Abstract
The incidence of diabetes mellitus is projected to continue to increase worldwide over the next 20 years leading to increased costs in the management of the disease and its associated co-morbidities. Insulin replacement is one of many treatment options that can help to bring about near normoglycemia in the patient with type 2 diabetes mellitus (T2DM). Glycemic control as close to normoglycemia as possible can help to reduce the risk of microvascular and macrovascular complications, yet less than one-half of patients with T2DM achieve glycemic targets as recommended by practice guidelines. The purpose of this review is to provide guidance to primary care physicians for the initiation and intensification of basal-bolus insulin therapy in patients with T2DM. Two treatment algorithms that can be both patient- and physician-driven are proposed: a stepwise approach and a multiple daily injections approach. Evidence shaping the two approaches will be discussed alongside management issues that surround the patient treated with insulin: hypoglycemia, weight gain, patient education, and quality of life.
| Abbreviations | ||
| ADA | = | American Diabetes Association |
| FPG | = | fasting plasma glucose |
| HbA1C | = | glycosylated hemoglobin |
| MDI | = | multiple daily injections |
| NPH | = | neutral protamine Hagedorn |
| OAD | = | oral antidiabetic drug |
| SMBG | = | self-monitored blood glucose |
| T1DM | = | type 1 diabetes mellitus |
| T2DM | = | type 2 diabetes mellitus |
| UKPDS | = | United Kingdom Prospective Diabetes Study |
Key messages
Basal insulin therapy combined with oral antidiabetic agents may not suffice for the achievement of guideline-recommended glycemic goals, thus necessitating the addition of prandial insulin.
Intensification of a basal insulin-only regimen can occur in a stepwise process or can become fully intensified to multiple daily injections by following an algorithm that is both patient- and physician-driven.
Issues impacting the patient, such as weight gain, hypoglycemia, and quality of life, are equally as important to the success of an insulin treatment regimen as is the choice of the treatment algorithm.
Introduction
Approximately 246 million people worldwide are presently afflicted with diabetes mellitus, and this number is expected to exceed 350 million within 20 years (Citation1,Citation2). Glycemic control is a vital factor in the effective management of both type 1 and type 2 diabetes mellitus (T1DM and T2DM, respectively); studies have demonstrated that maintenance of glycemic levels close to the non-diabetic range is associated with a reduction in diabetes-related co-morbidities, particularly microvascular complications (Citation3–8). Based on the strength of clinical evidence, the American Diabetes Association (ADA), the American Association of Clinical Endocrinologists, and the International Diabetes Federation recommend that patients with diabetes be as near to normoglycemia as possible (Citation9–11).
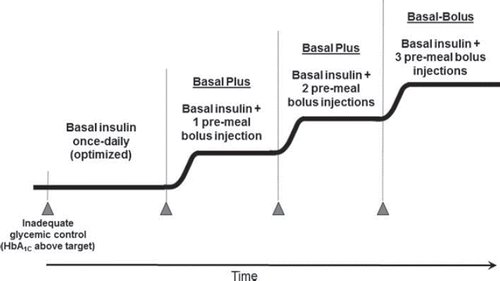
Despite guideline recommendations, at least 43% of patients are still unable to achieve glycemic control, thus emphasizing the on-going need for improvement in diabetes management (Citation12). The purpose of this review is to address the role and timeliness of intensive insulin therapy in both achieving glycemic targets and matching the progression of the disease, which is associated with increasing β-cell dysfunction. The advantages of a basal-bolus insulin regimen early in treatment will be discussed, and two regimens for the initiation and intensification of insulin therapy will be proposed: a stepwise approach and a multiple daily injections (MDI) approach.
Glycemic control in diabetes mellitus management
The United Kingdom Prospective Diabetes Study (UKPDS) demonstrated that tight glycemic control in patients with new-onset T2DM is associated with a reduced risk of diabetes-related complications, including a 25% relative risk reduction for microvascular complications compared with conventional treatment (Citation7). Long-term follow-up data over 10 years demonstrated continued significant reductions in the risk of microvascular disease (P = 0.001) and significant long-term risk reductions for myocardial infarction (P = 0.01) and death from any cause (P = 0.007) despite the inability to maintain glycosylated hemoglobin (HbA1C) levels achieved by those treated intensively during the original study (Citation13).
For many patients with T2DM, initial treatment involves diet and lifestyle modifications plus initiation of metformin therapy, followed by additional oral antidiabetic drugs (OADs), including insulin secretagogues, thiazolidinediones, dipeptidyl peptidase-IV inhibitors, and/or α-glucosidase inhibitors (Citation14). The injectable incretin mimetics (i.e. exenatide and liraglutide) have become available and may be used as monotherapy, but more often they are added to metformin monotherapy or combination therapy with metformin and a sulfonylurea.
Insulin therapy for the management of hyperglycemia
Data from UKPDS revealed that 3 years after diagnosis of T2DM, 50% of patients required treatment with more than one antidiabetic agent in order to achieve glycemic goals, with more than 75% of patients requiring multiple agents after 9 years of follow-up (Citation15). Insulin therapy, alone or in combination with OADs, should be considered when more intensive glucose lowering is needed to achieve HbA1C targets (Citation14,Citation16). A therapeutic strategy that includes early initiation of insulin therapy to control both fasting and postprandial hyperglycemia may translate to long-term benefits (Citation17). Weng et al. demonstrated that early, intensive insulin therapy with continuous subcutaneous insulin secretion or by MDI significantly improved glycemic remission rates compared to patients treated with oral hypoglycemic agents alone (Citation18). Remission rates, defined as the achievement of both fasting capillary blood glucose and 2-hour postprandial glucose targets for a 2-week period, were 51.1% for continuous subcutaneous insulin, 44.9% for MDI, and 26.7% for oral hypoglycemic agents (P = 0.0012 for insulin groups compared to oral agents).
