Abstract
Aims. To investigate the relationship of body mass index and serum adipokines with incidence of diabetes in men. Material and methods. Ten-year cohort study of a random population sample of 1011 men aged 35–69 years from the MONICA-Catalonia survey (1986–1988). WHO-MONICA protocol and the US Hispanic NHANES diabetes questionnaire were applied. Fasting serum glucose and lipids were measured by enzymatic methods, adipokines and insulin by Luminex xMAP technology,and hs-CRP by nephelometry in stored baseline samples (–80°C). Type2 diabetes was defined as fasting glucose ≥ 7.0 mmol/L or diagnosed diabetes. Incident diabetes was defined as absence of these criteria at baseline but presence at re-examination. Cox regression analysis was used. Results. Incidence of diabetes (n = 85) was 10.3/1000 person-years, increasing significantly with BMI but decreasing by quartiles of adiponectin. Incidence increased above median BMI and glucose (45.3/1000 person-years, OR = 19.97). Log-adiponectin associated with reduced risk of diabetes after multivariate adjustment (HR = 0.24, 95% CI 0.08–0.72), with significant modification of this effect by baseline glycaemia. C-reactive protein was not a significant factor. Leptin lost strength when adjusted for BMI. Conclusions. In a population with relatively high diabetes incidence, BMI and glucose were strong risk factors, while adiponectin protected against diabetes, especially in men with high glycaemic level.
Key messages
Incidence of type 2 diabetes is relatively high in Catalonia.
High body mass index is a risk factor for type 2 diabetes.
Serum adiponectin is a protective factor for type 2 diabetes, especially in men with a high glycaemic level.
Introduction
Obesity and type 2 diabetes mellitus are increasingly prevalent in many countries. Obesity is a risk factor for diabetes, cardiovascular diseases, and several other diseases (Citation1). Within the European Union, there is a 3–4-fold difference in diabetes mortality, with Spain showing higher rates (14.6 per 100,000 in 2005) than other European countries such as the United Kingdom (8.2 per 100,000). In the World Health Organization Multinational MONItoring of trends and determinants in CArdiovascular diseases (WHO-MONICA) study, we showed that prevalence of overweight, general and abdominal obesity in Catalonia, were high (Citation2) and increasing (Citation3). In previous studies, the prevalence of diabetes varied in adult men between 6% and 10% (Citation4,Citation5), but thus far the incidence of type 2 diabetes was unknown. The previous studies of diabetes prevalence in other parts of Spain had found discrepant rates, but these studies were mainly based on small samples (Citation6,Citation7).
Adipose tissue produces a variety of hormones, among which adipokines have received increasing attention over the recent years as they regulate processes ranging from glucose metabolism and insulin sensitivity to inflammation and atherogenesis. Adiponectin has important metabolic protective properties and is linked to the development of type 2 diabetes (Citation8,Citation9). Regarding leptin, another adipokine related to obesity and energy expenditure, there is some controversy over its potential relation to the risk of developing type 2 diabetes (Citation10,Citation11).
The objectives of the ADIPOCAT (ADIPOsity in MONICA-CATalonia) study were to investigate the incidence of type 2 diabetes and its relationship with body mass index (BMI), serum total adiponectin, leptin, and C-reactive protein in the men of the first MONICA-Catalonia cohort study carried out in 1986–1988.
Material and methods
Subjects and follow-up
Recruitment methods, baseline data and characteristics of the geographical area of the MONICA-Catalonia study were published previously (Citation12,Citation13). Briefly, men aged 35–69 years stratified by sex and age-groups were randomly selected from the population registers from nine cities of the province of Barcelona selected randomly with probability proportional to total population size. Of 1374 selected men, 37 were ineligible because they had moved out of the area or deceased prior to the survey, and 1011 accepted to participate (response rate 75.6%). The cohort was re-examined after 10 years of the baseline examination with the same survey methods or by telephone interview by the same health team as in the baseline survey. Out of 1011 men, 93 had died at the time of re-examination, 741 returned for physical examination, an additional 163 men replied to the follow-up questionnaire by telephone interview (response rate 89.4%), and 14 were lost to follow-up. Thus, after 10 years it was possible to establish the vital status and get information on diabetes in 98.6% of the original cohort. The surveys were conducted according to the Declaration of Helsinki, were approved by the study Steering Committee, and all subjects gave informed written consent.
