Abstract
Aim. This review compared the effect of high-dose nicotine replacement therapy (NRT) and combinations of NRT for increasing smoking abstinence rates compared to standard-dose NRT patch, varenicline, and bupropion on smoking abstinence.
Methods. Ten electronic databases were searched (up to January 2012) for randomized controlled trials (RCT) of standard-dose (≤ 22 mg) or high-dose nicotine patch therapy (> 22 mg), combination NRT (e.g. nicotine patch + nicotine inhaler), bupropion, and varenicline. Analysis consisted of random-effects pairwise meta-analysis and a Bayesian multiple treatment comparison (MTC).
Results. We identified 146 RCTs (65 standard-doses of the nicotine patch (≤ 22 mg); 6 high-dose NRT patch (> 22 mg); 5 high versus standard-dose NRT patch; 5 combination NRT versus inert controls; 6 combination versus single NRT patch; 48 bupropion; and 11 varenicline). The MTC found that all therapies offered treatment benefits at most time points over controls. Combination NRT and higher-dose NRT did not demonstrate consistent effects over other interventions. With the exception of varenicline, the benefits of treatments over standard-dose NRT were not retained in the long term.
Conclusions. All pharmacologic treatments were significantly more effective than inert controls. Varenicline was the only treatment demonstrating effects over other options. These results should be considered in the development of clinical practice guidelines.
Key messages
Several pharmacotherapies are available to increase smoking cessation. High-dose nicotine replacement therapy (NRT), combination NRT, and varenicline, in particular, are advocated as offering increased treatment effects.
However, the effectiveness of all available treatments, relative to each other, is not known.
Using a multiple treatment meta-analysis, there is clear evidence that some pharmacotherapies offer a larger treatment effect than others.
Introduction
Smoking is the leading cause of preventable mortality worldwide (Citation1). One in every two long-term smokers will die a smoking-related death (Citation2). Smoking cessation has a considerable impact on improving life expectancy, reducing morbidity, and reducing health care costs associated with treating smoking-related conditions (Citation3).
Several pharmacological interventions to assist with smoking cessation are available (Citation4). The most widely used product in Europe and the United States is nicotine replacement therapy (NRT). This is available both over the counter (OTC) and via prescription. NRT is recommended as a safe and effective intervention for the general population of smokers, adolescents, and smokers with cardiovascular disease (Citation5). NRT increases smoking cessation rates at 1 year by approximately 70% (odds ratio (OR) 1.70, 95% confidence interval (CI) 1.55–1.88) (Citation4,Citation6). Other effective pharmacotherapies available by prescription include bupropion and varenicline (Citation4,Citation7,Citation8). Among the currently available medications, varenicline is associated with the largest treatment effects for short-term and long-term cessation (Citation4,Citation9). Clinical trials have demonstrated statistically significant improvements in smoking cessation for varenicline versus bupropion (Citation10–12). Previous meta-analyses using indirect comparisons have demonstrated superiority of varenicline to standard doses of NRT (Citation4,Citation6,Citation9).
NRT monotherapy increases smoking abstinence rates but is not effective for all smokers. In attempts to increase the efficacy of NRT, higher doses of NRT (e.g. such as nicotine patch doses > 22 mg/d) and combination NRT (e.g. nicotine patch + inhaler) have been evaluated. However, the relative efficacy of high-dose nicotine patch therapy and combination therapy NRT interventions in comparison with bupropion and varenicline has not been explored (Citation13). We aimed to determine the comparative effectiveness of standard-dose nicotine patch therapy (≤ 22 mg), high-dose nicotine patch therapy (> 22 mg), combination NRT, bupropion, and varenicline at approximately 4, 12, 26, and 52 weeks after the target quit date. We undertook a systematic review, pairwise meta-analysis, and multiple treatment comparison (MTC) to compare the relevant pharmacotherapies.
Methods
Eligibility criteria
We included any randomized controlled trial (RCT) of NRT using a transdermal patch delivery evaluating lower and standard doses of NRT (≤ 22 mg), higher-dose NRT (> 22 mg), combination NRT, bupropion, or varenicline at licensed doses. We included only RCTs with at least 3 months post-target quit date (TQD) with biochemical confirmation of smoking abstinence (Citation14).