When patients fail to achieve glycemic control with OAD therapy, the question for practitioners is which strategy is best for starting insulin replacement. The 4-T study group sought to compare the safety and efficacy of three regimens (biphasic insulin aspart twice daily, prandial insulin aspart three times daily, or basal insulin detemir once daily (twice if required)) in 708 patients who failed prior OAD therapy (Citation19). The primary outcome in this randomized study was the HbA1C level at 3 years. After the first year of study, however, intensification of treatment occurred in subjects who were not at their HbA1C goal. At study end for biphasic insulin, prandial insulin, and basal insulin, median HbA1C decreased to 7.1%, 6.8%, and 6.9%, respectively, from a baseline of 8.6%, 8.6%, and 8.4%, with no significant difference between the three groups (P = 0.28). The proportion of patients achieving HbA1C ≤ 7% was significantly greater with prandial insulin and basal insulin compared with biphasic insulin (67.4% and 63.2% versus 49.4%; P ≤ 0.001 and P = 0.02, respectively). Though median HbA1C levels were near goal in the three treatment groups, 67.7% to 81.6% of patients across treatment arms required the addition of a second type of insulin to their regimen to achieve these outcomes. This seems to indicate that the choice of initial insulin therapy is not as important as recognizing the need to intensify initial therapy.
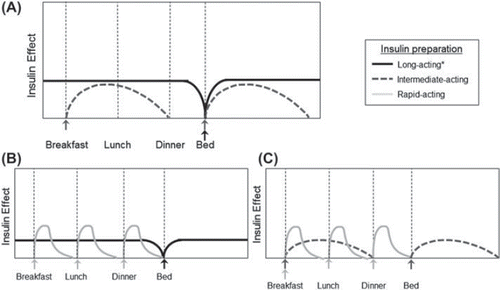
Insulin therapy is commonly initiated with a strategy to increase a patient's endogenous basal insulin level with injected basal insulin (Citation20), administered as a once-daily, long-acting insulin analogue preparation or as an injection of intermediate-acting human insulin administered once- or twice-daily (). While this approach leads to suppression of hepatic glucose production overnight and between meals, it does not mimic normal prandial insulin secretion and may not be sufficient to achieve and maintain HbA1C targets (Citation21). The Treat-to-Target Trial randomized patients with T2DM (n = 756) who had poor glycemic control on OAD therapy to insulin glargine or human neutral protamine Hagedorn (NPH) insulin using specific titration schedules based on self-monitored mean fasting plasma glucose (FPG). Insulin glargine helped 58.0% of patients to reach HbA1C ≤ 7% compared with 57.3% for human NPH insulin (Citation22). Nonetheless, approximately 40% of patients still did not achieve HbA1C targets, indicating the need to intensify basal insulin.
Figure 1. Insulin replacement regimens for the management of hyperglycemia. A: Once-daily, long-acting insulin analogue (black) or twice-daily, intermediate-acting human insulin (dashed) as basal replacement therapy. Insulin regimens can be intensified with the administration of a rapid-acting insulin analogue (light grey) at mealtimes in addition to long-acting (B) or intermediate-acting basal insulin (C). Arrows indicate insulin injections at mealtimes or bedtime. *As per the package insert, long-acting insulin glargine may be given at any time in the day, and insulin detemir once daily should be given in the evening. Adapted from De Witt et al. (Citation21) with permission.
Premixed preparations of intermediate- and rapid-acting insulin analogues in a single injection are one option to intensify a basal-only insulin regimen (Citation23). The 1-2-3 Study investigated whether the addition of biphasic insulin aspart 70/30 in patients failing OADs, with or without basal insulin, would help increase achievement of guideline-recommended glycemic targets (Citation24). The ADA goal achievement rate was 41% with one injection and increased to 70% and 77% with twice-daily and thrice-daily injections, respectively. Another option for basal-only intensification of insulin would be to consider twice-daily administration of a long-acting insulin analogue. In an open-label randomized study of 943 patients with T2DM, Swinnen et al. sought to determine whether insulin detemir given twice daily would be non-inferior to insulin glargine given once daily (Citation25). Patients randomized to twice-daily detemir saw a smaller proportion of patients achieve the primary outcome of HbA1C ≤ 7% without symptomatic hypoglycemia compared with insulin glargine (25.6% versus 27.5%, respectively). The treatment difference of 1.85% (95% confidence interval (CI), –3.78% to 7.48%) indicated the non-inferiority of this treatment option. Compared with insulin glargine, a larger proportion of patients treated with twice-daily detemir achieved the secondary end-points of HbA1C ≤ 7% (47.8% versus 44.1%, respectively; P = 0.254) and HbA1C ≤ 6.5% (22.7% versus 16.5%; P = 0.017). Premixed preparations and twice-daily long-acting insulin are options to consider in a patient who fails to achieve glycemic control with once-daily long-acting insulin and who may not want to begin self-administration of mealtime insulin. For all basal-only insulin regimens, however, dose titration to achieve fasting blood glucose targets should occur before considering this regimen a treatment failure. In clinical practice, basal insulin doses may not come close to the end-of-study doses used in clinical trials that treat towards specific glycemic targets (Citation26).