Survey methods
The WHO-MONICA questionnaire on risk factors and education was administered by trained nurses (Citation14). In addition, other chronic disease questions and measurements were included. For the diabetes assessment, the US Hispanic National Health and Nutrition Examination Survey II questionnaire was used (Citation15). Body height and weight were measured with a stadiometer and a SECA-713© (SECA, Hamburg, Germany) roman balance scale, with a precision of 1cm and 200g, respectively, without shoes and outer garments, in light clothing. BMI was calculated by the Quetelet index (kg/m2) (http://www.ktl.fi/publications/monica/bmi/bmiqa20.htm). Waist and hip circumference were not measured in the first WHO-MONICA surveys, but they were measured at the follow-up examination. Systolic and diastolic blood pressure were measured twice consecutively with a random zero mercury sphygmomanometer (Hawksley, Lancing, England) with the subjects in sitting position after 5 minutes of rest and choosing among three cuff sizes according to the arm circumference. Diastolic blood pressure (DBP) was defined as phase V of Korotkoff sounds. The mean of the two measurements was used for analysis. Education was assessed as the maximum number of years enrolled in formal learning.
Blood sampling
Study participants were given written instructions to keep a 12-hour fast before attending the survey. A 10 mL venous blood sample was taken from the antecubital arm into a Vacutainer© tube, in sitting position with minimal use of tourniquet, between 09.00 and 11.00 h. Blood was kept for 30 minutes at room temperature (20–24°C), centrifuged at 2500 rpm (10–15 minutes), and transported at 4°C to the biochemistry laboratory (Hospital de la Santa Creu i Sant Pau, Barcelona) within 4–6 hours of extraction and analysed for serum lipids and glucose the same day. Remaining serum aliquots were immediately frozen and stored at –40°C until 2005 and at –80° thereafter with monitoring of temperatures and alarm systems. Samples were kept frozen until the present analysis.
Glucose and lipid measurements
Fasting glucose was determined by the hexokinase method (Boehringer-Mannheim, Mannheim, Germany). Total cholesterol was analysed by the CHOD-PAP method (Boehringer- Mannheim, Mannheim, Germany), triglycerides by GPO-PAP (Boehringer-Mannheim, Mannheim, Germany). High-density lipoprotein (HDL)-cholesterol was measured the following day, by manual lipoprotein precipitation with the PEG 6000 method (Boehringer-Mannheim, Mannheim, Germany). Lipid measurements were subject to international quality control by the WHO International Lipid Reference Centre in Prague under the WHO-MONICA protocol (http://www./ktl.fi/publications/monica/tchol/tcholqa.htm,http://www.ktl.fi/publications/monica/hdl/hdlchol/hdlqa.htm). Glucose and lipids were also measured at re-examination using the same methods.
Adipokines and insulin measurements
Total adiponectin, leptin, insulin, and high-sensitivity C-reactive protein (hs-CRP) were determined in the serum aliquots stored frozen since the baseline. Adiponectin, leptin, and insulin concentrations were assayed using the Luminex xMAP technology with commercially available kits (MILLIPLEXTM MAP, Millipore, St Charles, MO, USA), on a multiplex suspension array system (Luminex 200 IS System). Briefly, adiponectin assay (HADK1 - 61K-A) was done on 1:400 diluted samples, insulin and leptin assay (HADK2 - 61K-B) on 1:50 diluted samples. The low detection limit of the assays was 145.5, 119.7, and 46.3 pg/mL for adiponectin, leptin, and insulin, respectively. All procedures were performed according to manufacturer's instructions with quality controls in the expected ranges for each assay. Standards were measured daily with a recovery observed/expected 100% (from 90% to 110%). One external control was repeatedly measured for each variable in ten different assays, and the inter-assay CV was 15.0% for adiponectin, 16.4% for leptin, and 13.7% for insulin.
