Abstract
Atherosclerotic renovascular disease (ARVD) is a common condition in both elderly patients and those with other vascular disease. No published randomized controlled trial has demonstrated an overall benefit of revascularization on any clinical or biochemical end-point, and optimal medical therapy in this condition is not clearly defined. In this review we consider the epidemiology of ARVD and discuss the evidence for current medical treatment. We also address the literature on revascularization, consider settings in which an interventional approach may still be considered, and touch upon on-going areas of research.
Key messages
Renal artery revascularization does not have a routine place in the management of atherosclerotic renovascular disease (ARVD).
Medical managements of patients with ARVD should include angiotensin blockade and statin therapy as first-line treatment.
In selected clinical settings, e.g. flash pulmonary edema, consideration for revascularization may be appropriate.
Introduction
Renovascular disease encompasses a spectrum of pathologies of which atheromatous renovascular disease (ARVD) is the most common manifestation. ARVD is often a silent disease, which can be under-recognized in the general population. In cases where signs or symptoms are detected, hypertension is the most common presentation. Despite its frequently indolent presentation, the prognostic implications of ARVD can be severe, with significant increases in cardiovascular mortality and morbidity related to the disease and associated abnormalities in other arterial trees.
Since the early 1980s, percutaneous techniques for renal artery revascularization have evolved and almost entirely replaced the surgical techniques pioneered in the late 1950s. Despite high rates of technical success, benefits of these procedures at a population level have not been demonstrated in a randomized controlled trial (RCT).
In this review we present an overview of the epidemiology of ARVD and then discuss diagnostic and treatment options.
Epidemiology
In the Western world renal artery stenosis (RAS) is largely atheromatous in nature, with around 10% of cases due to fibromuscular disease. This contrasts with the Far East and Indian subcontinent, where up to 60% of reported cases are secondary to vasculitis (Citation1). The appellation ARVD has been adopted to provide a distinction from these non-atheromatous causes and to provide descriptive accuracy for both partial and complete occlusions.
Population studies using ultrasound screening in asymptomatic adults aged over 65 years (mean blood pressure 135/72 mmHg) demonstrate a 7% disease prevalence (Citation2), with an annual incidence of 0.5% per annum described in analyses of Medicare claims data (1999–2001) for a comparable population (Citation3). Race does not appear to affect disease prevalence (Citation4). For patients with a history of coronary artery disease, congestive heart failure (CHF), or peripheral vascular disease (PVD), which are enriched populations of vascular disease, prevalence of ARVD can rise to 30%–40% (Citation5–7). Whilst United States Medicare claims data show a rise in the rate of diagnosis of ARVD between 1992 and 2004 (Citation8), subsequent US Renal Data System (USRDS) data (2004–2009) have described a progressive fall in incident (1.7% to 1.3%) and prevalent (1% to 0.7%) rates of end-stage renal disease secondary to ARVD (Citation9).
Despite strong associations with other vascular pathologies, traditional atherosclerotic risk factors such as smoking and diabetes mellitus may not exert the same influence in ARVD. A single-centre study comparing 249 age- and gender-matched patients investigated for ARVD did not demonstrate a significant difference in smoking status or diabetes prevalence between patients found to have ARVD and those with normal renal vessels (Citation10). In these data 68% of ARVD patients had a positive smoking history compared to 55% of non-ARVD patients (diabetes 28% versus 25%). Another study has also shown reduced smoking rates in ARVD patients compared to age- and renal function-matched controls (Citation11). However, information surrounding diabetes and ARVD is less consistent, with reports of increased prevalence of diabetes in ARVD patients compared to the general population (Citation12). These data are based on Medicare claims, and as such level of renal function was not considered between groups. Whether or not smoking and diabetes are implicated in the development of ARVD, their potential effects on prognosis should not be discounted.
Renovascular disease and hypertension
Although patients with ARVD usually have hypertension, the latter may be the cause of the ARVD rather than always being secondary to stimulation of the renin angiotensin aldosterone system (RAAS) due to organ hypoperfusion. A recent series of 62 biopsies from patients with >75% RAS who underwent nephrectomy for resistant hypertension (Citation13) may offer an insight. Here 50% of samples had evidence of intra-renal atherosclerotic disease (IRAVD), and 50% had evidence of intra-renal hypertensive vessel disease (IRHVD) in addition to IRAVD. Presence of IRHVD was associated with a significantly higher burden of glomerulosclerosis (but not related to blood pressure or age-adjusted serum creatinine). This disparity may potentially signal differences in the natural histories of these patients’ diseases. There were no histological features ‘unique’ to RAS.
There are no data specifically describing how many hypertensive patients in the general population have ARVD. Figures of 1% in the general population, rising to 5% in hospitalized patients have been suggested (Citation14) but are difficult to substantiate. More specific data are available when targeted patients groups are considered. In Germany, 161 patients presenting with severe hypertension (>180 mmHg systolic and/or >100 mmHg diastolic) to an emergency room were screened for secondary causes of elevated blood pressure; using duplex ultrasound, a significant RAS (defined as maximal renal artery flow ≥200 cm/hour) was found in 8.1% of patients (Citation15). Specific studies assessing young hypertensive patients or patients with abdominal bruits show a pooled prevalence of 14% for RAS (though a few of these patients have fibromuscular disease (FMD) not ARVD), rising to 20% in hypertensive diabetic patients (Citation12).
Other clinical presentations of ARVD
Flash pulmonary edema (FPE), rapid loss of renal function, and acute kidney injury (AKI) are other recognized manifestations/presentations of ARVD.
In our local database of over 1000 ARVD patients, evidence of presentation with FPE prior to diagnosis of ARVD can be found in approximately 7% of patients (Citation16). Arterial disease in this setting is invariably bilateral or affecting a single functioning kidney. In RAS, excess aldosterone secretion secondary to renal hypoperfusion results in increased vascular permeability and salt/water retention (Citation17). If the contralateral kidney is not diseased, it can reduce its renin secretion in response to elevations in aldosterone levels to prevent volume overload. In bilateral disease this response does not exist, and the resulting volume expansion, in combination with the left ventricular hypertrophy (Citation18) and increased vascular stiffness (Citation19) found in chronic kidney disease (CKD), leads to the dramatic decompensation that characterizes FPE.
Exact data on rates of AKI or rapid loss of function in ARVD are more challenging to estimate. Indeed, what constitutes rapid loss of function is ill-defined. Many patients do not have laboratory results available prior to diagnosis, making estimation of overall rates of loss of function difficult. When ARVD presents with AKI it is invariably in the presence of significant bilateral disease or a stenosis to a single functioning kidney (Citation20,Citation21). Anuric presentations of ARVD result from acute parenchymal ischaemic injury and happen when occlusions occur before the development of a collateral circulation (Citation22). In a chronic setting, where renal blood flow has fallen beneath the level at which autoregulation can preserve glomerular perfusion, a collateral circulation (e.g. from lumbar vessels) develops to maintain parenchymal viability. Where an additional insult further reduces perfusion, the collateral circulation cannot compensate, and AKI develops.