While premixed preparations and twice-daily long-acting insulin analogues offer an effective option for intensification of basal insulin, an even more physiologic approach is to add a rapid-acting insulin analogue at one or more mealtimes (or bolus insulin) to basal insulin analogue therapy in the form of an MDI regimen ( and C) (Citation21,Citation27,Citation28). The benefits of such regimens have been demonstrated in clinical trials. Relevant end-points and parameters of these trials are presented in . Rosenstock et al. randomly assigned 187 patients each who had failed prior insulin glargine with OAD therapy to prandial premixed therapy (50% lispro protamine/50% lispro) and to more traditional basal-bolus therapy with bedtime insulin glargine and prandial lispro (Citation29). Traditional basal-bolus insulin was associated with a significantly greater difference in the achievement of the primary outcome compared with prandial premixed therapy, change in HbA1C (–2.09% versus –1.87%, respectively; 90% CI –0.38% to –0.07%) from baseline HbA1C values of 8.89% and 8.83%. A significantly larger proportion of patients achieved HbA1C 7.0% in the basal-bolus therapy group (69% versus 54%, respectively; P = 0.009), although both groups were associated with statistically significant reductions in HbA1C from baseline. The incidence of severe hypoglycemia was 2.1% for basal-bolus treatment and 3.2% for prandial premixed therapy, and the incidence of all hypoglycemic events was 88.8% and 90.4%, respectively.
Table I. Parameters reported in studies involving insulin intensification or optimization.
The GINGER study group compared the efficacy and safety of an intensified basal-bolus regimen (insulin glargine once daily and prandial pre-meal insulin glulisine) with a regimen of twice-daily premixed insulin (NPH and lispro (70/30) or insulin NPH and aspart (75/25)) in 376 patients (Citation30). After 52 weeks of treatment, the change in HbA1C for the basal-bolus insulin regimen and twice-daily premixed regimen was –1.31% and –0.80%, respectively, from a baseline of 8.62% and 8.51% (P = 0.0001 for the adjusted mean difference). The incidence of severe hypoglycemic events was nearly equal at 7.8% and 7.6%, respectively (P = 0.9459), and the rate of all hypoglycemic events was 75.8% and 73.9% (P = 0.5641). In a subanalysis of AT.LANTUS, patients with T2DM who had been switched from a premixed insulin regimen to a regimen of a long-acting insulin glargine, with or without prandial insulin injections, demonstrated a significant reduction in mean HbA1C of 1.0% from a baseline value of 9.0% ± 1.3% (P < 0.001). HbA1C reductions of 1.22%, 1.61%, and 1.43% were observed for once-, twice-, and thrice-daily prandial insulin added on to insulin glargine, respectively, compared to 0.67% for insulin glargine alone. A significant reduction in mean FPG of 3.3 ± 2.8 mmol/L (60.2 ± 50.3 mg/dL) from a baseline value of 9.3 ± 2.8 mmol/L (167.1 ± 50.0 mg/dL) (P = 0.009) was also observed in the study (Citation31). The incidence of severe hypoglycemia was little changed, ranging from 0.0% to 1.7% in prandial insulin treatment arms compared to < 1% in patients receiving only basal insulin glargine. The rate of minor hypoglycemia ranged from 22.4% to 25.5%.
Intensifying insulin therapy in patients with using a basal-bolus regimen
For many patients with T2DM who are treated with insulin, the progressive decline of β-cell function will necessitate the eventual addition of prandial insulin. When given before meals, rapid-acting insulin analogues reduce postprandial glucose excursions and should reduce the risk of hypoglycemia compared with regular human insulin (Citation32–36). In a randomized trial comparing pre-meal administration of regular human insulin versus a rapid-acting insulin analogue in patients with T2DM, patients receiving the rapid-acting analogue demonstrated a smaller postprandial rise in serum glucose and a lower rate of hypoglycemia overall (P = 0.01) (Citation34,Citation35).
To identify patients who are candidates for basal-bolus therapy, an HbA1C level should be obtained in addition to reviewing blood glucose monitoring records. If HbA1C and glucose values are above the target individualized for the patient, prandial insulin injections should be added to basal insulin therapy. Owens et al. proposed more stringent criteria, stating that bolus insulin should be initiated in patients who do not reach an HbA1C < 7.0% in spite of lifestyle modifications, OAD therapy, and optimized basal insulin, which is defined as a fasting blood glucose < 5.6 mmol/L and/or a dose of > 0.7 units/kg (Citation37). Insulin replacement is one of many treatment options that can help to bring about near normoglycemia in the patient with T2DM. Herein, we propose two treatment algorithms for use in ambulatory patients for the addition of bolus insulin to a basal insulin regimen: a stepwise approach and an MDI approach. These approaches provide a framework for individualizing patient therapy during the insulin intensification process.
Optimizing basal-bolus insulin therapy
Stepwise approach
The progressive nature of diabetes mellitus suggests that a stepwise intensification of therapy would be a logical approach to treatment (Citation28). depicts the process of a stepwise approach. The simplest means of introducing bolus mealtime insulin, particularly in patients who may be reluctant to increase the number of self-injections, is to begin with a single injection before the largest meal of the day (Citation28,Citation38). The OPAL study demonstrated that a single dose of rapid-acting insulin administered either at breakfast or at the largest meal was effective in reducing HbA1C from baseline levels of 7.3%–7.4% to 6.9%–7.0% (Citation39). There was a non-significant trend towards better response when given at the main meal versus at breakfast (33.8% versus 27.8%). Of note, subgroup analysis showed that for patients whose HbA1C was > 7.0% at baseline, there was a significantly greater rate of patients achieving a target HbA1C of ≤ 7.0% in the group that received bolus insulin at the main meal versus at breakfast (52.2% versus 36.5%; P = 0.028).
Figure 2. Stepwise approach to the treatment of patients with type 2 diabetes mellitus. HbA1C = glycosylated hemoglobin. Adapted from Raccah et al. (Citation28) with permission.
