Abstract
Hereditary angioedema (HAE) is a potentially life-threatening autosomal dominant disease characterized by recurrent episodes of oedema, commonly occurring in the skin, abdomen, and upper respiratory tract. After many years during which limited treatment options were available, a range of newer therapies with proven efficacy have been approved in Europe by the European Commission for the treatment of HAE attacks. However, due to differing legislation and financial restrictions, these treatment options are not available in all countries. Home therapy and self-administration of treatment are recommended in order to minimize the burden of disease upon the patient, with the ideal treatment option being effective, well-tolerated, and easy to prepare and administer. Recently, the Hereditary Angioedema International Working Group (HAWK) consensus recommended early, on-demand treatment for HAE. This article reviews the current treatment options available, and considers the need for treatment guidelines to recommend the appropriate therapy.
Key messages
Recent developments have widened the treatment options for managing acute hereditary angioedema (HAE) attacks in Europe; however, regulations and financial restrictions mean that actual availability of these medications can vary widely.
Consultation between the patient and doctor is important to agree a suitable treatment strategy, taking into account points such as the preferences of the patient and the potential benefits of home therapy and self-administration.
The development of consensus guidelines offering clear recommendations and treatment algorithms describing how and when each treatment should be used is essential.
Living with hereditary angioedema
Hereditary angioedema (HAE) is a debilitating and potentially life-threatening autosomal dominant disorder of C1-inhibitor (C1-INH) deficiency characterized by recurrent episodes of oedema, commonly occurring in the skin, gut, and upper respiratory tract (Citation1). HAE attacks are a result of reduced levels of functional C1-INH protein, leading to elevated levels of bradykinin during HAE attacks, which in turn trigger increased vascular permeability and oedema (Citation2,Citation3). Attack onset is unpredictable, and the frequency, duration, and severity of attacks can vary considerably between individuals, even within the same family, and within the same individual from attack to attack (Citation4). Importantly, HAE is associated with mortality as a result of airway obstruction during laryngeal attacks (Citation5).
The unpredictable, painful and sometimes life-threatening nature of attacks can make HAE extremely stressful for patients and their families (Citation6,Citation7), with HAE attacks having a severe effect upon patients’ quality of life, restricting their school, work, or social life (Citation7). A survey of 457 HAE patients in the United States (US) found that HAE is also associated with high medical and economic costs related to the number of physician visits, missed work days, reduced productivity, hospital stays, chronic treatment, laboratory tests, and hospital procedures (Citation8). The estimated total costs per patient were US$42,000, ranging from US$14,000 for patients with mild attacks to US$96,000 for those with severe attacks. Assessment of the humanistic burden of illness associated with HAE confirmed significantly reduced health-related quality of life in HAE patients and markedly reduced productivity, including a 34% overall work impairment (Citation9). It was concluded that HAE results in a significant physical and mental burden to patients, negatively impacting education, career, and work productivity, and compounding the economic burden of the disease.
Due to the non-specific clinical symptoms and rareness of HAE, delays in receiving a diagnosis are common (Citation10,Citation11). During this delay HAE may be misdiagnosed and inappropriately treated; for example, antihistamines and corticosteroids may be administered to treat suspected allergies, and exploratory abdominal surgery may be carried out in response to abdominal oedema (Citation1). Even once a diagnosis of HAE has been achieved, patients can experience delays in receiving the correct treatment when attending a medical facility (Citation12). Aside from the time required to travel to hospital, once the patient has arrived they may find that the staff are unfamiliar with HAE and its treatment options, leading to a delay in treatment and other emergencies being prioritized over the HAE patient (Citation12). As most HAE attacks will resolve over time, patients may choose to remain at home and attempt self-management of their attacks with symptomatic treatment (potentially increasing their absenteeism from work or school) rather than attend a hospital for treatment (Citation12).
What HAE treatment options are available in the European Union?
Several treatment strategies have been developed to treat HAE: increasing C1-INH plasma levels, kallikrein inhibition, and blockade of bradykinin signalling ().