Studies had to enroll smokers at initiation of therapy and report smoking abstinence as either continuous or point prevalence smoking abstinence. From a clinical perspective, nicotine patch allows for continuous administration of NRT with acute administrations of nicotine gum, lozenge, inhaler, or nasal spray to be taken as needed to deal with cravings and urges. We focused only on NRT combination therapy trials involving use of a nicotine patch since from a clinical perspective it is most relevant to assess the impact of maintenance NRT patch therapy with the addition of an acute NRT formulation. For evaluations of combination versus single NRT, studies had to compare a NRT combination involving a nicotine patch versus NRT patch plus acute NRT placebo, since in clinical practice smokers should normally commence NRT monotherapy with maintenance NRT patch, rather than an acute formulation such as gum or nasal spray designed for use with breakthrough cravings. We excluded non-RCTs, post-hoc analyses, maintenance therapy of successful quitters, and studies with self-reported outcomes.
Study end-points
Our primary outcomes of interest were smoking abstinence at approximately 4, 12, 26, and 52 weeks after the TQD. These are the approximate time points reported in RCTs, although trials of bupropion and varenicline typically have a TQD as 1 week post-initiation of treatment. When both outcomes were available, we selected continuous tobacco abstinence over point prevalence tobacco abstinence.
Search strategy
In consultation with a medical librarian, we established a search strategy. We searched independently, in duplicate, the following 10 databases (from inception to 1 January 2012): MEDLINE, EMBASE, Cochrane CENTRAL, AMED, CINAHL, TOXNET, Development and Reproductive Toxicology, Hazardous Substances Databank, Psych-info, and Web of Science, and databases including the full text of journals (OVID, ScienceDirect, and Ingenta, including articles in full text from approximately 1700 journals since 1993). In addition, we searched the bibliographies of published systematic reviews and health technology assessments. Searches were not limited by language, sex, or age. The specific Ovid search strategy is provided in the web-appendix online.
Study selection
Two investigators (E.J.M., P.W.) independently and in duplicate scanned abstracts and thereafter obtained the full text reports of records indicating or suggesting that the study was a RCT evaluating a smoking abstinence therapy on the outcomes of interest. After obtaining full reports of the candidate trials the same reviewers independently assessed eligibility from full text papers.
Data collection
Two reviewers (P.W., E.J.M.) conducted data extraction independently using a standardized pre-piloted form, available from the authors upon request. Reviewers collected information about the smoking intervention, the population studied (age, sex, underlying conditions), treatment dosages and dosing schedules, the treatment effect at the specified time points, the specific measurement of abstinence (continuous or point prevalence), and the methods of biochemical confirmation of smoking abstinence. Study evaluation included general methodological quality features including allocation concealment, sequence generation, blinding status, intention-to-treat, and appropriate descriptions of loss to follow-up.
Data analysis
In order to assess inter-rater reliability on inclusion of articles, we calculated the phi statistic, which provides a measure of inter-observer agreement independent of chance (Citation5). Our analysis required two approaches: firstly, pairwise meta-analysis of all direct RCT evidence, and secondly, a multiple treatment comparison (MTC) meta-analysis that includes both the direct RCT evidence and indirect comparisons of those treatments. For pairwise meta-analysis, we calculated the relative risk (RR) and appropriate 95% confidence intervals (CIs) of outcomes according to the number of events of abstinence reported in the original studies or sub-studies. All studies were pooled using the DerSimonian–Laird random-effects method as this approach assumes there is variation in the treatment effect size between studies in a pairwise comparison (Citation15). The I2 statistic was calculated for each analysis as a measure of the proportion of the overall variation that is attributable to between-study heterogeneity. We considered an I2 value greater than 30% to be important and investigated the cause of heterogeneity using subgroup analysis and random-effects meta-regression. A-priori explanations of heterogeneity were: for NRT, whether the controls were placebo or open; for high-dose NRT patch and combination NRT, whether trials were of long-term smokers (high-dose NRT patch) or heavy-intake smokers (combination NRT); for bupropion, whether placebo control and confirmation of abstinence using cotinine detection; and, for varenicline, whether trials involved healthy smokers or smokers with an illness. As most trials of the different therapies provided the interventions for up to 12 weeks, we conducted a sensitivity analysis whereby we excluded any trials that provided the therapeutic intervention for longer than 16 weeks. We investigated whether continuous or point prevalence tobacco abstinence outcomes differed significantly by examining this issue using a random-effects meta-regression.