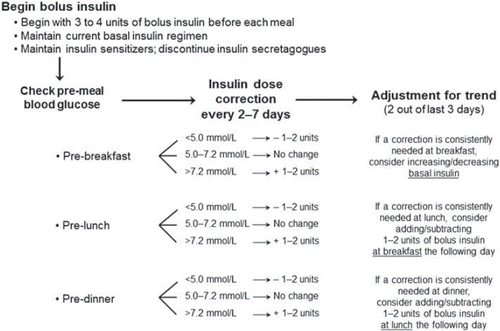
details a recommended stepwise approach for adding on the first bolus insulin dose to an existing basal insulin regimen. Self-monitoring of blood glucose levels (SMBG) 2 hours after meals for a period of up to 1 week before adding bolus insulin doses will help the practitioner to target which meal has the largest impact on postprandial blood glucose levels. We suggest an initial single dose of 4 units (Citation14) of insulin administered with the largest meal; for patients taking < 40 units/day of basal insulin, a starting dose of 3 units may be more appropriate. Patients should be maintained on their current basal insulin regimen when starting the stepwise approach. As an alternative to the above, bolus insulin has also been initiated on the basis of weight and 2-hour post-meal SMBG values (Citation40).
Figure 3. A stepwise method for the introduction of basal-bolus insulin therapy and bolus dose adjustment. *For a patient on < 40 units/day basal insulin, a starting dose of 3 units may be appropriate; †Dose corrections based on self-monitored blood glucose (SMBG) values at pre-meal (lunch and dinner) and bedtime; ‡Glycemic targets: pre-meal SMBG of 5.0–7.2 mmol/L (90–130 mg/dL); bedtime SMBG of 6.1–7.8 mmol/L (110–140 mg/dL); targets should be individualized; §ADA 2012 recommends treating to HbA1C < 7.0%; target should be individualized to the patient. FPG = fasting plasma glucose; HbA1C = glycosylated hemoglobin.
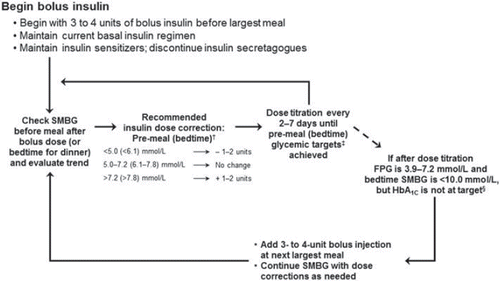
As weekly adjustment of basal-bolus insulin based on SMBG testing has been shown to produce significant reductions in HbA1C levels (Citation41,Citation42), the bolus dose at the largest meal should be adjusted at least every 7 days to achieve glycemic targets. Faster titration may be desirable in some clinical situations, although we recommend titrating bolus insulin no more frequently than every 2 days. Glycemic targets that we recommend using for the adjustment of bolus insulin doses, some of which are also recommended by ADA 2012 guidelines, include pre-meal SMBG of 5.0–7.2 mmol/L (90–130 mg/dL) and bedtime SMBG of 6.1–7.8 mmol/L (110–140 mg/dL). Dose titrations of ± 1–2 units or no change can be made according to the next pre-meal SMBG results (i.e. pre-lunch or pre-dinner for bolus insulin given before breakfast or lunch, respectively) or bedtime SMBG if bolus insulin is given before dinner. These glycemic targets should be individualized for the patient and viewed as flexible or adjustable by the clinician in certain situations (i.e. in elderly patients, some clinicians may prefer a higher range to target for pre-meal SMBG). Bolus insulin dose adjustment in fixed increments or based on 2-hour post-meal SMBG values is another option for optimizing insulin. Ampudia-Blasco et al. proposed an increment of 1 unit of bolus insulin if post-meal SMBG values were > 7.8 mmol/L (> 140 mg/dL) or increments of 1, 2, and 3 units for post- meal SMBG values in the range of 7.5–8.5 mmol/L (136–153 mg/dL), 8.6–10.0 mmol/L (154–180 mg/dL), and > 10 mmol/L (> 180 mg/dL), respectively (Citation40). Finally, dosing adjustments based on SMBG values could also be made on the basis of the size of the previous mealtime insulin dose, as has been performed in a randomized multicenter study by Bergenstal et al. (Citation41).
The decision to escalate in the stepwise approach from one pre-meal bolus dose to two and then possibly three doses should be made on the basis of HbA1C levels. ADA 2012 guidelines recommend targeting HbA1C < 7.0% as the goal for the treatment of hyperglycemia in patients with T2DM, or as close as possible to goal, provided one can do so safely and take individual circumstances into account (Citation9). A target HbA1C of < 7.0% may not be appropriate for all patients. In light of data from outcomes studies such as the UKPDS, ACCORD (Action to Control Cardiovascular Risk in Diabetes), ADVANCE (Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified-release Control Evaluation), and VADT (Veterans Affairs Diabetes Trials), HbA1C values should be individualized to the patient to minimize potential risks of clinical complications such as hypoglycemia, weight gain, and increased mortality (Citation43). Higher HbA1C values should be considered for the elderly, patients at high risk for hypoglycemia, patients with multiple co-morbidities, patients who are less motivated, or in patients who exhibit poor self-care. If patients have achieved recommended glycemic targets after their largest meal but continue to have an HbA1C above target, a second 3- to 4-unit injection may be added before the next largest meal. The second bolus dose should be titrated in the same manner as the first dose, and, if necessary, a third bolus injection of 3 to 4 units may be added and titrated. It should be noted that, in some cases, it may also be necessary to adjust the basal insulin dose during the stepwise approach to avoid nocturnal hypoglycemia and to maintain an acceptable daily insulin ratio of approximately 50% basal insulin/50% bolus insulin (Citation17,Citation28,Citation38). These adjustments should be made by the clinician in the course of normal office visits.