Figure 1. Simplified schematic of the biological cascades, regulated by C1-INH, which lead to oedema, indicating which stage each treatment option targets (Citation13). Adapted by permission from Macmillan Publishers Ltd: Zuraw et al. Nat Rev Drug Discov 2010;9(3):189–190, copyright 2010. Abbreviations: C1-INH = C1-inhibitor; HMMK = high-molecular mass kininogen; BK = bradykinin.
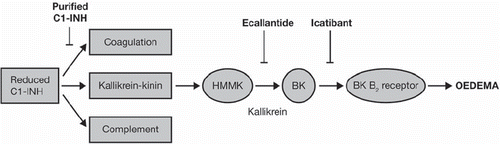
Antifibrinolytics and synthetic androgens have been used for many years; however, besides their considerable side-effects and limiting contraindications, there is no evidence for their efficacy and reliability for the treatment of acute attacks. In several European countries since the late seventies, HAE attacks have traditionally been treated with intravenous infusion of replacement plasma-derived (pd) C1-INH isolated from pooled human plasma. Measures have been taken in order to reduce the potential risk of virus transmission, including pasteurization and nanofiltration (Berinert®, CSL Behring Marburg, Germany; Cetor®/Cebitor®, Sanquin, Amsterdam, The Netherlands; Cinryze®, ViroPharma Inc., Exton, PA, USA), and a recombinant human (rh) C1-INH concentrate (Ruconest®, Pharming, Leiden, The Netherlands) has been developed. Since the introduction of the pasteurization step, pdC1-INH has been well tolerated, and viral transmission attributed to pdC1-INH has not been reported (Citation14,Citation15). Plasma-derived C1-INH has been approved in the European Union (EU) for intravenous self-administration to treat acute HAE attacks.
Ecallantide (Kalbitor®, Dyax Corp., Burlington, MA, USA) inhibits the action of kallikrein, preventing the release of bradykinin and inhibiting oedema formation (Citation16). Although ecallantide is administered subcutaneously rather than intravenously, it must be administered by a health care professional with appropriate medical support due to the risk of anaphylaxis (Citation17). Until November 2011 ecallantide was under review in the EU; however, Dyax have since withdrawn their application.
In July 2008, the bradykinin B2 receptor antagonist icatibant (Firazyr®, Shire HGT AB, Lund, Sweden), was licensed in the EU to treat type I and II HAE attacks in adults, becoming the first subcutaneous treatment option for HAE attacks. Instead of replacing C1-INH, icatibant blocks the action of bradykinin, the key mediator of the symptoms of HAE, by binding to the bradykinin B2 receptor, thereby interrupting and inhibiting oedema formation (Citation2,Citation3). The approval of icatibant for self-administration to treat acute HAE attacks in April 2011 has made it the only EU-approved acute HAE treatment option licensed for subcutaneous self-administration.
The efficacy of each of these new HAE treatment products has been demonstrated in clinical studies (Citation16,Citation18–24). The licensed indication and method of administration of each product are summarized in and .
Figure 2. Treatment options with licensed and non-licensed indications for HAE.
*Cinryze® licensed for prophylaxis; some clinicians prescribe Berinert® off-label for this purpose.
Table I. Overview of treatments available.
Which licensed treatment options are actually administered?
Although several different treatment options are licensed for use within the EU, this does not necessarily mean that they are available in all EU countries. In different countries, the registration and reimbursement regulations of individual local health authorities will affect which drugs are approved and recommended in each area, meaning that patients can find that their options are curtailed. The physicians themselves are also influenced by financial considerations, with 30% stating that cost was the most important factor in influencing treatment recommendations (Citation33).
For example, although pdC1-INH has been approved for the treatment of HAE attacks in several countries since 1979, it is only recently that treatment options have become more generally available (Citation14). While few pharmacies would stock more than one C1-INH concentrate product, treatment options with different modes of action should be stocked. Cost may be one factor in deciding which treatment options are offered, with each country having different criteria to consider. In Austria, for example, treatment costs in the hospital (both as in- and out-patients) are covered by the public, whilst treatment outside the hospital is paid for by the individual's social insurance system. Currently, HAE attacks are typically treated with infusions of replacement C1-INH within the hospital, with insurance companies reluctant to approve the use of a self-administered product which they would then have to pay for. Equally, although it is possible to administer higher doses of replacement C1-INH, doctors will usually administer only the lower dose, as doctors, patients, and the insurance companies are aware that this dose will be efficacious in most instances. But is this the ideal way to treat patients?