To inform comparative effectiveness between all interventions, we conducted a Bayesian multiple treatment comparison (MTC) meta-analysis (Citation16). We constructed a random-effects MTC allowing for different between-study variances based on the variance elicited in pairwise DerSimonian–Laird analyses. We used the pairwise between-study standard deviation estimates as the assumed ‘true’ between-study measures of variation, thereby enabling the random-effects MTC to mirror the differences in between-study heterogeneity observed in the pairwise treatment comparisons. We estimated the posterior densities for all unknown parameters using Markov chain Monte Carlo methods for each model. We assessed convergence based on trace plots and time series plots. The accuracy of the posterior estimates was done by calculating the Monte Carlo error for each parameter. As a rule of thumb, the Monte Carlo error for each parameter of interest is less than about 5% of the sample standard deviation. We calculated the probability of each intervention being best. All results for the MTC analysis are reported as posterior median with corresponding 95% credibility intervals (CrIs). Credibility intervals are the Bayesian equivalent of classical confidence intervals. We assessed the fit of our model using the deviance information criterion (DIC), a measure of model fit that penalizes for model complexity. An uninformed fixed-effects MTC was compared to the informed random-effects MTC in terms of model fit. The MTC results were assessed for consistency by comparing them with an adjusted indirect comparison (Citation17) and with the pairwise meta-analyses results. We evaluated inconsistency between direct estimates and indirect estimates by examining for statistical differences. As ORs are statistically superior to RRs (Citation18), we conducted the MTC with OR as the effect size and converted the OR to RRs using the formula: RR = OR/[(1–po) + po × OR] where po represents the baseline probability (or risk) in the reference group.
Analyses were conducted using Comprehensive Meta-analysis (version 2, http://www.meta-analysis.com) and WinBUGS version 1.4 (Medical Research Council Biostatistics Unit, Cambridge).
Results
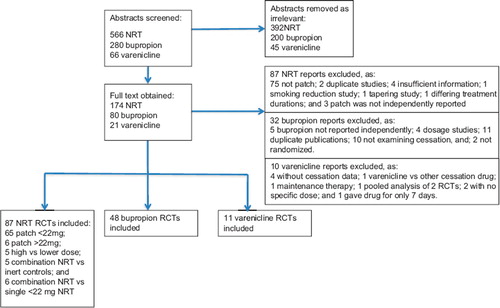
Our review identified 146 RCTs. We identified 65 RCTs that evaluated standard-dose nicotine patch therapy (≤ 22 mg); 6 RCTs evaluated high-dose nicotine patch therapy (> 22 mg) versus controls; 5 RCTs evaluated high versus standard-dose nicotine patch therapy; 5 RCTs evaluated combination NRT versus inert controls; 6 RCTs evaluated combination versus single NRT; 48 RCTs evaluated bupropion; and 11 RCTs evaluated varenicline. One RCT evaluated varenicline versus higher-dose NRT and one compared varenicline to standard-dose nicotine patch. Agreement on inclusion was excellent (phi = 0.95). displays the flow diagram of included studies. See Supplementary Table I to be found online at http://www.informahealthcare.com/doi/abs/10.3109/07853890.2012.705016 for characteristics of included trials and Supplementary appendix XIII to be found online at http://www.informahealthcare.com/doi/abs/10.3109/07853890.2012.705016 for the references of included trials.
The trial quality was variable, and older trials tended to miss reporting important methodological considerations. Specific details on methodological reporting are available from the authors. Most trials included participants who were attempting to quit. Most trials (75%) confirmed cessation using exhaled carbon monoxide (CO) confirmation, the remaining used salivary cotinine, serum cotinine, and urine sampling methods.