The stepwise addition of insulin to meals eases the transition from basal insulin therapy to a fully intensified regimen (Citation17,Citation44). It is also a process that can be patient-driven. In addition to serving as a guide for practitioners, the algorithm in (and also ) can serve as a hand-out or guide for patients on how to conduct their own bolus insulin dose adjustments. Previous studies involving patient-driven insulin regimens for basal insulin (Citation45) and prandial insulin (Citation46) have been associated with reductions in mean HbA1C levels (). In the TITRATE trial, 244 insulin-naive patients with T2DM who failed oral therapy received once-daily insulin detemir for 20 weeks and self-titrated doses on the basis of two FPG targets (3.9–5.0 and 4.4–6.1 mmol/L (70–90 and 79–110 mg/dL)) (Citation45). At study end, HbA1C was reduced in both treatment arms from 7.94% and 7.99%, respectively, to 6.77% and 7.00%. The STEP-Wise study in 296 patients poorly controlled on basal insulin and OADs also utilized self-titration for insulin detemir and add-on insulin aspart (Citation46). The basis of dose changes were SMBG values before meals in the SimpleSTEP group and 2-hours post-meal SMBG values in the ExtraSTEP group. HbA1C values decreased from 8.7% and 8.9% at baseline to 7.5% and 7.7%, respectively, after 36 weeks. To ensure that the patient is able to perform their own bolus dose adjustments, practitioners should review the algorithm with the patient, stressing that the trend in SMBG values (i.e. consistent readings at least 2 of the past 3 days) should serve as the basis for this decision. A daily log or diary of SMBG values can help to facilitate this process.
Multiple daily injections approach
In the MDI approach (), insulin therapy rapidly progresses from basal only to basal plus three bolus doses of insulin. We recommend maintaining the same amount of basal insulin while adding on 3 to 4 units of regular insulin before each meal. As in the stepwise approach, bolus insulin doses will be adjusted according to pre-meal and bedtime glucose values at least every 7 days, but no faster than every 2 days. The same glycemic values used for adjustment of pre-meal bolus insulin in the stepwise approach can be used for the MDI approach. In a fully intensified basal-bolus regimen the total daily insulin dose will resemble a ratio of approximately 50% basal insulin (intermediate- or long-acting analogue) and 50% divided into three equal bolus doses of rapid-acting insulin (Citation38,Citation47). As in the stepwise approach, adjustment of basal insulin during therapy may be required to achieve a desired 50%/50% ratio and to avoid nocturnal hypoglycemia; however, we recommend that these adjustments be handled by the practitioner.
If specific glycemic goals are not met, the corresponding pre-meal bolus doses should be adjusted by ± 1–2 units, with dose titration occurring every 3 days (). If SMBG levels are consistently high before breakfast, the bedtime dose of long-acting insulin should be increased to compensate, provided the glucose has not dropped below 3.9 mmol/L (70 mg/dL) during the night; if SMBG levels are high at lunchtime or dinner, increase the breakfast insulin dose or lunchtime dose, respectively. The same type of reasoning is applied if blood glucose testing indicates hypoglycemia, in which case insulin doses should be reduced accordingly. Thus, the dose of bolus insulin may vary from meal to meal, depending on fluctuations in blood glucose levels throughout the day, as well as mealtime carbohydrate intake (Citation38,Citation48).
The MDI approach to insulin therapy intensification offers the same opportunity for an individualized treatment regimen through titration of bolus doses similar to the stepwise approach. However, the immediate transition from once- or twice-daily injection of basal insulin to a complex regimen consisting of 4–5 total daily injections with the addition of three injections of bolus insulin may be initially challenging for some patients, thus making the stepwise approach a more attractive option. The success of the MDI approach outlined above also assumes consistent carbohydrate intake at each meal. For patients who wish to vary their carbohydrate intake from meal to meal and day to day, carbohydrate counting is recommended.
Bergenstal et al. conducted a study in patients with T2DM that compared the effectiveness of a simple algorithm to adjust bolus insulin dosing based on a weekly average of pre-meal SMBG levels versus an algorithm based on mealtime carbohydrate counting (Citation41). Both approaches resulted in similar levels of glycemic control (approximately a 1.5% reduction in HbA1C), with a low risk for severe hypoglycemia (4.9 versus 8.0 events/patient-year, respectively) (Citation41). Patients in the carbohydrate-counting group displayed a trend towards greater weight loss and lower daily insulin requirements, perhaps due to better matching of insulin doses to carbohydrate intake at each meal. Carbohydrate counting is an important aspect of managing insulin dosing and can provide patients with greater flexibility in food choices. However, for the method to be truly effective, the patient's ability and willingness to track carbohydrate intake and perform the necessary, sometimes complex, calculations should be considered (Citation41,Citation49).
Additional concerns
Insulin intensification and OADs
A common concern with progressing from basal insulin to adding on bolus insulin doses is whether to continue or discontinue OAD therapy. Treatment guidelines uniformly recommend to continue insulin sensitizers (biguanides and thiazolidinediones), but they recommend that insulin secretagogues be either discontinued from a regimen (Citation14) or else continued in patients with close monitoring for hypoglycemic events (Citation50). The same pattern is true in clinical trials. Sulfonylureas were discontinued from OAD therapy in the STEP-Wise study when initiating bolus insulin (Citation46), while patients in the OPAL study remained on their existing OAD regimen (Citation39). While each patient case must be handled individually, we would conservatively recommend discontinuing insulin secretagogues when adding on bolus insulin to a basal insulin regimen.