Many patients with significant RAS are first ‘uncovered’ by an acute fall in GFR in association with initiation of RAAS blockade with either angiotensin-converting enzyme inhibitors (ACE-i) or angiotensin receptor blockers (ARB). This is recognized in those with bilateral significant disease, or RAS to a solitary functioning kidney, but in the setting of unilateral RAS with a normally functioning contralateral kidney most patients avoid this fate. In view of the high prevalence of CKD and proteinuria in ARVD, ACE-i and ARB have much to offer ARVD patients in reducing cardiovascular events and mortality, and reduced chronic dialysis initiation (Citation23). Hence, the indication of renal revascularization to permit ACE-i or ARB use is an attractive one. However, experience of use of RAAS blockade in the setting of significant bilateral ARVD, without detriment to renal function, is now growing (Citation24).
Prognosis in ARVD
Although the overall rate of loss of renal function in ARVD is low (Citation25), a subset of patients lose renal function more rapidly and are at risk of progression to end stage kidney disease (ESKD). Little information exists to guide clinicians in identification of this patient group. Cohort data (preceding widespread adoption of RAAS blockade) suggested the only factor associated with an increased risk of loss of renal function was an elevation in baseline proteinuria (Citation26). This association was independent of the degree of RAS. Other studies have confirmed the absence of a relationship between degree of RAS and level of proteinuria, but found (as seen in CKD generally) a relationship between proteinuria and eGFR (Citation27,Citation28). As such, proteinuria is thought to be a marker of downstream hypertensive/ischaemic renal parenchymal injury in ARVD. This is supported by histological studies of ARVD patients which describe patterns of injury very similar to those seen in hypertensive patients (Citation29). This established parenchymal damage explains why patients with higher levels of proteinuria are more likely to progress to renal replacement therapy (RRT), or fail to respond to revascularization (Citation30,Citation31). In these data, a level of proteinuria as low as 0.6 g/24 hours was an independent risk factor for failure to improve renal function following revascularization, suggesting that parenchymal damage may be the main arbiter of renal outcome. If this hypothesis is accepted, the cause of proteinuria in ARVD becomes a key question. Long-term RAAS activation is linked to, amongst other things, oxidative stress, which can promote intra-renal atherosclerosis and glomerulosclerosis (Citation32). Hence, development of proteinuria in ARVD may represent progression through the natural history of the disease to its renal end-point (and thus beyond the help of revascularization). Equally proteinuria may reflect concurrent microvascular renal disease, e.g. diabetic/hypertensive, which has occurred independently of the stenosis (Citation33). A patient-level analysis of RCT data stratifying outcomes by level of proteinuria may be a worthwhile undertaking.
Mortality rates in ARVD are elevated, with RCT data describing an 8% annual mortality (compared to 3.7% in the general population) (Citation25). Where disparate vascular disease co-exists, mortality rates further increase. Patients with symptomatic PVD and an asymptomatic RAS of >60% have a 2.9-fold relative risk of death (Citation34), and patients with a positive screen for ARVD during diagnostic coronary angiography have a 4-year survival of 57% compared to 89% in patients with normal renal arteries (Citation35).
Diagnosis of ARVD
New imaging techniques have challenged the ‘gold standard’ position of direct angiography in diagnosis of ARVD, with use of this technique limited by the invasive nature of the procedure and the requirement for iodinated contrast. Three non-invasive imaging techniques are used in current practice—ultrasound, computed tomography angiography (CTA), and magnetic resonance angiography (MRA). Duplex ultrasound (DUS) has the advantage of requiring neither contrast nor radiation and offers high levels of positive and negative predictive accuracy for stenosis in a single renal vessel (Citation36). However, it is a time-consuming and operator-dependent technique, with bowel gas patterns and obese body habitus causing 10% technical failure rates even in experienced hands (Citation37).
The widespread availability of CTA and reproducibility of images have increased the uptake of this technique (although heavy vascular calcification often found in ARVD can make images difficult to interpret). Use of MRA has also increased—partly due to concerns over ionizing radiation and risk of contrast nephropathy with CTA. In head-to-head comparison, the two techniques have comparable sensitivity, specificity, and negative predictive accuracy (CTA 94%, 93%, 99%; MRA 90%, 94%, 98%, respectively) (Citation38). However, evidence linking the development of nephrogenic systemic fibrosis to gadolinium exposure (the contrast agent used in MRA) has become a major barrier to MRA use in patients with advanced CKD.
Current imaging research is targeted towards generation of a contrast-free MRI technique. Investigation into one such technique, arterial spin labelling, is at an early stage and is on-going (Citation39), but a greater depth of literature exists concerning blood oxygen level-dependent (BOLD) imaging. BOLD imaging provides a surrogate measurement of tissue oxygenation (calculated from levels of deoxyhaemoglobin), described by an R2* value. A reduced R2* value represents a fall in deoxyhaemoglobin levels and is seen in non-stenosed kidneys when the metabolic demand of the organ is reduced. An early study investigating BOLD in ARVD found that, following administration of furosemide, organs with significant stenosis but preserved volume had comparable changes in R2* values to non-stenosed kidneys, whilst organs which were stenosed and atrophied did not exhibit this same change (Citation40). This raised the possibility of a role for BOLD in selecting organs most likely to benefit from revascularization. Though baseline R2* values are similar in organs of patients with essential hypertension and ARVD (Citation41), a pilot study of 16 ARVD patients investigated with combined BOLD and isotope-GFR studies has shown promise in identifying patients most likely to receive renal functional improvement after intervention (Citation42). BOLD remains a promising research technique. However, the intra-renal haemodynamic effects of medications (e.g. acetazolamide), which can reduce both tissue oxygen demand and renal blood flow, must be better understood before we are able fully to interpret changes in R2* values (Citation43).
Medical therapy in ARVD
There are no randomized trial data that can define optimal medical therapy in ARVD. Instead, treatment decisions are based on observational data and historical perspective. The on-going Cardiovascular Outcomes in Renal Atherosclerotic Lesions (CORAL) trial (Citation44) has standardized medical therapy to anti-platelet and lipid-lowering therapy in all patients, with use of an angiotensin II blocker as the first-line anti-hypertensive agent. This protocol accurately reflects current opinion and practice.
Anti-platelet therapy
Anti-platelet therapies have the longest historical standing in ARVD and the smallest body of evidence. The significant systemic burden of atheroma associated with ARVD is accepted as indication for anti-platelet therapy (Citation45), with current research focused on anti-platelet strategies peri-revascularization.
When performing renal artery angioplasty and stenting, microemboli sufficiently large to cause renal parenchymal damage are released (Citation46). One study has shown a significant reduction in the rate of generation of these platelet-rich emboli when clopidogrel is added to standard aspirin therapy prior to intervention (Citation47). However, no study has assessed long-term renal functional outcomes with dual anti-platelet therapy. Other work suggests that ARVD patients most likely to develop platelet-rich emboli during stenting have higher circulating levels of soluble CD40 ligand, a marker of platelet activation (Citation48). This finding may suggest a future role of glycoprotein IIa/IIIb inhibition during renal artery stenting, a technique that has shown promise when combined with an embolic protection device (Citation49).