Pairwise comparisons
We examined pairwise comparisons of all interventions with available head-to-head data. The results are reported in . For any direct comparison with an I2 value of greater than 30%, we assessed heterogeneity. For NRT versus placebo, heterogeneity was explained by the use of placebo or not and reduced the I2 to approximately 0% (4 weeks: I2 = 0%, 3 months: I2 = 3%, 6 months: I2 = 16%, 12 months: I2 = 0%). For high-dose nicotine patch therapy (> 22 mg) versus placebo, meta-regression identified a trial of long-term smokers (Citation19) as contributing to observed heterogeneity. When this trial was removed from the pairwise analyses the observed heterogeneity was eliminated or substantially reduced at all time points (4 weeks: I2 = 0%, 3 months: I2 = 0%, 6 months: I2 = 12%, 12 months: I2 = 0%). For high-dose versus standard doses of nicotine patch therapy, at the 3 months time point there were too few RCTs to statistically evaluate heterogeneity, and no particular attribute was visually apparent. For combination NRT versus single NRT, at 4 weeks and 12 months the inclusion of one trial with the heaviest smoking participants appeared to account for the observed heterogeneity. When this study was excluded from the pairwise analyses, the observed heterogeneity was eliminated or reduced close to zero (4 weeks: I2 = 0%, 3 months: I2 = 1%, 6 months: I2 = 0%, 12 months: I2 = 0%). For bupropion versus inert control, heterogeneity was explained by the use of placebo or not at the first three time points, reducing the I2 to 0%; whereas at 12 months, differences in outcome adjudication explained a significant proportion of heterogeneity. For varenicline, heterogeneity at all time points was explained by the inclusion of studies focusing on smoking cessation in patients with established illness (CVD or COPD), which reduced the I2 to 0%.
Table I. Pairwise meta-analysis results for smoking abstinence.
Multiple treatment meta-analysis
Our multiple treatment meta-analysis included data from all RCTs. displays the geometric distribution of the utilized studies. The deviance information criteria for the random-effects model had a value of 1397.4, 1415.0, 1383.9, and 1006.9 for the defined time points, which represented a better fit of the data compared to the fixed-effects model sensitivity analysis (1471.2, 1640.6, 1465.1, 1078.6). The between-study standard deviations for the analysis for the defined time points are available from the authors. displays the study comparison results at the specified time points from the random-effects MTC. displays the probability that each treatment is best at the specified time points. At all four time points, the probability of being the best treatment is consistently led by varenicline.
Figure 2. Geometric distribution of included studies in MTC analysis. Nodes represent the study drugs. Links between nodes represent direct comparisons from RCTs. The numbers beside the links represent the number of RCTs for each comparison.
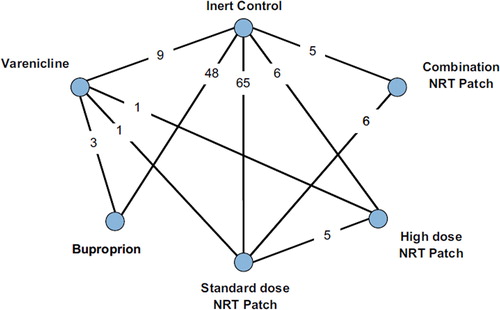
Table II. Estimated relative risk (RR) and 95% credibility intervals (CrI) produced by the random-effects multiple treatment comparison (MTC) analysis.
Table III. Probability of each treatment being best, derived on the basis of the random-effects multiple treatment comparison (MTC) model for smoking cessation.
Interpretation of MTC
Standard-dose NRT patch, high-dose NRT patch, bupropion, and varenicline were all significantly better than placebo and inert controls at all time points. Combination NRT was only significantly better than placebo/controls at 4 weeks and 6 months, but not at the other time points. Combination NRT was not significantly different to high-dose patch or bupropion in terms of smoking abstinence rates at any time point. Bupropion was not significantly better than any other active pharmacotherapy at any time point, apart from versus standard-dose NRT at 4 weeks. Varenicline was associated with statistically significant improvements in smoking abstinence compared to all interventions at all time points except at 6 months compared to high-dose (> 22 mg) nicotine patch therapy and combination NRT.
Sensitivity analysis
As a sensitivity analysis of the pairwise meta-analysis comparisons, we removed trials that had a period of treatment greater than 16 weeks. Although there was a trend towards increased effect sizes, this was inconsistent and negligible. We additionally examined pairwise meta-analysis effects using only trials that reported continuous abstinence rates, by events, and the results were not importantly different to the main analysis (Supplementary Table VIII provides the pairwise results). For the MTC, we performed an uninformed fixed-effects analysis (Supplementary Table IX to be found online at http://www.informahealthcare.com/doi/abs/10.3109/07853890.2012.705016), and the results were consistent with the informed random-effects MTC (). We compared the MTC analysis results to the adjusted indirect comparison findings (Supplementary Table X to be found online at http://www.informahealthcare.com/doi/abs/10.3109/07853890.2012.705016). Supplementary Table VII–X to be found online at http://www.informahealthcare.com/doi/abs/10.3109/07853890.2012.705016 display the fixed- and random-effects (odds ratio) MTC analysis, the adjusted indirect comparison sensitivity analysis, and pairwise outcomes only using continuous abstinence. The two analyses were consistent for most treatment comparisons, with no major divergent results. We examined the coherence between direct, indirect estimates, and MTC estimates and the discrepancies are explained in Supplementary appendix XI to be found online at http://www.informahealthcare.com/doi/abs/10.3109/07853890.2012.705016 and in the discussion below.