A high-sensitivity assay was used for serum C-reactive protein (N Latex CRP Mono; DADE Behring, Deerfield, IL, USA) using a nephelometer (DADE Behring, Deerfield, IL, USA). The lineal detection limit of the assay ranged between 0.04 and 5.0 mg/L, and the inter-assay CV was 8.5%.
Hormonal measurements and hs-CRP were available in 964 of the 1011 subjects. The reason for missing determinations was insufficient amount of available serum (46 subjects), and one additional person was excluded because of outlying abnormally high hormonal values. All biochemical determinations were carried out by technicians blinded to the diabetes status.
Incident diabetes
Prevalent diabetes at baseline was defined as either having a fasting glucose ≥ 7.0 mmol/L (Citation16) or a report of medical diagnosis or treatment for diabetes (n = 42 and n = 39 out of 1011 subjects, respectively). Incident cases were men who did not fulfil these criteria at baseline but did at re-examination, or those who, during the follow-up period, had been hospitalized for or died from diabetes as the main diagnosis. All incident cases were considered to have type 2 diabetes. Prevalent cases of diabetes at baseline were excluded from the denominator for the calculation of the incidence and for the study of the association of diabetes with adipokines and insulin. Incidence rates were calculated as person-time (cases/1000 person-years). Length of follow-up was defined as the time interval between the dates of two surveys or between the date of baseline examination and date of death.
Statistical analyses
Means (standard deviation), medians (interquartile range), or proportions were computed for all men and by age-groups (35–44, 45–54, 55–64, and 65–69 years). Triglycerides, adiponectin, leptin, insulin, and hs-CRP were log-transformed for analysis that required the assumptions of normal distributions, and median and interquartile range are presented for these variables. Quartiles of adiponectin and glucose distribution excluding prevalent diabetes were defined. Chi-squared test was used for univariate baseline cross-sectional analysis of association with qualitative variables, and the analysis of variance for linear trend was used for quantitative variables.
For longitudinal analysis, Cox multivariate proportional hazard model regression was used to calculate the hazard ratios (HR). Men were censored at the time of death or at the date of follow-up interview or re-examination. HRs are presented per one unit or per 1 log unit difference in the case of log-transformed variables. Several models were built using different continuous variables as covariates to assess the principal effects of BMI and adipokines. In the first model, age and BMI were used as covariates to study the effects of log-adiponectin and of log-leptin, respectively, on the incidence of diabetes. In the second model, age, BMI, and either log-leptin or log-adiponectin were included. In the third, years of school and serum insulin were added, and in the fourth, HDL-cholesterol, log-triglycerides, and DBP were added to the previous covariates. To study the mediating effect of fasting glucose, another two models were built by using glucose instead of insulin but keeping all the other covariates. Interaction terms insulin × glucose and glucose × log-adiponectin were also tested in the fifth model. Finally, to analyse the mediating effects of inflammation, hs-CRP was added to the different models. As a sensitivity analysis, we repeated all regressions using a logistic regression instead of a Cox model. All tests were two-tailed, and P values lower than 0.05 were considered statistically significant. Statistical analysis was carried out with SPSS version13 for Windows (SPSS Inc., Chicago, IL, USA).