Statin therapy
Lipid-lowering therapy in ARVD seems a rational choice based on the burden of concomitant vascular disease, slower rates of loss of renal function in all-cause CKD patients treated with statins (Citation50,Citation51), and data from the recent Study of Heart and Renal Protection (SHARP) trial which demonstrated a reduction in the rate of major cardiovascular events in CKD patients treated with simvastatin 20 mg + ezetimibe 10 mg (Citation52). Specifically within ARVD, the Single Operator, Single Centre, Renal Stent Registry Study demonstrated a reduced hazard for death in revascularized patients treated with lipid-lowering therapy (hazard for death 0.17, P = 0.019) but did not consider medically managed patients (Citation53). Other cohort data (followed over an 11-year period) have been used to compare dyslipidaemic ARVD patients treated with statin therapy (n = 68) with ARVD patients with normal lipid profiles (n = 36) (Citation54). With comparable proportions of patients revascularized within each group, the significantly reduced mortality for statin-treated patients (6% versus 36%, P < 0.05) and improved renal survival suggests a pleiotropic role of statins irrespective of lipid profile. Although truly randomized outcome data are lacking (and now unlikely ever to become available), there are potential pathophysiological explanations for the benefits described in statin-treated ARVD patients. Cohort analysis has demonstrated a 0.28 relative risk of stenosis progression (average follow-up period 27 months) for patients receiving statin therapy (Citation55), and lower levels of renal fibrosis develop in statin-treated animals in porcine RAS models (Citation56).
Renin angiotensin blockade
Target blood pressure in CKD is often defined as <130/80 mmHg (Citation57), with angiotensin blockade considered first-line therapy due to putative renal protection in excess of the benefits provided solely by blood pressure reduction (Citation58). In ARVD, angiotensin blockade is the most effective and well-tolerated anti-hypertensive pharmacotherapy (Citation59,Citation60). As these agents can adversely affect renal function in the presence of bilateral RAS, many clinicians have concerns regarding their use in ARVD, limiting their use (Citation61). Given a growing body of evidence describing reduced mortality in revascularized and non-revascularized ARVD patients treated with angiotensin blockade (Citation23,Citation24,Citation62), potential under-use of these agents is a genuine clinical concern. Whilst a reduction in GFR can be precipitated when they are introduced, this change is reversible upon withdrawal of the agent (Citation63), and the majority of ARVD patients (including those with significant bilateral disease) can tolerate supervised introduction of renin angiotensin blockade without a clinically significant deterioration of their renal function (Citation24,Citation64).
Data on second-line anti-hypertensive agents is scarcer. Within CORAL, diuretics are second-line agents, with calcium-channel blockers and beta-blockers third-line. Mechanistically, the addition of a diuretic to relieve salt and water retention secondary to excess activation of the renin-angiotensin-aldosterone system is a logical approach, and it may be that many cases of resistant hypertension in CKD are due to reticence in the use of diuretic agents (Citation65). The design of CORAL advocates use of a thiazide unless there is significant renal impairment (serum creatinine >2 mg/dL), when a loop diuretic is advocated. This is an important consideration given the lack of efficacy of thiazides in moderate to advanced CKD (Citation66). The neurohormonal over-stimulation seen in ARVD is not limited to the RAAS, with evidence of excess sympathetic activation driving elevations in serum noradrenaline levels (Citation67). This increased sympathetic activity may have a link to cardiovascular mortality (Citation68), and some small studies suggest beta-blockade may slow progression of the degree of RAS (Citation69) or offer benefits to eGFR following revascularization (Citation70).
Emerging therapies
With an appreciation of the role ischaemic endothelial dysfunction plays in the development of renal parenchymal damage, there is interest in the potential utility of endothelial progenitor cells (EPC) as an adjunctive therapy to revascularization. In pig models of RAS with EPC given 6 weeks after induction of stenosis, significant benefits in cortical volume, renal blood flow, and eGFR have been described (Citation71,Citation72). Similar benefits (again in porcine models) have been described with chronic endothelin-A blockade (Citation73). In these data, preserved renal haemodynamics and microvascular density were described in the context of up-regulated angiogenic growth factors such as vascular endothelial growth factor (VEGF).
Loss of VEGF (an endothelial specific growth factor) is associated with development of glomerulosclerosis and tubulointerstitial fibrosis (Citation74). Pig models with intra-renal VEGF given at the time of creation of the stenosis (Citation75) and time of stenosis and at angioplasty (Citation76) have shown benefit to clinical, histological, and microvascular parameters. Though it may suggest a preventative rather than therapeutic role, this remains an exciting area of continuing research.
Revascularization for ARVD
To date, five published RCTs have compared medical therapy with medical therapy plus revascularization in ARVD. The three earliest trials focused on alterations in blood pressure as a primary end-point (Citation77–79), whilst the two most recent (Citation25,Citation80) have considered renal functional outcomes as their primary end-point with blood pressure changes a pre-specified secondary end-point.
Revascularization to control hypertension
None of the five RCT has demonstrated an overall difference in blood pressure outcomes between medically managed and revascularized patients.
The three earliest studies are not comparable with current practice as each used balloon angioplasty without stenting, an approach now known to be associated with worse angiographic outcomes and higher re-stenosis rates (Citation81–83). Furthermore, patient numbers and follow-up periods were small, cross-over rates were high, and each study had significant differences from modern practice in utilization of RAAS blockade.
The two more recent trials published in 2009 better reflect current practice. The smaller of these, the Stenting in Renal Dysfunction Caused by Atherosclerotic Renovascular Disease (STAR) trial (n = 140) did not demonstrate a blood pressure response to revascularization (although this was not the primary end-point) (Citation80); and ASTRAL (with 806 patients recruited worldwide) demonstrated beyond reasonable doubt the parity of medical and interventional therapy in blood pressure control (Citation25). In ASTRAL, with baseline blood pressures of 149/76 mmHg in the revascularization group and 152/76 mmHg in the medical therapy group, systolic and diastolic blood pressure slopes were nearly identical between groups (). This finding has been further emphasized in meta-analysis of the five published RCTs (Citation84).
Figure 1. A: Mean change in systolic blood pressure over time in ASTRAL. B: Mean change in diastolic blood pressure over time in ASTRAL. Top graph: blood pressures for revascularized and medically managed patients. Bottom graph: difference in blood pressure between groups. (Wheatley, K. et al. Revascularization versus medical therapy for renal-artery stenosis. N Engl J Med, 2009. Reproduced with permission.)
