muscle health. Recently, a review stated that vitamin D affects muscle strength and function by several processes, including calcium homeostasis, cell proliferation, cell differentiation, fibre size, prevention of fatty degeneration, protection against insulin resistance, and arachidonic acid mobilization (Citation12).
In line with previous data in patients with end-stage pulmonary diseases (Citation18), we showed that plasma 25(OH)D concentration was positively associated with walking distance after adjustment for potential confounders. Moreover, Ringbaek et al. (Citation30) evaluated the influence of vitamin D on the endurance shuttle walk test in 311 out-patients with COPD who participated in pulmonary rehabilitation. They demonstrated that lower plasma 25(OH)D concentrations were associated with smaller improvements in the endurance shuttle walk test.
Research on the relationship between plasma 25(OH)D concentration and skeletal muscle strength is scarce in patients with COPD. Our data showed no association between plasma 25(OH)D concentration and quadriceps muscle strength. In addition, Førli et al. (Citation18) demonstrated that plasma 25(OH)D concentration was not associated with hand grip strength after adjustment for potential confounders in patients with end-stage pulmonary diseases.
In healthy subjects, cross-sectional data on the relationship between vitamin D and muscle function are conflicting (Citation31–34). This is partly due to variances in subjects studied and methods used. Recently, a meta-analysis demonstrated that vitamin D supplementation had a positive effect on muscle strength in subjects with plasma 25(OH)D concentrations below 25 nmol/L and no effect in subjects with plasma 25(OH)D concentrations above 25 nmol/L (Citation35). However, well-designed randomized controlled trials on the effect of vitamin D supplementation on muscle fibre composition, strength, and exercise capacity are still lacking.
Some methodological considerations should be mentioned. Primarily, there could be a selection bias due to the inclusion of patients who were referred to pulmonary rehabilitation. However, the included COPD patients represented different stages of disease severity. In addition, a comparable prevalence of vitamin D deficiency was found in COPD patients recruited at a university hospital in Belgium (Citation16). Secondly, no causal relationship could be established due to the cross-sectional design of the study. Thirdly, we did not include a healthy control group.
The strengths of our study are that all patients were included in summer, well-characterized and diagnosed with COPD according to the GOLD guidelines. By contrast, previous studies on the role of vitamin D in osteoporosis and muscle dysfunction included patients with several different pulmonary diseases (Citation17,Citation18).
Although randomized controlled trials on vitamin D supplementation in patients with COPD are lacking, we advise chest physicians to be alert for vitamin D deficiency in patients with COPD. It is already known that osteoporosis and muscle dysfunction influence morbidity and mortality in patients with COPD (Citation7,Citation10). Our data demonstrated that the prevalence of vitamin D deficiency is high in COPD and that vitamin D status is associated with bone mineral density and functional exercise capacity, suggesting a link between vitamin D and COPD-related osteoporosis and muscle dysfunction. Indeed, in healthy individuals randomized controlled trials have already demonstrated beneficial effects of vitamin D supplementation on bone health and skeletal muscle function. However, further research on the role of vitamin D in bone and muscle health in patients with COPD is warranted.
To conclude, the majority of patients with COPD entering pulmonary rehabilitation had vitamin D deficiency. Additionally, vitamin D status was positively associated with bone mineral density and functional exercise capacity as measured by 6MWD. In future, intervention studies will be necessary to determine whether vitamin D supplementation might be of benefit in the prevention or treatment of osteoporosis and poor exercise capacity in patients with COPD.
Declaration of interest: The authors report no conflicts of interest.
References
- Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, . Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176:532–55.
- Barnes PJ, Celli BR. Systemic manifestations and comorbidities of COPD. Eur Respir J. 2009;33:1165–85.
- Graat-Verboom L, Wouters EF, Smeenk FW, van den Borne BE, Lunde R, Spruit MA. Current status of research on osteoporosis in COPD: a systematic review. Eur Respir J. 2009;34:209–18.
- Seymour JM, Spruit MA, Hopkinson NS, Natanek SA, Man WD, Jackson A, . The prevalence of quadriceps weakness in COPD and the relationship with disease severity. Eur Respir J. 2010;36:81–8.
- Bossenbroek L, de Greef MH, Wempe JB, Krijnen WP, Ten Hacken NH. Daily physical activity in patients with chronic obstructive pulmonary disease: a systematic review. COPD. 2011;8:306–19.
- WHO Scientific Group on the Prevention and Management of Osteoporosis. Prevention and management of osteoporosis; report of a WHO scientific group. 2003. http://whqlibdoc.who.int/trs/WHO_TRS_921.pdf
- Lehouck A, Boonen S, Decramer M, Janssens W. COPD, bone metabolism, and osteoporosis. Chest. 2011;139:648–57.
- Vieth R. The role of vitamin D in the prevention of osteoporosis. Ann Med. 2005;37:278–85.
- Trivedi DP, Doll R, Khaw KT. Effect of four monthly oral vitamin D3 (cholecalciferol) supplementation on fractures and mortality in men and women living in the community: randomised double blind controlled trial. BMJ. 2003;326:469.
- Schols AM, Broekhuizen R, Weling-Scheepers CA, Wouters EF. Body composition and mortality in chronic obstructive pulmonary disease. Am J Clin Nutr. 2005;82:53–9.
- Rabinovich RA, Vilaro J. Structural and functional changes of peripheral muscles in chronic obstructive pulmonary disease patients. Curr Opin Pulm Med. 2010;16:123–33.