Results
Of the 1011 men, 81 had diabetes at baseline (crude prevalence 8.0%, 95% CI 6.3–9.7) and were excluded for statistical analysis. shows the comparison of selected baseline characteristics among the incident cases of diabetes (n = 85; 3 of them assessed by a telephone questionnaire) and men who remained free of diabetes. The mean duration of follow-up was 9.4 ± 1.4 years (range 0.32–12.5). The average incidence of diabetes was 10.3/1000 person-years, and it rose with age (7.7, 13.3, 9.8, and 16.2 for age-groups 35–44, 45–54, 55–64, and 65–69, respectively (P for trend = 0.0001)). The incidence of diabetes was higher among men with lower education level (primary school or less) (9.9 versus 6.4/1000 person-years, age-adjusted HR = 1.32, 95% CI 1.03–1.68). Diabetes incidence rose significantly by BMI categories () or by BMI entered as a continuous variable (). The incidence of diabetes increased with glucose at baseline. There was a joint effect between baseline glucose and BMI (incidence 45.3/1000 person-years (95% CI 33.9–56.6) among subjects above the median glucose (5.4mmol/L) and median BMI (26.1 kg/m2) compared with 2.3/1000 person years (95% CI 0.8–3.7) below the medians).
Table I. Mean (± standard deviation) or median (interquartile range) of baseline characteristics in men aged 35–69 years by incident diabetes in the ADIPOCAT study.
Table II. Baseline characteristics by quartiles of adiponectin in non-diabetic men aged 35–69 in the ADIPOCAT study.
Figure 1. Incidence rates of diabetes in men aged 35–69 years in the ADIPOCAT study (a) by BMI categories (kg/m2); (b) by quartiles of serum adiponectin(μg/mL).
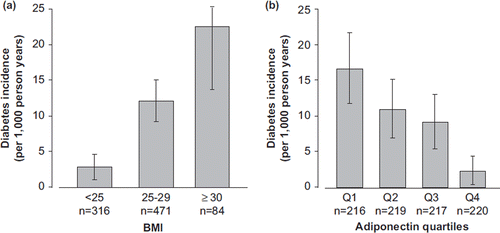
Table III. Cox proportional hazard ratios and 95% confidence intervals between BMI and incident diabetes in men aged 35–69 years in the ADIPOCAT study.
All variables included in the analysis decreased by quartiles of adiponectin except years of school, systolic blood pressure, total cholesterol, and hs-CRP. HDL-cholesterol increased with adiponectin (). Adiponectin varied by glucose quartiles (Q1 = 23.1, Q2 = 21.2, Q3 = 20.2, Q4 = 19.4 μg/mL, F = 5.7, P < 0.001) and vice versa (). Obese men (BMI ≥ 30 kg/m2) had lower levels of adiponectin than overweight (25 < BMI < 30 kg/m2) or normal weight (BMI < 25 kg/m2) men (17.6 ± 8.8, 20.1 ± 9.4, 23.7 ± 12.2 µg/mL, P < 0.001, respectively).
BMI was a significant predictor of diabetes (age-adjusted HR = 1.18, 95%CI 1.12–1.25). HR somewhat decreased but remained statistically significant after adjusting for adipokines and, in addition, for the metabolic syndrome-related variables such as DBP, HDL-cholesterol, and log-triglycerides ().
The incidence of diabetes decreased significantly by quartiles of adiponectin from 16.7/1000 person-years in the lowest to 2.3/1000 person-years in the highest quartile (P for trend < 0.0001) (incidence rate ratio between highest and lowest quartiles = 0.14) (). In Cox proportional hazard models, log-adiponectin was a strong protector of incident diabetes (models 1 and 2, ). Adjustment for log-leptin (model 3, ) practically did not change the HR, and the inclusion of insulin and years of school attenuated it only slightly (model 4, ). Log-adiponectin was not modified after further adjustment for metabolic syndrome-related variables (model 5, ). A further adjustment for hs-CRP did not change the results either (HR = 0.26, 95% CI 0.09–0.78). However, when fasting glucose was entered into the model instead of insulin, log-adiponectin lost part of its protection (models 6 and 7, ). Stratifying for baseline glycaemia (model 6; normoglycaemia < 6.1 mmol/L, impaired fasting glucose (IFG) 6.1–7.0 mmol/L) showed a significant effect modification. Log-adiponectin HR in normoglycaemic people was 0.28 (95% CI 0.06–1.40) and in people with IFG, 0.40 (95% CI 0.09–1.90, chi-square = 13.05,P < 0.0001).