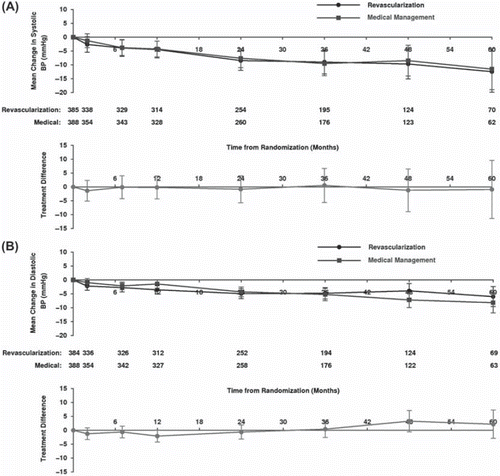
Despite the lack of overall benefit from revascularization in the treatment of hypertension, there remain selected clinical settings in which its use is still considered. In patients with refractory hypertension (blood pressure >160/90 mmHg despite three different anti-hypertensives) it can be challenging to achieve blood pressure control. Whilst no RCT has specifically considered the role of revascularization in this patient group, it is inevitable that some patients included in the published data will have met this definition. Indeed average baseline patient characteristics in the Dutch Renal Artery Stenosis Intervention Cooperative Group (DRASTIC) were blood pressure 180/103 mmHg despite 3.25 anti-hypertensive medications (Citation79). Although blood pressure at 12 months was comparable between groups (angioplasty 160/93 mmHg, medical 162/88 mmHg), more patients in the angioplasty group had an overall blood pressure improvement, defined as reduction in DBP >10 mmHg (68% versus 38%), and fewer had a blood pressure deterioration by the same definition (9% versus 33%, P = 0.002). Although this finding is in the context of >40% cross-over from the medical to interventional arm, it is also noteworthy that the 14 patients who crossed over from the medical to interventional arm due to uncontrolled blood pressure at 3 months exhibited blood pressure improvements at 12 months (190/111 mmHg versus 169/102 mmHg). Post-hoc analysis of published data, combined with future information from CORAL, may form a valuable future resource to address this issue further.
Revascularization may also facilitate safe use of angiotensin blockade in patients previously intolerant to this therapy. There are retrospective data demonstrating the efficacy of revascularization in permitting the use of ACE-i and angiotensin receptor blockers in patients who previously could not tolerate these agents (Citation24,Citation85). Given the wider benefits of these agents, this may be a therapeutic approach more commonly seen in the future.
Revascularization for other end-points
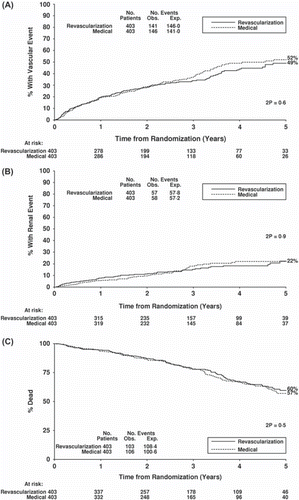
In line with the findings for blood pressure, no RCT has shown a benefit of revascularization on renal function, cardiovascular event rate, or mortality rate. In ASTRAL (Citation25), average baseline age was 70.5 years, creatinine 178 μmol/L (2 mg/dL), eGFR 40 mL/min, and blood pressure 150/76 mmHg. Using repeated measures analysis no significant difference was seen in the primary end-point (reciprocal of creatinine over time, to assess renal function) (). A similar pattern was seen for the secondary end-points of cardiovascular events, renal events, and overall survival (), which were identical. Although the STAR trial included far fewer patients than ASTRAL, with a larger proportion in the revascularization arm failing to undergo the procedure, results were similar to those seen in ASTRAL (Citation80).
Figure 2. Reciprocal of serum creatinine over time in ASTRAL (primary study end-point). Top graph: reciprocal of serum creatinine for revascularized and medically managed patients. Bottom graph: difference in reciprocal of serum creatinine between groups. (Wheatley, K. et al. Revascularization versus medical therapy for renal-artery stenosis. N Engl J Med, 2009. Reproduced with permission.)
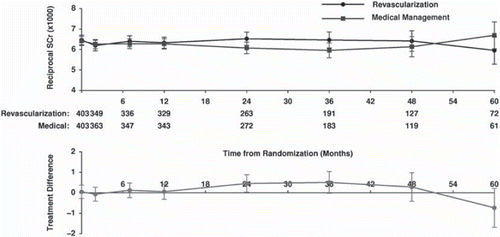
Figure 3. A: Survival curve for time to first vascular event in ASTRAL. End-points of myocardial infarction, stroke, vascular death, hospitalization for angina, fluid overload or cardiac failure, coronary artery procedure, or other arterial procedure. B: Survival curve for time to first renal event in ASTRAL. End-points of acute renal failure, commencement of dialysis, transplantation, nephrectomy, or renal death. C: Overall patient survival curve in ASTRAL. Solid line = revascularization; dotted line = medical therapy. (Wheatley, K. et al. Revascularization versus medical therapy for renal-artery stenosis. N Engl J Med, 2009. Reproduced with permission.)
Limitations of trial data
Although over 1000 ARVD patients are described in RCT data, the published results have a number of important limitations. The three earliest studies (Citation77–79) suffer from small patient numbers, short follow-up periods, significant cross-over rates, and the use of now out-dated interventional techniques and approaches to pharmacotherapy. Specific criticism has been levelled at ASTRAL over the lack of a reference laboratory to validate CTA/MRA findings. However, whilst over-estimation of RAS on indirect angiography is a recognized problem (Citation86), only 8% of patients randomized to the interventional arm of the study did not receive revascularization due to low disease burden (Citation25). Per protocol analysis of data (i.e. including only those who had been revascularized in the revascularized arm) replicated the overall outcomes of ASTRAL exactly.
The most important limitation of published RCTs is that of patient selection. Within ASTRAL, patients could only be randomized if their treating physician was uncertain of the benefits of revascularization, and so many high-risk patients are likely to have been excluded. Although ASTRAL suggests that revascularization does not benefit ARVD patients with stable CKD, it must be acknowledged that no study addresses the role of intervention in the highest-risk patient groups. Published guidelines provide support for revascularization in patients with FPE, resistant hypertension (RH), and some cases of declining renal function (Citation87). These recommendations are primarily based upon observational data describing e.g. reduced hospital admissions following revascularization for FPE (Citation88). Other non-randomized series describe outcomes opposed to RCT results, with reports of long-term improvements in renal function and blood pressure following intervention (Citation89), or benefit from stenting in patients with more severe renal impairment (Citation90). Although ASTRAL did consider some high-risk patients (sub-analyses of patients with > 70% stenosis bilaterally/to single functioning kidney and of patients with rapid loss of renal function), there is a need for future studies to be designed around patients most likely to benefit rather than a further ‘all-comers’ approach. Finally, although outcomes within the two arms have been similar for the initial follow-up period, long-term outcomes remain uncertain. With a slow rate of loss of renal function in ASTRAL, the lack of difference in renal end-points at 5 years is not unsurprising, and the possibility of e.g. 10-year benefit cannot yet be discounted.
Success and complications of renal artery revascularization
When assessing any interventional procedure, in addition to analysis of the clinical effects, consideration must also be given to technical success rates and risk of complications.