How do we use these treatment options?
As awareness of HAE has increased and new treatment options have become available, international consensus recommendations have been published to share expert opinion and educate physicians on the diagnosis and treatment of HAE (Citation14,Citation34). However, due to the rarity of HAE and the lack of head-to-head studies, these documents are only able to offer diagnostic algorithms and information on the different treatment options available. There are currently no clear treatment guidelines for physicians to follow, making it difficult for those physicians who are not specialists in the field to select the appropriate first- or second-line treatment option. However, the future World Allergy Organization (WAO) guidelines may provide clearer guidance for physicians.
It is also debatable how aware physicians are of the current treatment options and guidelines. A recent survey in the US of 172 physicians between October 2009 and February 2010 illustrated the wide variability of treatment of patients with HAE, albeit with the continued use of older therapies (Citation35). It should be noted that this survey reflects the situation in the US shortly after C1-INH concentrate had been approved for acute attacks, and ecallantide was approved during the survey period; icatibant and recombinant C1-INH were not available in the US at the time the survey was conducted. The US survey found that although C1-INH concentrate was the most common treatment for acute attacks, used by 49.4% of physicians, similar usage rates were reported for fresh frozen plasma (40.1%), intravenous hydration (45.4%), and oral analgesics (35.5%). Additionally, 9.3% of respondents indicated they did not recommend any treatment for acute attacks. Most physicians were familiar with the different (pasteurized and recombinant) C1-INH concentrate products (69.7%–80.2%), with just over half familiar with ecallantide (52.3%) and icatibant (55.2%). Interestingly, approximately a quarter of all respondents indicated they were unlikely to prescribe either ecallantide (26.7%) or icatibant (22.1%); this may have been due to a lack of knowledge about these treatment agents (Citation35). The recently published Hereditary Angioedema International Working Group (HAWK) consensus recommendations will hopefully provide more guidance to physicians (Citation34).
In theory, we might expect results of a similar survey in Europe, where C1-INH concentrate has been available for decades, to be somewhat different. A current survey by the WAO, covering 153 physicians predominantly from Asia, Europe, and South America, notes that although familiarity with emerging therapies was variable, many respondents were likely to use novel therapies if they had access to them (Citation33). However, the clinical variability seen in HAE treatment in Europe may also reflect both the variation in availability of different therapies and the lack of familiarity (or perhaps confidence) with newer therapies by physicians. It appears that information on new treatments is better spread from patient to patient (e.g. through both international and national patient organizations) than between doctors.
Thus, the development of treatment algorithms that provide guidance on how and when to use recently approved treatment options would be of great assistance to physicians and could improve the management of this debilitating disease. The HAWK consensus recommends early, on-demand treatment for HAE (Citation34), but questions remain as to which drug to use, the rationale for each choice, and when each should be given.
So how should we treat HAE?
The requirement to attend a medical facility to receive treatment for an HAE attack can result in treatment delays and disruptions to patients’ lives (Citation12,Citation36). The ability to self-administer treatment confers a number of advantages, including the possibility of earlier access to treatment when an attack occurs. Guidelines have been laid out in the HAE International Home Therapy Consensus Document, which suggest that self- (and home) administration offers the prospect to HAE patients of minimal disruption while living a healthy and productive life (Citation12). Self-administration in particular has the possibility to provide patients with the opportunity to control their HAE, and not be controlled by their disease.