Table IV. Cox proportional hazard ratios and 95% confidence intervals between serum adiponectin and incident diabetes in men aged 35–69 years in the ADIPOCAT study.
Leptin was a strong risk factor for incident diabetes (age- adjusted log-leptin HR = 3.7, 95% CI 2.0–6.8), but the risk attenuated after adjusting for BMI (HR = 1.82, 95% CI 0.92–3.61) or for BMI together with other variables (HR = 1.59, 95% CI 0.93–3.85).
Age-adjusted log-hs-CRP was not a significant risk factor for incident diabetes in this study (age-adjusted HR = 1.36, 95% CI 0.86–2.14), although there was a trend with increasing hs-CRP.
Results of all models using logistic regression instead of Cox model showed similar pattern of results (data not shown).
Discussion
This study shows the incidence of type 2 diabetes of 10.3/1000 person-years among men aged 35–69 years in Catalonia. This means that approximately 13,800 men aged 35–69 would develop type 2 diabetes every year in Catalonia with a current total population of > 7,600,000. This level of incidence could be an underestimation of the actual incidence due to the outdated survey timeframe (1986–1988), but only if incidence rates would have been growing in the region or because diabetes ascertainment was not based on an oral glucose tolerance test. Comparisons of the incidence rates with other studies in this region of the world are not straightforward, since age range, diabetes definition, sampling frames, follow-up duration, and other methods are not strictly comparable. However, the rates of the Asturias study (Citation6) would seem to be closer to ours, although their confidence limits were more imprecise.
We found a strong inverse relation between serum adiponectin levels and onset of diabetes in adult men. This is in accordance with a recent meta-analysis (Citation17), but ours is the first study showing the protection of total adiponectin on diabetes in white Mediterranean-based men beyond that reported in other populations (Citation18–21). The median total adiponectin in men was 19 μg/mL. These levels were similar to other studies using the same recent Luminex xMAP technology (Citation22,Citation23), but higher than previous studies using radioimmunoassay or ELISA (Citation24,Citation25).
The effect of adiponectin upon diabetes incidence was independent of leptin, insulin, and metabolic syndrome factors. Our novel results indicate that glycaemia may be a more powerful factor than insulinaemia in mediating effects of adiponectin on diabetes risk, since the hazard ratio was halved when controlling for glucose, but not when adjusted for insulin. To our knowledge, only the Hoorn study suggested an effect of glucose on adiponectin but only in women, and the potential mediating effects of insulin and glucose were not disentangled in that study (Citation19). A very recent collaborative analysis of the KORA and the Framingham Offspring studies (Citation26) showed that lower levels of adiponectin were associated with higher risk of type 2 diabetes in insulin-resistant but not in insulin-sensitive individuals, which is in line with our results. Mechanisms of the protective action of adiponectin are still being unravelled. Adiponectin has insulin-mimetic and insulin-sensitizing properties in the liver and skeletal muscle but was also found to mediate glucose transport via mechanisms other than insulin and insulin resistance (Citation9). Injection of adiponectin promoted a reduction in blood glucose levels without affecting insulin levels in animal studies (Citation27). In addition, in-vivo experimental studies, using the insulin clamp technique, demonstrated that adiponectin inhibited glucose production without affecting glucose uptake (Citation28). It can be hypothesized that a reciprocal biological interaction may also be plausible, namely that glucose can down-regulate adiponectin levels. In fact, glucose has been shown to inhibit adiponectin expression in adipocytes in vitro (Citation29). Furthermore, hyperglycaemia has been shown to decrease the expression of the adipokine receptor AdipoR1 and reduce the metabolic effects of glomerular adiponectin in the skeletal muscle (Citation9). Interestingly, glucose loading in subjects with normal glucose tolerance or impaired fasting glucose induced a significant decrease in adiponectin, while an oral fat load did not have any effect (Citation30).