Technical success in renal artery revascularization is judged by the degree of post-procedure stenosis. With no consensus definition on exactly what degree of stenosis should be considered clinically significant, it is challenging to provide an exact definition of angiographic success. Meta-analysis of 14 studies highlights the broad range of definitions employed (with definitions of success ranging between < 10% and < 50% residual stenosis), but demonstrates success rates of > 94% irrespective of the definition applied (Citation91), indicating that technical success can be consistently achieved.
Less information exists regarding long-term stent patency. One study using DUS surveillance demonstrated 12-month patency rates to be as low as 50%, with a further reduction to 40% at 18 months (Citation92). In light of these figures there has been interest in the use of drug-eluting stents (DES), with reports of successful deployment to treat renal artery in-stent re-stenosis (Citation93). One study has compared re-stenosis rates in ARVD between bare metal and sirolimus-eluting stents. In the Sirolimus-Eluting versus Bare-Metal Low Profile Stent for Renal Artery Treatment (GREAT trial) angiographic outcomes for 105 patients randomized between stent types were compared at 6 months and 2 years (Citation94). Although no statistically significant difference was observed between device types, a trend toward lower re-stenosis rates was observed in the DES group (6.7% versus 14.6%).
Complication rates from intervention in atherosclerotic RAS can be significant. Within ASTRAL, serious adverse events were reported in 6.8% of patients (Citation25). These included renal artery occlusion, haemorrhage requiring hospitalization, and two deaths. This significant risk is again demonstrated in STAR, where in 46 revascularization episodes, 3 deaths were observed (Citation80). Meta-analysis of 687 patients of the three RCT and single-centre studies that preceded STAR and ASTRAL showed that 9% of patients suffered a significant complication of percutaneous revascularization (e.g. renal failure post-procedure, renal infarction, significant haemorrhage), with an overall mortality of 1% (Citation91). These data underscore the need for a robust rationale prior to undertaking revascularization.
Future studies
Several RCTs are on-going (i.e. CORAL, and long-term follow-up of ASTRAL), and data from these are eagerly anticipated. Other work is focused on technical developments such as usefulness of embolic protection devices and alternative device types, new therapies, and using biomarkers to improve diagnostic capability. In two small studies, brain natriuretic peptide (BNP) has been shown potentially to have a role in predicting blood pressure response to renal artery revascularization in humans (Citation95,Citation96). Although these data are limited as date of onset of arterial hypertension cannot be accurately defined in either study, these are promising results which merit further investigation.
A further emerging technique is renal denervation. The direct mechanism through which renal sympathetic activity (RSA) affects blood pressure is not fully defined in humans. However, there are data demonstrating a two-way relationship between urinary sodium excretion and RSA (Citation97,Citation98), a reduction in renin release with β-adrenergic blockade (Citation99), and an effect of sympathetic activity on GFR (Citation100). In humans with refractory hypertension, pilot studies of renal denervation with renal nerve ablation therapy showed beneficial effects on blood pressure (and possibly GFR) (Citation101,Citation102), with the small initial RCT confirming blood pressure but not GFR benefits (Citation103). No human data describe this technique in ARVD patients, but in experimental Goldblatt rat models (2-kidneys 1-clip) beneficial effects on blood pressure have been described (Citation104). This technique may (in isolation or in combination with revascularization) have a future role in treatment of RH in ARVD.
Conclusion
Renal artery revascularization does not benefit the majority of patients with atherosclerotic RAS. Robust RCT data clearly demonstrate parity of blood pressure and renal functional outcomes between revascularized and medically managed patients, but the patient populations studied have not focused on high-risk clinical presentations that might be more likely to benefit such as severe hypertension, heart failure, or declining renal function; the complication rates of the procedure demand better outcome data. However, in selected circumstances, renal revascularization may have an important part to play. For the few patients requiring but unable to tolerate angiotensin blockade without serious deterioration of renal function, revascularization can facilitate safe usage of these agents. There may also be a role for revascularization in cases of refractory hypertension, but more dedicated RCT data are required in these patients, as well as in other high-risk subgroups. In particular, patients with heart failure (either CHF or FPE) and RAS theoretically have a lot to gain from revascularization, and future efforts need to be concentrated on studying these patients. However, for the majority of patients with ARVD the mainstay of treatment has to be vascular protection with statins and anti-platelet therapy, and blood pressure control with renin angiotensin blockade at the centre of therapy.
Declaration of interest: The authors report no conflicts of interest and received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- Cheung CM, Hegarty J, Kalra PA. Dilemmas in the management of renal artery stenosis. Br Med Bull. 2005;73–74: 35–55.
- Hansen KJ, Edwards MS, Craven TE, Cherr GS, Jackson SA, Appel RG, . Prevalence of renovascular disease in the elderly: a population-based study. J Vasc Surg. 2002;36:443–51.
- Kalra PA, Guo H, Kausz AT, Gilbertson DT, Liu J, Chen S-C, . Atherosclerotic renovascular disease in United States patients aged 67 years or older: risk factors, revascularization, and prognosis. Kidney Int. 2005;68:293–301.
- Jazrawi A, Darda S, Burke P, Daccarett M, Stehlik J, David S, . Is race a risk factor for the development of renal artery stenosis? Cardiol Res Pract. 2009;2009:817987.
- Khosla S, Kunjummen B, Manda R, Khaleel R, Kular R, Gladson M, . Prevalence of renal artery stenosis requiring revascularization in patients initially referred for coronary angiography. Cathet Cardiovasc Intervent. 2003;58:400–3.
- MacDowall P, Kalra PA, O'Donoghue DJ, Waldek S, Mamtora H, Brown K. Risk of morbidity from renovascular disease in elderly patients with congestive cardiac failure. Lancet. 1998;352:13–16.
- Olin JW, Melia M, Young JR, Graor RA, Risius B. Prevalence of atherosclerotic renal artery stenosis in patients with atherosclerosis elsewhere. Am J Med. 1990;88:46N–51N.
- Kalra PA, Guo H, Gilbertson DT, Liu J, Chen S-C, Ishani A, . Atherosclerotic renovascular disease in the United States. Kidney Int. 2009;77:37–43.
- Collins AJ, Foley RN, Herzog C, Chavers BM, Gilbertson D, Ishani A, . Excerpts from the US Renal Data System 2009 Annual Data Report. Am J Kidney Dis. 2010;55: A6–7.
- Shurrab AE, Mamtora H, O'Donoghue D, Waldek S, Kalra PA. Increasing the diagnostic yield of renal angiography for the diagnosis of atheromatous renovascular disease. Br J Radiol. 2001;74:213–8.
- Scoble JE, de Takats D, Ostermann ME, Connolly JO, Scott NR, Beeso JA, . Lipid profiles in patients with atherosclerotic renal artery stenosis. Nephron. 1999;83:117–21.
- de Mast Q, Beutler JJ. The prevalence of atherosclerotic renal artery stenosis in risk groups: a systematic literature review. J Hypertens. 2009;27:1333–40.
- Keddis M, Garovic V, Bailey K, Wood CM, Raissian Y, Grande JP. Ischaemic nephropathy secondary to atherosclerotic renal artery stenosis: clinical and histopathological correlates. Nephrol Dial Transplant. 2010;25:3615–22.