Faster symptom relief and attack resolution have been observed with self-administration of C1-INH concentrate compared with attacks prior to the start of self-administration, potentially due to the reduced attack-to-treatment time (Citation37). Early treatment with C1-INH has also been implicated in the prevention of severe attacks and reduced consumption of C1-INH concentrate (Citation38). Experience with self- or assisted-infusion of C1-INH concentrate has demonstrated a significant improvement in quality of life, with patients feeling that they had gained control of their disease and their lives and experiencing reduced fear of life-threatening upper-airway attacks and painful abdominal attacks (Citation7). Additionally, as patients trained in self-administration of C1-INH concentrate show reduced hospitalization, the reported health care and personal expenses related to HAE may be potentially reduced (Citation7,Citation8).
Ideally, self-administration should be a simple process, easily carried out at the first signs of an attack. At present, intravenous pdC1-INH and subcutaneous icatibant have been licensed for self-administration within the EU, offering a choice of both treatment and administration technique. Whilst C1-INH concentrate can be used for either self- or assisted-administration outside of a medical setting, reconstitution of lyophilized C1-INH concentrate is time-consuming, and transportation, self-cannulation, and administration are not ideal. Additionally, intravenous administration carries the risk of complications such as phlebitis, infiltration, and extravasation (Citation39). In contrast, the technique for subcutaneous injection is easily taught, and the risks associated with poor technique are minimal (Citation40). There is a high likelihood of injection site reactions with subcutaneous administration, although these are self-limiting and resolve without further intervention (Citation22,Citation23). However, patients may prefer to self-administer the treatment they are most familiar with, regardless of whether it is intravenously or subcutaneously administered, and will also consider the perceived efficacy and absence of relapse. Therefore, the final choice of treatment should be reached between the patient and their doctor.
It is of course important to consider the patient's preferences and agree to a suitable treatment strategy with them. Home treatment with C1-INH concentrate has been available for years, with some patients being taught to self-administer whilst others rely on helpers. Assessment of the impact of self-administration of C1-INH concentrate on quality of life found that patients felt they had gained control of their disease and their lives (Citation7). As awareness of the benefits of home therapy spreads via patient networks, many patients are now requesting it from their physicians (Citation36). Thus, physicians and health authorities should be fully aware of the benefits that self-administration holds, for both patients (direct benefits) and hospitals (indirect benefits), and consider these benefits when deciding upon a preferred treatment option.
Where do we go from here?
Treatment options for managing acute HAE attacks have been available in many European countries for some time. Although recent developments have widened the options available, regulations and financial restrictions mean that actual availability of these medications can vary widely. Health-economic data showing the cost-benefits of these treatment options are needed to enable patients across Europe to access their chosen treatment option. Additionally, where these treatments are available, there may be a reluctance to adopt newer therapies due to a lack of familiarity with them. As such, treatment algorithms describing how and when each treatment should be used would offer invaluable assistance to many physicians.
The management of HAE is changing, with treatment options that can be self-administered allowing patients to decide when to treat their HAE attacks with early and on-demand treatment as recommended by the HAWK consensus (Citation34).
Notice of Correction
The version of this article that was published online on 11 Jul 2012. contained an error in Table I. The author has added the following footnote to the Table: Although the wording in each product SPC differs, the general advice for these products is to only use during pregnancy if the potential benefit justifies the potential risk to the foetus, and caution should be exercised when administering to nursing women (Citation17,Citation28–32). The author also revised the considerations in the table so that icatibant and ecallantide are not contraindicated during pregnancy or lactation.
Declaration of interest: Medical writing support was provided by Claire Crouchley at Prime Medica Ltd during the preparation of this paper, supported by Shire Human Genetic Therapies, Inc. (HGT). Responsibility for opinions, conclusions, and interpretation of data lies with the author.
W. Aberer has acted as a medical advisor and speaker for Shire HGT and CSL Behring; received funding to attend conferences and other educational events, received donations to his departmental fund, and participated in clinical trials for Shire HGT.
References
- Agostoni A, Aygören-Pürsün E, Binkley KE, Blanch A, Bork K, Bouillet L, . Hereditary and acquired angioedema: problems and progress: proceedings of the third C1 esterase inhibitor deficiency workshop and beyond. J Allergy Clin Immunol. 2004;114:S51–131.
- Kaplan AP, Joseph K. The bradykinin-forming cascade and its role in hereditary angioedema. Ann Allergy Asthma Immunol. 2010;104:193–204.