Leptin has been found to be associated with diabetes, but not independently of other factors. The Atherosclerosis Risk in Communities (ARIC) study found that serum leptin turned from having a statistically significant risk factor for diabetes to a factor that was associated with a significantly lower risk after adjusting for obesity, metabolic syndrome, adiponectin, insulin metabolism, and inflammation factors (Citation10). Obviously, there is multicollinearity in these associations due to the high correlation between leptin and BMI. We could not replicate this reverse change of effect even though our median leptin levels were the same as in ARIC (4.4 ng/mL). The Mauritian study (Citation11) showed leptin to be a significant risk factor for diabetes in men, but not in women, after adjusting for age, ethnicity, waist circumference, and other metabolic variables.
It has been suggested that inflammatory markers might have an independent effect on diabetes risk and that adiponectin might act as a modulating inflammation factor (Citation31). In our study, we could not see a correlation of hs-CRP with adiponectin, nor that hs-CRP predicted the incidence of diabetes. Other studies with higher prevalence of obesity and higher median values of hs-CRP than ours (Citation21,Citation32,Citation33) did not find such an association either, and in the ARIC study hs-CRP lost its significance when obesity was taken into account (Citation31).
Our study has several advantages. First, it has a longer duration of follow-up than several other studies, and hence it can contribute to the sound evidence for the temporality of the association. Second, it has a full cohort design which yields a stronger statistical power compared with nested case-control studies (Citation8). Furthermore, the follow-up coverage at 10 years was nearly complete (98.6%), leaving practically no room for selection bias in the ascertainment of incident diabetes. Several of the previous studies had much lower response rates in the follow-up re-examinations or smaller number of incident diabetes. A limitation of our study was the absence of a glucose tolerance test, which would have allowed us to assess the effect of adipokines on post-challenge hyperglycaemia, the major abnormality in glucose regulation abnormalities in the early stage. Another limitation was the absence of abdominal obesity measures in the baseline survey, although current knowledge shows both general and abdominal obesity equally predictive of incident diabetes (Citation34). Indeed, BMI and waist circumference were highly correlated in the re-examination survey (r = 0.88, P < 0.0001). Other risk factors for diabetes such as family history, physical activity, smoking and drinking habits could modulate the relationship of adipokines with diabetes, but their study was out of the focus of this paper.
In conclusion, the incidence of diabetes is high among men in Catalonia. Increased BMI and plasma/serum glucose are strong risk factors for diabetes, and an increased level of both multiplies the incidence by a factor of 20. Our study shows that serum adiponectin is a significant protector of diabetes independently of BMI, lipids, metabolic syndrome variables, and hs-CRP, and it originally demonstrates that fasting glucose is a mediator of adipokine action on diabetes.
Acknowledgements
The MONICA-Catalonia survey and the follow-up were funded by the Catalonia Department of Health. The ADIPOCAT study was funded by CIBEROBN CB06/03 (to L.B.), SAF 2006 - 10091 and SAF2010 - 16549 (to L.B.), Lilly Foundation (to L.B.), FIS-PI10/01115 (to T.P.), and Fundación ‘Jesús Serra’. We acknowledge the contribution to field data collection of Ana Rodés and José M. Borrás (first survey), Guillermo Paluzie and Teresa Puig (re-examination survey), Luisa Balañá (both surveys), and to laboratory determinations of Juan Antonio Gómez Gerique (lipids, glucose) and Francisco Javier Rodríguez (hormones).
Declaration of interest: The authors report no conflicts of interest.
References
- James PT, Rigby N, Leach R; International Obesity Task Force. The obesity epidemic, metabolic syndrome and future prevention strategies. Eur J Cardiovasc Prev Rehabil. 2004;11:3–8.