Hypoglycemia
Unique hypoglycemic concerns may be associated with each of the two insulin intensification options. Although basal-bolus insulin therapy strives closely to approximate physiologic insulin secretion, patients may still experience hypoglycemia. highlights the incidence of hypoglycemia in several studies involving intensification of insulin therapy and/or optimization of treatment. With the exception of the 1-2-3 Study, rates of major hypoglycemia events were relatively low, ranging from 0.0% to 1.7% compared with 7% in the 1-2-3 Study.
Patient education is key, as the inability to recognize an impending hypoglycemic event is an important risk factor for severe hypoglycemia, potentially requiring emergency intervention (Citation51). The use of rapid-acting insulin analogues with improved pharmacokinetic/pharmacodynamic profiles, and more convenient dosing closer to mealtimes (including immediately after meals) than regular human insulin, can also help to reduce the risk of hypoglycemia. Hypoglycemia may be a concern following missed or delayed meals, when the carbohydrate content of the meal is lower than usual, or when there is an expectation that the upcoming meal will be carbohydrate rich and is not. These conditions may necessitate a change in the bolus insulin dose and require an appropriate diet strategy to complement insulin therapy (e.g. consistent carbohydrate intake or carbohydrate counting). The involvement of a nutritionist and certified diabetes educator registered nurse or registered nurse practitioner is often helpful in teaching and implementing such strategies.
Increased or unexpected exercise may also lead to hypoglycemic episodes, particularly when exogenous insulin levels are at their peaks and physical activity is prolonged; however, this does not mean that exercise should be avoided (Citation52–54). The ADA recommends that patients monitor blood glucose levels prior to and during activity, and for several hours after being active, to map the glycemic response to exercise (Citation53). If pre-exercise glucose levels are < 5.6 mmol/L (< 100 mg/dL), then 15 g of additional carbohydrates should be ingested. If exercise is planned, then a conservative reduction in insulin may be considered (Citation55).
Weight gain
Weight gain is often a common side-effect of insulin usage. More intensive therapy aimed at tighter glucose control is usually associated with more weight gain when compared with less intensive treatment. In the 1-2-3 Study, the mean body-weight increase across the once-daily through thrice-daily biphasic insulin aspart 70/30 treatment arms was found to be 5 kg (Citation24), while in STEP-Wise and AT.LANTUS, weight gain increases were smaller with intensification (Citation31,Citation46). In STEP-Wise, increases of + 2.0–2.7 kg were observed in patients who were receiving prandial insulin in addition to once-daily insulin detemir (Citation46), while in the AT.LANTUS subanalysis a statistically significant increase from baseline in body-weight of + 0.8 kg (P < 0.001) was observed in patients treated with prandial insulin in addition to insulin glargine (Citation31).
Patient education and quality of life
Instruction in insulin management and diabetes self-care skills, including self-adjustment of insulin dose, exercise, and appropriate treatment of hypo- and hyperglycemia, has been shown to be associated with improvements in HbA1C, patient empowerment, and quality of life (Citation56). In patients newly switched to insulin therapy, participation in a diabetes treatment and teaching program contributed to reported improvements in quality of life, fewer daily struggles, and fewer worries about the future (Citation57). Thus, the availability of education and support programs can impact patient treatment satisfaction, and may play a significant role in the overall success of a basal-bolus insulin regimen.
Conclusion
Although the benefits of good glycemic control in T2DM are well known, many patients do not achieve and maintain recommended HbA1C goals (Citation58,Citation59). It is essential that patients and physicians are aware of the need to achieve these goals in order to reduce the morbidity and mortality associated with T2DM. While there are a number of different approaches to the intensification and optimization of insulin therapy, the stepwise approach may be the easiest to implement. Gradual introduction of bolus insulin at mealtimes helps to ease the transition to full intensive therapy, while patient-directed dose titration provides a measure of flexibility in terms of diet- and exercise-related insulin needs. The recommendations presented here for the stepwise and MDI approaches are meant to serve as a guide-post. Ultimately, an approach that individualizes patient goals and proceeds conservatively and with great care when adding on and titrating insulin will help to increase the success of insulin therapy in the achievement of recommended goals.
Acknowledgements
The authors thank Robert Schupp, PharmD, and Alan J. Klopp, PhD, of inScience Communications, Springer Healthcare, for providing medical writing support.
Declaration of interest: Preparation of this manuscript was supported by Novo Nordisk, US. The authors did not receive any compensation for this work.
During the past year, Martin Abrahamson has served as an advisory board consultant for Boehringer Ingelheim, Halozyme, and Novo Nordisk.
Anne Peters has served as an advisory board consultant for Amylin Pharmaceuticals, Eli Lilly, and Novo Nordisk and has spoken for/is on the Speaker's Bureau of Amylin Pharmaceuticals, Boehringer Ingelheim, Dexcom, Eli Lilly, Novo Nordisk, and Takeda.
References
- International Diabetes Federation. Diabetes Backgrounder. Available at: http://www.eatlas.idf.org/webdata/docs/background_opening_pc.pdf [accessed 2 September 2011].
- Centers for Disease Control and Prevention. Centers for Disease Control detailed data for diagnosed diabetes. Available at: http://www.cdc.gov/diabetes/statistics/prev/national/tnumage.htm [accessed 15 September 2011].
- Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, . Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560–72.
- The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–86.
- Nathan DM, Cleary PA, Backlund JY, Genuth SM, Lachin JM, Orchard TJ, . Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353:2643–53.
- Ohkubo Y, Kishikawa H, Araki E, Miyata T, Isami S, Motoyoshi S, . Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non-insulin-dependent diabetes mellitus: a randomized prospective 6-year study. Diabetes Res Clin Pract. 1995;28:103–17.
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352:837–53.
- UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet. 1998;352:854–65.