- Nussberger J, Cugno M, Amstutz C, Cicardi M, Pellacani A, Agostoni A. Plasma bradykinin in angio-oedema. Lancet. 1998;351:1693–7.
- Weis M. Clinical review of hereditary angioedema: diagnosis and management. Postgrad Med. 2009;121:113–20.
- Nzeako UC, Frigas E, Tremaine WJ. Hereditary angioedema: a broad review for clinicians. Arch Intern Med. 2001;161: 2417–29.
- Bracho FA. Hereditary angioedema. Curr Opin Hematol. 2005;12:493–8.
- Bygum A, Andersen KE, Mikkelsen CS. Self-administration of intravenous C1-inhibitor therapy for hereditary angioedema and associated quality of life benefits. Eur J Dermatol. 2009;19: 147–51.
- Wilson DA, Bork K, Shea EP, Rentz AM, Blaustein MB, Pullman WE. Economic costs associated with acute attacks and long-term management of hereditary angioedema. Ann Allergy Asthma Immunol. 2010;104:314–20.
- Lumry WR, Castaldo AJ, Vernon MK, Blaustein MB, Wilson DA, Horn PT. The humanistic burden of hereditary angioedema: impact on health-related quality of life, productivity, and depression. Allergy Asthma Proc. 2010;31:407–14.
- Roche O, Blanch A, Caballero T, Sastre N, Callejo D, López-Trascasa M. Hereditary angioedema due to C1 inhibitor deficiency: patient registry and approach to the prevalence in Spain. Ann Allergy Asthma Immunol. 2005;94:498–503.
- Lunn ML, Santos CB, Craig TJ. Is there a need for clinical guidelines in the United States for the diagnosis of hereditary angioedema and the screening of family members of affected patients? Ann Allergy Asthma Immunol. 2010;104: 211–4.
- Longhurst HJ, Farkas H, Craig T, Aygören-Pürsün E, Bethune C, Bjorkander J, . HAE international home therapy consensus document. Allergy Asthma Clin Immunol. 2010;6:22.
- Zuraw B, Yasothan U, Kirkpatrick P. Ecallantide. Nat Rev Drug Discov. 2010;9:189–90.
- Bowen T, Cicardi M, Farkas H, Bork K, Longhurst HJ, Zuraw B, . 2010 International consensus algorithm for the diagnosis, therapy and management of hereditary angioedema. Allergy Asthma Clin Immunol. 2010;6:24.
- De Serres J, Gröner A, Lindner J. Safety and efficacy of pasteurized C1 inhibitor concentrate (Berinert P) in hereditary angioedema: a review. Transfus Apher Sci. 2003;29:247–54.
- Cicardi M, Levy RJ, McNeil DL, Li HH, Sheffer AL, Campion M, . Ecallantide for the treatment of acute attacks in hereditary angioedema. N Engl J Med. 2010;363:523–31.
- Kalbitor prescribing information. Available from: http://www.kalbitor.com/pdf/KalbitorFullPrescribingInformation.pdf (accessed 15 July 2011).
- Craig TJ, Levy RJ, Wasserman RL, Bewtra AK, Hurewitz D, Obtułowicz K, . Efficacy of human C1 esterase inhibitor concentrate compared with placebo in acute hereditary angioedema attacks. J Allergy Clin Immunol. 2009;124: 801–8.
- Wasserman RL, Levy RJ, Bewtra AK, Hurewitz D, Craig TJ, Kiessling PC, . Prospective study of C1 esterase inhibitor in the treatment of successive acute abdominal and facial hereditary angioedema attacks. Ann Allergy Asthma Immunol. 2011;106:62–8.
- Zuraw BL, Busse PJ, White M, Jacobs J, Lumry W, Baker J, . Nanofiltered C1 inhibitor concentrate for treatment of hereditary angioedema. N Engl J Med. 2010;363:513–22.