Discussion
Our pairwise meta-analysis and MTC confirm the effectiveness of all pharmacotherapies for smoking cessation over inert controls in maintaining short-term and long-term smoking cessation. Our MTC analysis provides robust findings on the relative effectiveness of interventions by drawing power from all available trials and provides inferences on the relative effectiveness of each intervention. In our MTC analysis, we found that varenicline exhibited the largest and sustained treatment effects. The MTC did not demonstrate a statistically superior advantage of combination NRT or higher-dose NRT compared with other active therapies.
We have previously evaluated these treatments as monotherapies versus placebo or inert controls and found similar therapeutic effects at the short- and long-term cessation periods (Citation4,Citation9). In this study, several of the pairwise (head-to-head) evaluations demonstrated a statistically significant effect which was not maintained in the MTC findings. This is usually due to a smaller number of trials in the head-to-head analysis. Simulation studies have demonstrated that low numbers of studies can result in spurious conclusions that are rejected when further evidence is added to the analyses (Citation20). MTC analysis improves the power of comparative analysis by borrowing strength from the network of evidence. Supplementary appendix XI to be found online at http://www.informahealthcare.com/doi/abs/10.3109/07853890.2012.705016 explains the inconsistency in detail.
Our study has several important strengths. Our comprehensive search strategy improved the likelihood that we identified all relevant trials. Duplicate extraction of trial data reduced the potential for error in the data abstraction component of the synthesis process. By limiting our analysis to randomized trial evidence we reduce the likelihood of systematic error, which increases internal validity. By applying meta-regression we were able to distinguish features that led to initial trial level heterogeneity. Finally, by applying the multiple treatment comparison (both fixed- and random-effects) and performing adjusted indirect comparisons (see Supplementary Table VII–X to be found online at http://www.informahealthcare.com/doi/abs/10.3109/07853890.2012.705016) to demonstrate consistency, we were able to determine treatment effects in the absence of head-to-head evaluations. A random-effects model was chosen as the base case MTC analysis since it enables the modeling of between-study variation and places greater weight on smaller studies, thus being more conservative. The findings between the fixed- and random-effects models were not importantly different.
Limitations of this analysis include the potential for publication bias, in particular the possibility that small negative studies would not be published. However, it is unlikely that the presence of these studies would have altered the findings of the analysis given the large number of studies included and the consistency with the findings between short- and long-term cessation meta-analyses (Citation4,Citation9). We limited our search to English language databases, although we did exclude non-English articles if identified so the possibility of quality studies in other languages does exist. We used the multiple treatment meta-analysis approach, which includes a mix of direct head- to-head RCT evidence and indirect RCT evidence. Some readers may be unfamiliar with the MTC approach over pairwise meta-analysis. The MTC approach permits inferences over a network of connected trials that may or may not have been compared to each other directly. Although this approach is typically statistically intense, it requires several underlying assumptions that may be more complex than pairwise meta-analysis. At a basic level, it requires a homogeneity assumption (such that the populations and diseases are appropriate to combine), a similarity assumption (that the trials measure similar outcomes), and a consistency assumption (that the findings of direct and indirect evidence are not importantly different) (Citation21,Citation22).
In brief, in order to address the homogeneity and similarity assumptions, a random-effects MTC model was performed to accommodate the variation detected in some of the pairwise comparisons which may be due to differences in, for example, populations studied, doses of NRT prescribed, and how smoking abstinence was defined. Since the standard random-effects MTC model assumes a common variance across all treatment comparisons, the DerSimonian–Laird standard deviation data estimates used in the pairwise meta-analysis comparisons were used to inform the current random-effects MTC analysis to model more accurately the diversity in between-study variation observed across the different pairwise comparisons.
The inconsistencies observed between the pairwise comparisons, MTC, and adjusted indirect comparison sensitivity analysis are discussed in the Supplementary appendix XI to be found online at http://www.informahealthcare.com/doi/abs/10.3109/07853890.2012.705016. The main reason for observed inconsistencies appears to be the addition of more NRT standard-dose patch and NRT higher-dose patch trials that were available for comparison with other treatments in the MTC network than was the case in either the pairwise or adjusted indirect comparison analyses.