- American Diabetes Association. Standards of medical care in diabetes—2012. Diabetes Care. 2012;35 Suppl 1:S11–63.
- International Diabetes Federation. Global guideline for type 2 diabetes. Available at: http://www.idf.org/webdata/docs/IDF%20GGT2D.pdf. [accessed 2 September 2011].
- Rodbard HW, Blonde L, Braithwaite SS, Brett EM, Cobin RH, Handelsman Y, . American Association of Clinical Endocrinologists medical guidelines for clinical practice for the management of diabetes mellitus. Endocr Pract. 2007;13 Suppl 1:1–68.
- Hoerger TJ, Segel JE, Gregg EW, Saaddine JB. Is glycemic control improving in U.S. adults? Diabetes Care. 2008;31:81–6.
- Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577–89.
- Nathan DM, Buse JB, Davidson MB, Ferrannini E, Holman RR, Sherwin R, . Medical management of hyperglycaemia in type 2 diabetes mellitus: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia. 2009;52:17–30.
- Turner RC, Cull CA, Frighi V, Holman RR; UK Prospective Diabetes Study (UKPDS) Group. Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus: progressive requirement for multiple therapies (UKPDS 49). JAMA. 1999;281:2005–12.
- Riddle MC. Glycemic management of type 2 diabetes: an emerging strategy with oral agents, insulins, and combinations. Endocrinol Metab Clin North Am. 2005;34:77–98.
- Hirsch IB, Bergenstal RM, Parkin CG, Wright E, Buse JB. A real-world approach to insulin therapy in primary care practice. Clin Diabetes. 2005;23:78–86.
- Weng J, Li Y, Xu W, Shi L, Zhang Q, Zhu D, . Effect of intensive insulin therapy on beta-cell function and glycaemic control in patients with newly diagnosed type 2 diabetes: a multicentre randomised parallel-group trial. Lancet. 2008;371:1753–60.
- Holman RR, Farmer AJ, Davies MJ, Levy JC, Darbyshire JL, Keenan JF, . Three-year efficacy of complex insulin regimens in type 2 diabetes. N Engl J Med. 2009;361: 1736–47.
- Nathan DM, Buse JB, Davidson MB, Ferrannini E, Holman RR, Sherwin R, . Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2009;32: 193–203.
- DeWitt DE, Hirsch IB. Outpatient insulin therapy in type 1 and type 2 diabetes mellitus: scientific review. JAMA. 2003;289:2254–64.
- Riddle MC, Rosenstock J, Gerich J. The treat-to-target trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes Care. 2003;26:3080–6.
- Raskin P, Allen E, Hollander P, Lewin A, Gabbay RA, Hu P, . Initiating insulin therapy in type 2 diabetes: a comparison of biphasic and basal insulin analogs. Diabetes Care. 2005;28:260–5.
- Garber AJ, Wahlen J, Wahl T, Bressler P, Braceras R, Allen E, . Attainment of glycaemic goals in type 2 diabetes with once-, twice-, or thrice-daily dosing with biphasic insulin aspart 70/30 (the 1-2-3 Study). Diabetes Obes Metab. 2006;8:58–66.
- Swinnen SG, Dain MP, Aronson R, Davies M, Gerstein HC, Pfeiffer AF, . A 24-week, randomized, treat-to-target trial comparing initiation of insulin glargine once-daily with insulin detemir twice-daily in patients with type 2 diabetes inadequately controlled on oral glucose-lowering drugs. Diabetes Care. 2010;33:1176–8.
- Sasali A, Leahy JL. Insulin therapy for type 2 diabetes. Curr Diabetes Rep. 2003;3:378–85.
- Nathan DM, Buse JB, Davidson MB, Heine RJ, Holman RR, Sherwin R, . Management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2006;29:1963–72.
- Raccah D, Bretzel RG, Owens D, Riddle M. When basal insulin therapy in type 2 diabetes mellitus is not enough—what next? Diabetes Metab Res Rev. 2007;23:257–64.
- Rosenstock J, Ahmann AJ, Colon G, Scism-Bacon J, Jiang H, Martin S. Advancing insulin therapy in type 2 diabetes previously treated with glargine plus oral agents: prandial premixed (insulin lispro protamine suspension/lispro) versus basal/bolus (glargine/lispro) therapy. Diabetes Care. 2008; 31:20–5.
- Fritsche A, Larbig M, Owens D, Haring H-U. Comparison between a basal-bolus and a premixed insulin regimen in individuals with type 2 diabetes—results of the GINGER study. Diabetes Obes Metab. 2010;12:115–23.
- Davies M, Sinnassamy P, Storms F, Gomis R. Insulin glargine-based therapy improves glycemic control in patients with type 2 diabetes sub-optimally controlled on premixed insulin therapies. Diabetes Res Clin Pract. 2008;79:368–75.
- Rolla A. Pharmacokinetic and pharmacodynamic advantages of insulin analogues and premixed insulin analogues over human insulins: impact on efficacy and safety. Am J Med. 2008;121:S9–19.
- Hartman I. Insulin analogs: impact on treatment success, satisfaction, quality of life, and adherence. Clin Med Res. 2008;6:54–67.
- Anderson JH Jr, Brunelle RL, Keohane P, Koivisto VA, Trautmann ME, Vignati L, . Mealtime treatment with insulin analog improves postprandial hyperglycemia and hypoglycemia in patients with non-insulin-dependent diabetes mellitus. Multicenter Insulin Lispro Study Group. Arch Intern Med. 1997;157:1249–55.
- Anderson JH Jr, Brunelle RL, Koivisto VA, Trautmann ME, Vignati L, DiMarchi R. Improved mealtime treatment of diabetes mellitus using an insulin analogue. Multicenter Insulin Lispro Study Group. Clin Ther. 1997;19:62–72.