- Dirks-Naylor AJ, Lennon-Edwards S. The effects of vitamin D on skeletal muscle function and cellular signaling. J Steroid Biochem Mol Biol. 2011;125:159–68.
- Costa EM, Blau HM, Feldman D. 1,25-dihydroxyvitamin D3 receptors and hormonal responses in cloned human skeletal muscle cells. Endocrinology. 1986;119:2214–20.
- Bischoff-Ferrari HA, Dawson-Hughes B, Staehelin HB, Orav JE, Stuck AE, Theiler R, . Fall prevention with supplemental and active forms of vitamin D: a meta-analysis of randomised controlled trials. BMJ. 2009;339:b3692.
- Janssens W, Lehouck A, Carremans C, Bouillon R, Mathieu C, Decramer M. Vitamin D beyond bones in chronic obstructive pulmonary disease: time to act. Am J Respir Crit Care Med. 2009;179:630–6.
- Janssens W, Bouillon R, Claes B, Carremans C, Lehouck A, Buysschaert I, . Vitamin D deficiency is highly prevalent in COPD and correlates with variants in the vitamin D-binding gene. Thorax. 2010;65: 215–20.
- Forli L, Halse J, Haug E, Bjortuft O, Vatn M, Kofstad J, . Vitamin D deficiency, bone mineral density and weight in patients with advanced pulmonary disease. J Intern Med. 2004;256:56–62.
- Forli L, Bjortuft O, Boe J. Vitamin D status in relation to nutritional depletion and muscle function in patients with advanced pulmonary disease. Exp Lung Res. 2009;35:524–38.
- Hopkinson NS, Li KW, Kehoe A, Humphries SE, Roughton M, Moxham J, . Vitamin D receptor genotypes influence quadriceps strength in chronic obstructive pulmonary disease. Am J Clin Nutr. 2008;87:385–90.
- Kuchuk NO, Pluijm SM, van Schoor NM, Looman CW, Smit JH, Lips P. Relationships of serum 25-hydroxyvitamin D to bone mineral density and serum parathyroid hormone and markers of bone turnover in older persons. J Clin Endocrinol Metab. 2009;94:1244–50.
- Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–81.
- Franco CB, Paz-Filho G, Gomes PE, Nascimento VB, Kulak CA, Boguszewski CL, . Chronic obstructive pulmonary disease is associated with osteoporosis and low levels of vitamin D. Osteoporos Int. 2009;20:1881–7.
- Graat-Verboom L, Spruit MA, van den Borne BE, Smeenk FW, Martens EJ, Lunde R, . Correlates of osteoporosis in chronic obstructive pulmonary disease: an underestimated systemic component. Respir Med. 2009;103:1143–51.
- Spruit MA, Watkins ML, Edwards LD, Vestbo J, Calverley PM, Pinto-Plata V, . Determinants of poor 6-min walking distance in patients with COPD: the ECLIPSE cohort. Respir Med. 2010;104:849–57.
- van Dam RM, Snijder MB, Dekker JM, Stehouwer CD, Bouter LM, Heine RJ, . Potentially modifiable determinants of vitamin D status in an older population in the Netherlands: the Hoorn Study. Am J Clin Nutr. 2007;85:755–61.
- Black PN, Scragg R. Relationship between serum 25-hydroxyvitamin d and pulmonary function in the third national health and nutrition examination survey. Chest. 2005;128:3792–8.
- Zosky GR, Berry LJ, Elliot JG, James AL, Gorman S, Hart PH. Vitamin D deficiency causes deficits in lung function and alters lung structure. Am J Respir Crit Care Med. 2011;183:1336–43.
- Herr C, Greulich T, Koczulla RA, Meyer S, Zakharkina T, Branscheidt M, . The role of vitamin D in pulmonary disease: COPD, asthma, infection, and cancer. Respir Res. 2011;12:31.
- Graat-Verboom L, van den Borne BE, Smeenk FW, Spruit MA, Wouters EF. Osteoporosis in COPD outpatients based on bone mineral density and vertebral fractures. J Bone Miner Res. 2011;26:561–8.
- Ringbaek T, Martinez G, Durakovic A, Thogersen J, Midjord AK, Jensen JE, . Vitamin D status in patients with chronic obstructive pulmonary disease who participate in pulmonary rehabilitation. J Cardiopulm Rehabil Prev. 2011;31:261–7.
- Marantes I, Achenbach SJ, Atkinson EJ, Khosla S, Melton LJ, III, Amin S. Is vitamin D a determinant of muscle mass and strength? J Bone Miner Res. 2011.
- Ceglia L, Chiu GR, Harris SS, Araujo AB. Serum 25-hydroxyvitamin D concentration and physical function in adult men. Clin Endocrinol (Oxf). 2011;74:370–6.
- Houston DK, Cesari M, Ferrucci L, Cherubini A, Maggio D, Bartali B, . Association between vitamin D status and physical performance: the InCHIANTI study. J Gerontol A Biol Sci Med Sci. 2007;62:440–6.
- Gerdhem P, Ringsberg KA, Obrant KJ, Akesson K. Association between 25-hydroxy vitamin D levels, physical activity, muscle strength and fractures in the prospective population-based OPRA Study of Elderly Women. Osteoporos Int. 2005;16:1425–31.
- Stockton KA, Mengersen K, Paratz JD, Kandiah D, Bennell KL. Effect of vitamin D supplementation on muscle strength: a systematic review and meta-analysis. Osteoporos Int. 2011;22:859–71.