When evaluating the current MTC results it is particularly important to consider the role of the standard-dose NRT patch node in the network, the largest information source. We observe that high-dose NRT patch and combination NRT do less well versus standard-dose nicotine patch therapy at some time points than is the case in the relevant pairwise comparisons. The standard-dose NRT node consists of the largest number of trials (n = 65), with a broad range of NRT doses (range 2 mg–22 mg), the majority compared against placebo. Thirty-one (48%) standard-dose NRT patch trials included doses up to 21 or 22 mg. Although the range starts from 2 mg, this reflects the base titration rate for the standard-dose NRT patch studies. All NRT standard-dose studies starting from a base rate of 2, 5, or 7 mg (n = 28; 43%) were titrated up to at least 14 mg, and many (23/28; 82%) were titrated up to 21 mg during the life of the studies concerned. Therefore most NRT doses in the standard-dose arm in the MTC were in the range of 14–21 mg. In contrast, very few trials in the high-dose NRT patch and combination NRT trials compared against doses (21 or 22 mg) of standard NRT patch. Specifically, 5/6 (83%) standard-dose arms in high-dose patch trials had 15 mg doses, and 4/6 (67%) standard-dose arms in combination therapy NRT trials had a maximum titration of 15 mg. Therefore in the pairwise analyses, the high-dose NRT patch and combination NRT arms achieve a greater effect size difference compared to the predominantly lower-dose standard NRT patch comparator treatments. However, the relatively higher doses of NRT in the standard-dose NRT patch node in the MTC appear to contribute to a reduction in effect size differences when compared to combination NRT and high-dose patch NRT, relative to the treatment differences observed in the relevant pairwise comparisons.
The large group of NRT patch trials is therefore more representative of standard-dose NRT patch treatment as a whole than the more limited set of standard-dose NRT patch trials included in the pairwise and adjusted indirect comparisons of the other active treatments of interest (such as high-dose NRT and combination NRT). Therefore, we believe the MTC provides a more comprehensive assessment of how the active treatments of interest (high-dose NRT patch, combination NRT, bupropion, and varenicline) fare against standard-dose NRT patch than either the pairwise or adjusted indirect comparisons alone can provide, and the apparent inconsistencies between the three analyses for these treatment comparisons can be viewed in this context.
There is now some discussion in the methods community as to whether head-to-head trials provide the strongest level of inference regarding intervention superiority compared to indirect comparisons. Some argue that the MTC approach can reduce bias if it includes more trials for a comparison than are available in a head-to-head pairwise meta-analysis of the same treatment comparison, and so may reduce the impact of small outlying studies (Citation23).
A recent Cochrane review found slightly different results than our publication (Citation24). In that analysis, the authors used only pairwise meta-analysis to examine treatment differences. The Cochrane review of NRT therapies for smoking cessation found a statistically significant advantage for combination NRT versus single NRT/no treatment at ‘long-term cessation’ (RR 1.35, 95% CI 1.11–1.63). The review included studies where the single NRT arm was an acute treatment (gum monotherapy and inhaler monotherapy). This is in contrast to our publication that focuses on making a comparison with a maintenance NRT monotherapy, i.e. patch. Further, the Cochrane review definition of ‘long-term cessation’ combined 6-month and 12-month data as a composite outcome, whereas our review examines smoking abstinence at specific time points.
There has been much expectation about the combined effects of NRT as well as higher-dose NRT (Citation25). Case series have indicated important treatment effects that provide compelling inferences of treatment effects (Citation26). In RCTs comparing combination NRT or higher-dose NRT with placebo/inert controls, there is clear evidence of treatment effects. However, these effects are not clearly superior compared with standard dosing of NRT. We did not find any factorial (2 × 2) RCTs evaluating combination NRT, that, if well-powered, would increase the internal validity of a trial by allowing the determination of whether adding non-patch interventions to patch significantly increases effectiveness. It is likely that there is only a small benefit from increased doses of NRT and that treatment effects for most patients may not be dose-dependent.
In conclusion, although most pharmacotherapies provide significantly improved cessation over inert controls over the short and longer term in the pairwise analyses, our MTC demonstrates that varenicline is significantly more effective than other active pharmacotherapy interventions at most time points including at the long-term follow-up. In light of the current economic climate and the drive towards efficiency savings in health services across the world, policy-makers and clinicians should consider the relative costs of the different treatment options when making decisions about selection of appropriate treatments. While considering that not all smokers tolerate medications and that a variety of treatment options should remain available, our data may be used to help guide the development of guidelines and policies to promote the selection of the most efficacious first-line medications for smoking cessation.