- Derkx FH, Schalekamp MA. Renal artery stenosis and hypertension. Lancet. 1994;344:237–9.
- Börgel J, Springer S, Ghafoor J, Arndt D, Duchna H-W, Barthel A, . Unrecognized secondary causes of hypertension in patients with hypertensive urgency/emergency: prevalence and co-prevalence. Clin Res Cardiol. 2010;99:499–506.
- Green D, Kalra PA. The heart in atherosclerotic renovascular disease. Front Biosci (Elite Ed). 2012;4:856–64.PMID 22201919
- Guazzi M, Brambilla R, Agostoni P, Guazzi MD. Influence of ACE-inhibition on salt-mediated worsening of pulmonary gas exchange in heart failure. Br J Clin Pharmacol. 2001;51: 482–7.
- de Simone G. Left ventricular geometry and hypotension in end-stage renal disease: a mechanical perspective. J Am Soc Nephrol. 2003;14:2421–7.
- Marchais SJ, Guerin AP, Pannier BM, Levy BI, Safar ME, London GM. Wave reflections and cardiac hypertrophy in chronic uremia. Influence of body size. Hypertension. 1993;22:876–83.
- Cheung CM, Wright JR, Shurrab AE, Mamtora H, Foley RN, O'Donoghue DJ, . Epidemiology of renal dysfunction and patient outcome in atherosclerotic renal artery occlusion. J Am Soc Nephrol. 2002;13:149–57.
- Dwyer KM, Vrazas JI, Lodge RS, Humphery TJ, Schlicht SM, Murphy BF, . Treatment of acute renal failure caused by renal artery occlusion with renal artery angioplasty. Am J Kidney Dis. 2002;40:189–94.
- Chrysochou C, Sinha S, Chalmers N, Kalra PR, Kalra PA. Anuric acute renal failure and pulmonary oedema: a case for urgent action. Int J Cardiol. 2009;132:e31–3.
- Hackam DG, Duong-Hua ML, Mamdani M, Li P, Tobe SW, Spence JD, . Angiotensin inhibition in renovascular disease: a population-based cohort study. Am Heart J. 2008;156: 549–55.
- Chrysochou C, Foley RN, Young JF, Khavandi K, Cheung CM, Kalra PA. Dispelling the myth: the use of renin-angiotensin blockade in atheromatous renovascular disease. Nephrol Dial Transplant. 2012;27:1403–9.
- ; ASTRAL InvestigatorsWheatley K, Ives N, Gray R, Kalra PA, Moss JG, . Revascularization versus medical therapy for renal-artery stenosis. N Engl J Med. 2009;361: 1953–62.
- Wright J, Shurrab A, Cheung C, Waldek S, O'Donoghue D, Foley R, . A prospective study of the determinants of renal functional outcome and mortality in atherosclerotic renovascular disease. Am J Kidney Dis. 2002;39:1153–61.
- Wright JR, Shurrab AE, Cooper A, Kalra PR, Foley RN, Kalra PA. Progression of cardiac dysfunction in patients with atherosclerotic renovascular disease. QJM. 2009;102: 695–704.
- Suresh M, Laboi P, Mamtora H, Kalra PA. Relationship of renal dysfunction to proximal arterial disease severity in atherosclerotic renovascular disease. Nephrol Dial Transplant. 2000;15:631–6.
- Textor SC. Renovascular hypertension in 2007: where are we now? Curr Cardiol Rep. 2007;9:453–61.
- Chrysochou C, Kalra PA. Epidemiology and natural history of atherosclerotic renovascular disease. Prog Cardiovasc Dis. 2009;52:184–95.
- Chrysochou C, Cheung CM, Durow M, Middleton RJ, Solomon LR, Craig A, . Proteinuria as a predictor of renal functional outcome after revascularization in atherosclerotic renovascular disease (ARVD). QJM. 2009;102:283–8.
- Chade AR, Lerman A, Lerman LO. Kidney in early atherosclerosis. Hypertension. 2005;45:1042–9.
- Meier P. Atherosclerotic renal artery stenosis: update on management strategies. Curr Opin Cardiol. 2011;26:463–71.
- Amighi J, Schlager O, Haumer M, Dick P, Mlekusch W, Loewe C, . Renal artery stenosis predicts adverse cardiovascular and renal outcome in patients with peripheral artery disease. Eur J Clin Invest. 2009;39:784–92.
- Conlon PJ, Athirakul K, Kovalik E, Schwab SJ, Crowley J, Stack R, . Survival in renal vascular disease. J Am Soc Nephrol. 1998;9:252–6.
- Hansen KJ, Tribble RW, Reavis SW, Canzanello VJ, Craven TE, Plonk GW, . Renal duplex sonography: evaluation of clinical utility. J Vasc Surg. 1990;12:227–36.
- Kohler TR, Zierler RE, Martin RL, Nicholls SC, Bergelin RO, Kazmers A, . Noninvasive diagnosis of renal artery stenosis by ultrasonic duplex scanning. J Vasc Surg. 1986;4: 450–6.
- Rountas C, Vlychou M, Vassiou K, Liakopoulos V, Kapsalaki E, Koukoulis G, . Imaging modalities for renal artery stenosis in suspected renovascular hypertension: prospective intraindividual comparison of color Doppler US, CT angiography, GD-enhanced MR angiography, and digital substraction angiography. Ren Fail. 2007;29:295–302.
- Kiefer C, Schroth G, Gralla J, Diehm N, Baumgartner I, Husmann M. A feasibility study on model-based evaluation of kidney perfusion measured by means of FAIR prepared true-FISP arterial spin labeling (ASL) on a 3-T MR scanner. Acad Radiol. 2009;16:79–87.
- Textor SC, Glockner JF, Lerman LO, Misra S, McKusick MA, Riederer SJ, . The use of magnetic resonance to evaluate tissue oxygenation in renal artery stenosis. J Am Soc Nephrol. 2008;19:780–8.
- Gloviczki ML, Glockner JF, Lerman LO, McKusick MA, Misra S, Grande JP, . Preserved oxygenation despite reduced blood flow in poststenotic kidneys in human atherosclerotic renal artery stenosis. Hypertension. 2010;55: 961–6.
- Chrysochou C, Mendichovszky IA, Buckley DL, Cheung CM, Jackson A, Kalra PA. BOLD imaging: a potential predictive biomarker of renal functional outcome following revascularization in atheromatous renovascular disease. Nephrol Dial Transplant. 2012;27:1013–9.
- Warner L, Glockner JF, Woollard J, Textor SC, Romero JC, Lerman LO. Determinations of renal cortical and medullary oxygenation using blood oxygen level-dependent magnetic resonance imaging and selective diuretics. Invest Radiol. 2011;46:41–7.
- Cooper C, Murphy T, Matsumoto A, Steffes M, Cohen D, Jaff M, . Stent revascularization for the prevention of cardiovascular and renal events among patients with renal artery stenosis and systolic hypertension: Rationale and design of the CORAL trial. Am Heart J. 2006;152:59–66.