- Molarius A, Seidell J C, Sans S, Tuomilehto J, Kuulasmaa K; WHO MONICA Project. Varying sensitivity of waist action levels to identify subjects with overweight or obesity in 19 populations of the WHO MONICA Project. J Clin Epidemiol. 1999;52:1213–24.
- Silventoinen K, Sans S, Tolonen H, Monterde D, Kuulasmaa K, Kesteloot H, . Trends in obesity and energy supply: an ecological analysis from the WHO-MONICA Project. Int J Obesity. 2004;28:710–8.
- Castell C, Treserras R, Serra J, Goday A, Lloveras G, Salleras L. Prevalence of diabetes in Catalonia (Spain): an oral glucose tolerance test-based population study. Diabetes Res Clin Pract. 1999;43:33–40.
- Masiá R, Sala J, Rohlfs I, Piulats R, Manresa JM, Marrugat J. Prevalencia de diabetes mellitus en la provincia de Girona, España: el estudioREGICOR. Rev Esp Cardiol. 2004;57:261–4.
- Valdés S, Botas P, Delgado E, Alvarez F, Díaz Cárdena F. Population-based incidence of type 2 diabetes in northern Spain: the Asturias Study. Diabetes Care. 2007;30:2258–63.
- Soriguer F, Rojo-Martinez G, Almaraz MC, Esteva I, Ruiz de Adana MS, Morcillo S, . Incidence of type 2 diabetes in Southern Spain (Pizarra study). Eur J Clin Investig. 2008;38:126–33.
- Lindsay RS, Funahashi T, Hanson RL, Matsuzawa Y, Tanaka S, Tataranni PA, . Adiponectin and development of type 2 diabetes in the Pima Indian population. Lancet. 2002;360:57–8.
- Kadowaki T, Yamauchi T. Adiponectin and adiponectin receptors. Endocr Rev. 2005;26:439–51.
- Schmidt MI, Duncan B, Vigo A, Pankow JS,Couper D, Ballantyne CM, . Leptin and incident type 2 diabetes: risk or protection? Diabetologia. 2006;49:2086–96.
- Soderberg S, Zimmet P, Tuomilehto J, Chitson P, Gareboo H, Alberti KGMM, . Leptin predicts the development of diabetes in Mauritian men, but not women: a population-based study. Int J Obes. 2007;31: 1126–33.
- Rodés A, Sans S, Balañà LL, Paluzie G, Aguilera R, Balaguer-Vintró I. Recruitment methods and differences in early, late and nonrespondents in the first MONICA-Catalonia population survey. Rev Epidemiol Santé Publique. 1990;38:447–54.
- Sans S, Paluzie G,Balañá L, Puig T, Balaguer-Vintró I. Trends in prevalence, awareness, treatment and control of arterial hypertension between 1986 and 1996: the MONICA-Catalonia study. Med Clin (Barc). 2001;117:246–53. Erratum in Med Clin (Barc). 2001;117:731.
- World Health Organization MONICA Manual, version 1.1., CVD/MNC. Geneva: World Health Organization; 1986.
- US Department of Health and Human Services. Public Health Service. National Center for Health Statistics Data collection forms of the Hispanic NHANES. Hyattsville, Maryland: US Department Printing Office; 1983.
- World Health Organization/International Diabetes Federation Definition and diagnosis of diabetes mellitus and intermediate hyperglycaemia: Report of a WHO/IDF consultation. Geneva: World Health Organization; 2006.
- Li S, Shin HJ, Ding E, van Dam RM. Adiponectin levels and risk of type 2 diabetes. A systematic review and meta-analysis. JAMA. 2009;302: 179–88.
- Wanamatee SG, Lowe GD, Rumley A, Cherry L, Whincup PH, Sattar N. Adipokines and risk of type 2 diabetes in older men. Diabetes Care. 2007;30:1200–5.
- Snijder MB, Heine RJ, Seidell J, Bouder LM, Stehouwer CDA, Neipels G, . Associations with adiponectin level with impaired glucose metabolism and type 2 diabetes in older men and women. The Hoorn Study. Diabetes Care. 2006;29:2498–503.