- Zuraw B, Cicardi M, Levy RJ, Nuijens JH, Relan A, Visscher S, . Recombinant human C1-inhibitor for the treatment of acute angioedema attacks in patients with hereditary angioedema. J Allergy Clin Immunol. 2010;126:821–7.e14.
- Cicardi M, Banerji A, Bracho F, Malbrán A, Rosenkranz B, Riedl M, . Icatibant, a new bradykinin-receptor antagonist, in hereditary angioedema. N Engl J Med. 2010;363: 532–41.
- Lumry WR, Li HH, Levy RJ, Potter PC, Farkas H, Moldovan D, . Randomized placebo-controlled trial of the bradykinin B2 receptor antagonist icatibant for the treatment of acute attacks of hereditary angioedema: the FAST-3 trial. Ann Allergy Asthma Immunol. 2011;107:529–37.
- Levy RJ, Lumry WR, McNeil DL, Li HH, Campion M, Horn PT, . EDEMA4: a phase 3, double-blind study of subcutaneous ecallantide treatment for acute attacks of hereditary angioedema. Ann Allergy Asthma Immunol. 2010;104:523–9.
- Gompels MM, Lock RJ, Abinun M, Bethune CA, Davies G, Grattan C, . C1 inhibitor deficiency: consensus document. Clin Exp Immunol. 2005;139:379–94.
- Cyklokapron summary of product characteristics. Available from: http://www.medicines.org.uk/emc/medicine/16512/SPC/ (accessed 17 August 2011).
- Caballero T, Farkas H, Bouillet L, Bowen T, Gompel A, Fagerberg C, . International consensus and practical guidelines on the gynecologic and obstetric management of female patients with hereditary angioedema caused by C1 inhibitor deficiency. J Allergy Clin Immunol. 2012;129:308–20.
- Cinryze summary of product characteristics. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/001207/WC500108895.pdf (accessed 15 July 2011).
- Berinert prescribing information. Available from: http://www.berinert.com/docs/Berinert_pi.pdf (accessed 15 July 2011).
- Cetor summary of product characteristics. Available from: http://www.sanquin.nl/sanquin-eng/sqn_products_plasma.nsf/All/F197E/$FILE/common-spc-summaryofproductcharacteristicscetor500uen.pdf (accessed 15 July 2011).
- Ruconest summary of product characteristics. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/001223/WC500098542.pdf (accessed 15 July 2011).
- Firazyr summary of product characteristics. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000899/WC500022966.pdf (accessed 15 July 2011).
- Craig T, Katelaris C, Longhurst H, Grumach A, Maurer M. Hereditary angioedema worldwide epidemiology and treatment practices: interim results of a WAO survey. Allergy. 2011;66(Suppl 94):1–104. (Abstract no. 164).
- Cicardi M, Bork K, Caballero T, Craig T, Li HH, Longhurst H, . Evidence-based recommendations for the therapeutic management of angioedema owing to hereditary C1 inhibitor deficiency: consensus report of an International Working Group. Allergy. 2012;67:147–57.
- Riedl M, Gower RG, Chrvala CA. Current medical management of hereditary angioedema: results from a large survey of US physicians. Ann Allergy Asthma Immunol. 2011;106:316–22.e4.
- Longhurst HJ, Carr S, Khair K. C1-inhibitor concentrate home therapy for hereditary angioedema: a viable, effective treatment option. Clin Exp Immunol. 2007;147:11–17.
- Levi M, Choi G, Picavet C, Hack CE. Self-administration of C1-inhibitor concentrate in patients with hereditary or acquired angioedema caused by C1-inhibitor deficiency. J Allergy Clin Immunol. 2006;117:904–8.
- Rusicke E, Martinez-Saguer E, Aygören-Pürsün E, Kreuz W. Home treatment in patients with hereditary angioedema (HAE). J Allergy Clin Immunol. 2006;117:S180. (Abstract no. 700).
- Gabriel J. Infusion therapy. Part two. Prevention and management of complications. Nurs Stand. 2008;22:41–8.
- Dagen C, Craig TJ. Treatment of hereditary angioedema: items that need to be addressed in practice parameter. Allergy Asthma Clin Immunol. 2010;6:11.