- Colyer WR, Cooper CJ. Management of renal artery stenosis: 2010. Curr Treat Options Cardiovasc Med. 2011;13: 103–13.
- Hiramoto J, Hansen KJ, Pan XM, Edwards MS, Sawhney R, Rapp JH. Atheroemboli during renal artery angioplasty: an ex vivo study. J Vasc Surg. 2005;41:1026–30.
- Kanjwal K, Cooper CJ, Virmani R, Haller S, Shapiro JI, Burket MW, . Predictors of embolization during protected renal artery angioplasty and stenting: role of antiplatelet therapy. Cathet Cardiovasc Intervent. 2010;76:16–23.
- Haller S, Adlakha S, Reed G, Brewster P, Kennedy D, Burket MW, . Platelet activation in patients with atherosclerotic renal artery stenosis undergoing stent revascularization. Clin J Am Soc Nephrol. 2011;6:2185–91.
- Cooper CJ, Haller ST, Colyer W, Steffes M, Burket MW, Thomas WJ, . Embolic protection and platelet inhibition during renal artery stenting. Circulation. 2008;117: 2752–60.
- Shah S, Paparello J, Danesh FR. Effects of statin therapy on the progression of chronic kidney disease. Adv Chronic Kidney Dis. 2005;12:187–95.
- Huskey J, Lindenfeld J, Cook T, Targher G, Kendrick J, Kjekshus J, . Effect of simvastatin on kidney function loss in patients with coronary heart disease: findings from the Scandinavian Simvastatin Survival Study (4S). Atherosclerosis. 2009;205:202–6.
- Baigent C, Landray M, Reith C, Emberson J, Wheeler DC, Tomson C, . The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet. 2011;377: 2181–92.
- Bates MC, Campbell JE, Stone PA, Jaff MR, Broce M, Lavigne PS. Factors affecting long-term survival following renal artery stenting. Cathet Cardiovasc Intervent. 2007;69: 1037–43.
- Silva VS, Martin LC, Franco RJS, Carvalho FC, Bregagnollo EA, Castro JH, . Pleiotropic effects of statins may improve outcomes in atherosclerotic renovascular disease. Am J Hypertens. 2008;21:1163–8.
- Cheung CM, Patel A, Shaheen N, Cain S, Eddington H, Hegarty J, . The effects of statins on the progression of atherosclerotic renovascular disease. Nephron Clin Pract. 2007;107:c35–42.
- Chade AR, Zhu X-Y, Grande JP, Krier JD, Lerman A, Lerman LO. Simvastatin abates development of renal fibrosis in experimental renovascular disease. J Hypertens. 2008;26: 1651–60.
- Agarwal R. Blood pressure goal in chronic kidney disease: what is the evidence? Curr Opin Nephrol Hypertens. 2011;20:229–32.
- Jafar TH, Schmid CH, Landa M, Giatras I, Toto R, Remuzzi G, . Angiotensin-converting enzyme inhibitors and progression of nondiabetic renal disease. A meta-analysis of patient-level data. Ann Intern Med. 2001;135:73–87.
- Franklin SS, Smith RD. Comparison of effects of enalapril plus hydrochlorothiazide versus standard triple therapy on renal function in renovascular hypertension. Am J Med. 1985;79:14–23.
- Burke TA, Sturkenboom MC, Lu S-E, Wentworth CE, Lin Y, Rhoads GG. Discontinuation of antihypertensive drugs among newly diagnosed hypertensive patients in UK general practice. J Hypertens. 2006;24:1193–200.
- Bart BA, Gattis WA, Diem SJ, O'Connor CM. Reasons for underuse of angiotensin-converting enzyme inhibitors in patients with heart failure and left ventricular dysfunction. Am J Cardiol. 1997;79:1118–20.
- Losito A. Long-term follow-up of atherosclerotic renovascular disease. Beneficial effect of ACE inhibition. Nephrol Dial Transplant. 2005;20:1604–9.
- van de Ven PJ, Beutler JJ, Kaatee R, Beek FJ, Mali WP, Koomans HA. Angiotensin converting enzyme inhibitor-induced renal dysfunction in atherosclerotic renovascular disease. Kidney Int. 1998;53:986–93.
- Hackam DG, Spence JD, Garg AX, Textor SC. Role of renin-angiotensin system blockade in atherosclerotic renal artery stenosis and renovascular hypertension. Hypertension. 2007;50:998–1003.
- Singer GM, Izhar M, Black HR. Goal-oriented hypertension management: translating clinical trials to practice. Hypertension. 2002;40:464–9.
- Faselis C, Doumas M, Papademetriou V. Common secondary causes of resistant hypertension and rational for treatment. Int J Hypertens. 2011;2011:236239.
- Johansson M, Elam M, Rundqvist B, Eisenhofer G, Herlitz H, Lambert G, . Increased sympathetic nerve activity in renovascular hypertension. Circulation. 1999;99:2537–42.
- Johansson M, Herlitz H, Jensen G, Rundqvist B, Friberg P. Increased cardiovascular mortality in hypertensive patients with renal artery stenosis. Relation to sympathetic activation, renal function and treatment regimens. J Hypertens. 1999; 17(Pt 1):1743–50.
- Cianci R, Martina P, Borghesi F, di Donato D, Polidori L, Lai S, . Revascularization versus medical therapy for renal artery stenosis: antihypertensive drugs and renal outcome. Angiology. 2011;62:92–9.
- Duranay M, Kanbay M, Akay H, Unverdi S, Sürer H, Altay M, . Nebivolol improves renal function in patients who underwent angioplasty due to renal artery stenosis: a pilot study. Nephron Clin Pract. 2010;114:c213–7.
- Chade AR, Zhu X, Lavi R, Krier JD, Pislaru S, Simari RD, . Endothelial progenitor cells restore renal function in chronic experimental renovascular disease. Circulation. 2009;119:547–57.
- Chade AR, Zhu X-Y, Krier JD, Jordan KL, Textor SC, Grande JP, . Endothelial progenitor cells homing and renal repair in experimental renovascular disease. Stem Cells. 2010;28:1039–47.
- Kelsen S, Hall JE, Chade AR. Endothelin-A receptor blockade slows the progression of renal injury in experimental renovascular disease. Am J Physiol Renal Physiol. 2011;301: F218–25.
- Schrijvers BF, Flyvbjerg A, De Vriese AS. The role of vascular endothelial growth factor (VEGF) in renal pathophysiology. Kidney Int. 2004;65:2003–17.
- Iliescu R, Fernandez SR, Kelsen S, Maric C, Chade AR. Role of renal microcirculation in experimental renovascular disease. Nephrol Dial Transplant. 2010;25:1079–87.
- Chade AR, Kelsen S. Renal microvascular disease determines the responses to revascularization in experimental renovascular disease. Circ Cardiovasc Interv. 2010;3:376–83.
- Webster J, Marshall F, Abdalla M, Dominiczak A, Edwards R, Isles CG, . Randomised comparison of percutaneous angioplasty vs continued medical therapy for hypertensive patients with atheromatous renal artery stenosis. Scottish and Newcastle Renal Artery Stenosis Collaborative Group. J Hum Hypertens. 1998;12:329–35.