- Heller S, Bode BW, Kozlovski P, Svendsen AL. Examining the glycemic and hypoglycemic benefits with rapid-acting insulin analogs: a meta-analysis of insulin aspart vs regular insulin in randomized controlled trials [abstract 505-P]. Presented at: American Diabetes Association 69th Scientific Sessions, 5–9 June 2009, New Orleans, LA, USA.
- Owens DR, Schalkwyk C, Smith P, Beer S, Goenka N, Bain SC, . Algorithm for the introduction of rapid-acting insulin analogues in patients with type 2 diabetes on basal insulin therapy. Pract Diab Int. 2009;26:70–7.
- Pearson J, Powers MA. Systematically initiating insulin: the staged diabetes management approach. Diabetes Educ. 2006;32:19S–28S.
- Lankisch MR, Ferlinz KC, Leahy JL, Scherbaum WA. Introducing a simplified approach to insulin therapy in type 2 diabetes: a comparison of two single-dose regimens of insulin glulisine plus insulin glargine and oral antidiabetic drugs. Diabetes Obes Metab. 2008;10:1178–85.
- Ampudia-Blasco FJ, Rossetti P, Ascaso JF. Basal plus basal-bolus approach in type 2 diabetes. Diabetes Technol Ther. 2011;13 Suppl 1:S75–83.
- Bergenstal RM, Johnson M, Powers MA, Wynne A, Vlajnic A, Hollander P, . Adjust to target in type 2 diabetes: comparison of a simple algorithm with carbohydrate counting for adjustment of mealtime insulin glulisine. Diabetes Care. 2008;31:1305–10.
- Yki-Jarvinen H, Kauppinen-Makelin R, Tiikkainen M, Vahatalo M, Virtamo H, Nikkila K, . Insulin glargine or NPH combined with metformin in type 2 diabetes: the LANMET study. Diabetologia. 2006;49:442–51.
- Ismail-Beigi F, Moghissi E, Tiktin M, Hirsch IB, Inzucchi SE, Genuth S. Individualizing glycemic targets in type 2 diabetes mellitus: implications of recent clinical trials. Ann Intern Med. 2011;154:554–9.
- Monnier L, Colette C. Addition of rapid-acting insulin to basal insulin therapy in type 2 diabetes: indications and modalities. Diabetes Metab. 2006;32:7–13.
- Blonde L, Merilainen M, Karwe V, Raskin P. Patient-directed titration for achieving glycaemic goals using a once-daily basal insulin analogue: an assessment of two different fasting plasma glucose targets—the TITRATE study. Diabetes Obes Metab. 2009;11:623–31.
- Meneghini L, Mersebach H, Kumar S, Svendsen AL, Hermansen K. A comparison of two intensification regimens with rapid-acting insulin aspart in type 2 diabetes inadequately controlled by once-daily insulin detemir and oral antidiabetes drugs: the STEP-Wise randomized study. Endocr Pract. 2011;17:727–36.
- Palumbo PJ. The case for insulin treatment early in type 2 diabetes. Cleve Clin J Med. 2004;71:385–6, 391–2.
- Mayfield JA, White RD. Insulin therapy for type 2 diabetes: rescue, augmentation, and replacement of beta-cell function. Am Fam Physician. 2004;70:489–500.
- Davis NJ, Wylie-Rosett J. Death to carbohydrate counting? Diabetes Care. 2008;31:1467–8.
- National Institute for Health and Clinical Excellence. The management of type 2 diabetes. Available at: http://www.nice.org.uk/nicemedia/live/12165/44322/44322.pdf [accessed 10 January 2010].
- Akram K, Pedersen-Bjergaard U, Borch-Johnsen K, Thorsteinsson B. Frequency and risk factors of severe hypoglycemia in insulin-treated type 2 diabetes: a literature survey. J Diabetes Complications. 2006;20:402–8.
- Colberg S. The impact of exercise on insulin action in type 2 diabetes mellitus: relationship to prevention and control. Insulin. 2006;1:85–98.
- Sigal RJ, Kenny GP, Wasserman DH, Castaneda-Sceppa C, White RD. Physical activity/exercise and type 2 diabetes: a consensus statement from the American Diabetes Association. Diabetes Care. 2006;29:1433–8.
- Plockinger U, Topuz M, Riese B, Reuter T. Risk of exercise-induced hypoglycaemia in patients with type 2 diabetes on intensive insulin therapy: comparison of insulin glargine with NPH insulin as basal insulin supplement. Diabetes Res Clin Pract. 2008;81:290–5.
- Pearson T. Exercise, insulin, and type 2 diabetes. Medscape Diabetes Endocrinol. 2008. Available at: http://www.medscape.com/viewarticle/572219 [accessed 26 June 2012].
- Lowe J, Linjawi S, Mensch M, James K, Attia J. Flexible eating and flexible insulin dosing in patients with diabetes: results of an intensive self-management course. Diabetes Res Clin Pract. 2008;80:439–43.
- Braun A, Samann A, Kubiak T, Zieschang T, Kloos C, Muller UA, . Effects of metabolic control, patient education and initiation of insulin therapy on the quality of life of patients with type 2 diabetes mellitus. Patient Educ Couns. 2008;73:50–9.
- Grant RW, Buse JB, Meigs JB. Quality of diabetes care in U.S. academic medical centers: low rates of medical regimen change. Diabetes Care. 2005;28:337–442.
- Resnick HE, Foster GL, Bardsley J, Ratner RE. Achievement of American Diabetes Association clinical practice recommendations among U.S. adults with diabetes, 1999–2002: the National Health and Nutrition Examination Survey. Diabetes Care. 2006;29:531–7.