- Brancati FL, Kao WH, Folsom A, Watson RL, Szklo M. Incident type 2 diabetes mellitus in African American and white adults. The Atherosclerosis RIsk in Communities study. JAMA. 2000;283:2253–9.
- Ley SH, Harris SB, Connelly PW, Mamakeesick M, Gittelson J, Hegele RA, . Adipokines and incident type 2 diabetes in an aboriginal Canadian population. Diabetes Care. 2008;31:1410–5.
- Dullaart RPF, deVries R, vanTol A, Sluiter WJ. Lower plasma adiponectin is a marker of increased intima-media thickness associated with type-2 diabetes mellitus and with male gender. Eur J Endocrinol. 2007;156:387–94.
- Wang J, Li H, Franco OH, Yu Z, Liu Y, Lin X. Adiponectin and metabolic syndrome in middle-age and elderly Chinese. Obesity. 2008;16:172–8.
- Salas-Salvado J, Granada M, Bullo M, Corominas A, Casas P, Foz M. Plasma adiponectin distribution in a Mediterranean population and its association with cardiovascular risk factors and metabolic syndrome. Metabolism. 2007;56:1486–92.
- Duncan BB, Schmidt MI, Pankow JS, Bang H, Couper D, Ballantyne CM, . Adiponectin and the development of type 2 diabetes: the Atherosclerosis RIsk in Communities study. Diabetes. 2004;53: 2473–8.
- Hivert MH, Sullivan LM, Shrader P, Fox CS, Nathan DM, D’Agostino R, . Insulin resistance influences the association of adiponectin levels with diabetes incidence in two population-based cohorts: the Cooperative Health Research in the Region of Augsburg (KORA) S4/F4 study and the Framingham Offspring Study. Diabetologia. 2011;54: 1019–24.
- Berg AH, Du Combs TPX, Brownlee M, Scherer PE. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat Med. 2001;7:947–53.
- Combs TP, Berg AH, Obici S, Scherer PE, Rossetti L. Endogenous glucose production is inhibited by the adipose-derived protein ACRP30. J Clin Invest. 2001;108:1875–81.
- Sun J, Xu J, Dai Z, Sun Y. Intermittent high glucose stimulate MCP-1, IL-18 and PAI-1, but inhibit adiponectin expression and secretion in adipocytes dependent of ROS. Cell Biochem Biophys. 2009;55: 173–80.
- Ozeki H, Hara K, Yatsuka C, Nakano T, Matsumoto S, Suetsugu M, . Serum high-molecular weight adiponectin decreases abruptly after an oral glucose load in subjects with normal glucose tolerance or impaired fasting glucose, but not those with impaired glucose tolerance or diabetes mellitus. Metabolism. 2009;58:1470–6.
- Duncan BB, Schmidt MI, Pankow JS, Ballantyne C, Couper D, Vigo A, . Low-grade systemic inflammation and the development of type 2 diabetes. The Atherosclerosis Risk in Communities Study. Diabetes. 2003;52:1799–805.
- Koenig W, Khuseyinova N, Baumer J, Meisinger C, Löwel H. Serum concentrations of adiponectin and risk of type 2 diabetes mellitus and coronary heart disease in apparently healthy middle aged men. Results from the 18-year follow-up of a large cohort from Southern Germany. JAm CollCardiol. 2006;48:1369–77.
- Krakoff J, Funahashi T, Stehouwer CDA, Schalkwijk CJ, Tanaka S, Matsuzawa Y, . Inflammatory markers, adiponectin and risk of type 2 diabetes in the Pima Indian. Diabetes Care. 2003;26:1745–51.
- Vázquez G, Duval S, Jacobs D, Silventoinen K. Comparison of body mass index, waist circumference, and waist/hip ratio in predicting incident diabetes: a meta-analysis. Am J Epidemiol. 2007;29:115–28.