- Plouin PF, Chatellier G, Darné B, Raynaud A. Blood pressure outcome of angioplasty in atherosclerotic renal artery stenosis: a randomized trial. Essai Multicentrique Medicaments vs Angioplastie (EMMA) Study Group. Hypertension. 1998;31:823–9.
- van Jaarsveld BC, Krijnen P, Pieterman H, Derkx FH, Deinum J, Postma CT, . The effect of balloon angioplasty on hypertension in atherosclerotic renal-artery stenosis. Dutch Renal Artery Stenosis Intervention Cooperative Study Group. N Engl J Med. 2000;342:1007–14.
- Bax L, Woittiez A-JJ, Kouwenberg HJ, Mali WPTM, Buskens E, Beek FJA, . Stent placement in patients with atherosclerotic renal artery stenosis and impaired renal function: a randomized trial. Ann Intern Med. 2009;150:840–8, W150–1.
- Dorros G, Prince C, Mathiak L. Stenting of a renal artery stenosis achieves better relief of the obstructive lesion than balloon angioplasty. Cathet Cardiovasc Diagn. 1993;29:191–8.
- Rundback JH, Manoni T, Rozenblit GN, Poplausky M, Maddineni S, Crea G, . Balloon angioplasty or stent placement in patients with azotemic renovascular disease: a retrospective comparison of clinical outcomes. Heart Dis. 1999;1:121–5.
- Lederman RJ, Mendelsohn FO, Santos R, Phillips HR, Stack RS, Crowley JJ. Primary renal artery stenting: characteristics and outcomes after 363 procedures. Am Heart J. 2001;142: 314–23.
- Kumbhani DJ, Bavry AA, Harvey JE, de Souza R, Scarpioni R, Bhatt DL, . Clinical outcomes after percutaneous revascularization versus medical management in patients with significant renal artery stenosis: a meta-analysis of randomized controlled trials. Am Heart J. 2011;161:622–30.e1.
- Khosla S, Ahmed A, Siddiqui M, Trivedi A, Benatar D, Salem Y, . Safety of angiotensin-converting enzyme inhibitors in patients with bilateral renal artery stenosis following successful renal artery stent revascularization. Am J Ther. 2006;13:306–8.
- Subramanian R, White CJ, Rosenfield K, Bashir R, Almagor Y, Meerkin D, . Renal fractional flow reserve: a hemodynamic evaluation of moderate renal artery stenoses. Catheter Cardiovasc Interv. 2005;64:480–6.
- Hirsch AT, Haskal ZJ, Hertzer NR, Bakal CW, Creager MA, Halperin JL, . ACC/AHA Guidelines for the Management of Patients with Peripheral Arterial Disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Associations for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (writing committee to develop guidelines for the management of patients with peripheral arterial disease)—summary of recommendations. J Vasc Interv Radiol. 2006;17:1383–97; quiz 1398.
- Kane GC, Xu N, Mistrik E, Roubicek T, Stanson AW, Garovic VD. Renal artery revascularization improves heart failure control in patients with atherosclerotic renal artery stenosis. Nephrol Dial Transplant. 2010;25:813–20.
- Hegde U, Rajapurkar M, Gang S, Khanapet M, Durugkar S, Gohel K, . Fifteen years’ experience of treating atherosclerotic renal artery stenosis by interventional nephrologists in India. Semin Dial. 2012;25:97–104.
- Kalra PA, Chrysochou C, Green D, Cheung CM, Khavandi K, Sixt S, . The benefit of renal artery stenting in patients with atheromatous renovascular disease and advanced chronic kidney disease. Cathet Cardiovasc Intervent. 2010;75: 1–10.
- Leertouwer TC, Gussenhoven EJ, Bosch JL, van Jaarsveld BC, van Dijk LC, Deinum J, . Stent placement for renal arterial stenosis: where do we stand? A meta-analysis. Radiology. 2000;216:78–85.
- Corriere MA, Edwards MS, Pearce JD, Andrews JS, Geary RL, Hansen KJ. Restenosis after renal artery angioplasty and stenting: incidence and risk factors. J Vasc Surg. 2009;50: 813–9.e1.
- Kiernan TJ, Yan BP, Eisenberg JD, Ruggiero NJ, Gupta V, Drachman D, . Treatment of renal artery in-stent restenosis with sirolimus-eluting stents. Vasc Med. 2010;15:3–7.
- Zähringer M, Sapoval M, Pattynama PMT, Rabbia C, Vignali C, Maleux G, . Sirolimus-eluting versus bare-metal low-profile stent for renal artery treatment (GREAT Trial): angiographic follow-up after 6 months and clinical outcome up to 2 years. J Endovasc Ther. 2007;14:460–8.
- Silva JA, Chan AW, White CJ, Collins TJ, Jenkins JS, Reilly JP, . Elevated brain natriuretic peptide predicts blood pressure response after stent revascularization in patients with renal artery stenosis. Circulation. 2005;111:328–33.
- Staub D, Zeller T, Trenk D, Maushart C, Uthoff H, Breidthardt T, . Use of B-type natriuretic peptide to predict blood pressure improvement after percutaneous revascularisation for renal artery stenosis. Eur J Vasc Endovasc Surg. 2010;40:599–607.
- Friberg P, Meredith I, Jennings G, Lambert G, Fazio V, Esler M. Evidence for increased renal norepinephrine overflow during sodium restriction in humans. Hypertension. 1990;16: 121–30.
- Meredith IT, Esler MD, Cox HS, Lambert GW, Jennings GL, Eisenhofer G. Biochemical evidence of sympathetic denervation of the heart in pure autonomic failure. Clin Auton Res. 1991;1:187–94.
- Kaye D, Esler M, Kingwell B, McPherson G. Functional and neurochemical evidence for partial cardiac sympathetic reinnervation after cardiac transplantation in humans. Circulation. 1993;88:1110–8.
- Esler MD, Nestel PJ. Sympathetic responsiveness to head-up tilt in essential hypertension. Clin Sci. 1973;44:213–26.
- Krum H, Schlaich M, Whitbourn R, Sobotka PA, Sadowski J, Bartus K, . Catheter-based renal sympathetic denervation for resistant hypertension: a multicentre safety and proof-of-principle cohort study. Lancet. 2009;373:1275–81.
- Simonetti G, Spinelli A, Gandini R, Da Ros V, Gaspari E, Coco I, . Endovascular radiofrequency renal denervation in treating refractory arterial hypertension: a preliminary experience. Radiol Med. 2012;117:426–44.
- , Symplicity HTN-2 InvestigatorsEsler MD, Krum H, Sobotka PA, Schlaich MP, Schmieder RE, . Renal sympathetic denervation in patients with treatment-resistant hypertension (The Symplicity HTN-2 Trial): a randomised controlled trial. Lancet. 2010;376:1903–9.
- DiBona GF, Esler M. Translational medicine: the antihypertensive effect of renal denervation. Am J Physiol Regul Integr Comp Physiol. 2010;298:R245–53.