Notice of Correction
The version of this article published online ahead of print on 6 Aug 2012 contained an error on page 5 Table I and page 6, Figure 2. The heading in the ‘Number of studies’ column read Bupropion versus varenicline but should have read Varenicline versus bupropion. In Figure 2, the numbers didn't add up to what was in the text. The error has been corrected for this version.
Supplementary Appendix XIII
Download PDF (271.8 KB)Declaration of interest: This study received funding from Pfizer Ltd. Kristian Thorlund receives salary support from the Canadian Institutes of Health Research via the Drug Safety and Effectiveness Network (NETMAN). Edward Mills's salary is provided by the Canadian Institutes of Health Research via a Canada Research Chair.
Ian Lockhart is an employee of Pfizer. Edward Mills, Kristian Thorlund, and Ping Wu have consulted to Pfizer Ltd in previous reports. Jon O. Ebbert has received support to conduct clinical trials with varenicline from Pfizer. Milo A. Puhan reports no conflicts of interest. None of the researchers have any connections with the tobacco, alcohol, gaming industries, or any body substantially funded by one of these organizations.
References
- Peto R, Lopez AD, Boreham J, Thun M, Heath C Jr, Doll R. Mortality from smoking worldwide. Br Med Bull. 1996;52:12–21.
- Doll R, Peto R, Wheatley K, Gray R, Sutherland I. Mortality in relation to smoking: 40 years’ observations on male British doctors. BMJ. 1994;309:901–11.
- Asaria P, Chisholm D, Mathers C, Ezzati M, Beaglehole R. Chronic disease prevention: health effects and financial costs of strategies to reduce salt intake and control tobacco use. Lancet. 2007;370:2044–53.
- Wu P, Wilson K, Dimoulas P, Mills EJ. Effectiveness of smoking cessation therapies: a systematic review and meta-analysis. BMC Public Health. 2006;6:300.
- Meade MO, Cook RJ, Guyatt GH, Groll R, Kachura JR, Bedard M, . Interobserver variation in interpreting chest radiographs for the diagnosis of acute respiratory distress syndrome. Am J Respir Crit Care Med. 2000;161:85–90.
- Eisenberg MJ, Filion KB, Yavin D, Belisle P, Mottillo S, Joseph L, . Pharmacotherapies for smoking cessation: a meta-analysis of randomized controlled trials. CMAJ. 2008;179:135–44.
- Hays JT, Ebbert JO. Varenicline for tobacco dependence. N Engl J Med. 2008;359:2018–24.
- Hays JT, Ebbert JO. Bupropion for the treatment of tobacco dependence: guidelines for balancing risks and benefits. CNS Drugs. 2003;17:71–83.
- Mills EJ, Wu P, Spurden D, Ebbert JO, Wilson K. Efficacy of pharmacotherapies for short-term smoking abstinance: a systematic review and meta-analysis. Harm Reduct J. 2009;6:25.
- Gonzales D, Rennard SI, Nides M, Oncken C, Azoulay S, Billing CB, . Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: a randomized controlled trial. JAMA. 2006;296:47–55.
- Nides M, Oncken C, Gonzales D, Rennard S, Watsky EJ, Anziano R, . Smoking cessation with varenicline, a selective alpha4beta2 nicotinic receptor partial agonist: results from a 7-week, randomized, placebo- and bupropion-controlled trial with 1-year follow-up. Arch Intern Med. 2006;166:1561–8.
- Jorenby DE, Hays JT, Rigotti NA, Azoulay S, Watsky EJ, Williams KE, Billing CB, Gong J, Reeves KR. Varenicline Phase 3 Study Group. http://www.ncbi.nlm.nih.gov/pubmed/16820547 Efficacy of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation: a randomized controlled trial. JAMA. 2006;296: 56–63.
- Fiore MC, Jaén CR, Baker TB, Bailey WC, Benowitz N, Curry SJ, . Treating tobacco use and dependence: 2008 update. Clinical practice guideline. Rockville, MD: U.S. Department of Health and Human Services, Public Health Service; 2008, http://www.ahrq.gov/clinic/tobacco/treating_tobacco_use08.pdf.
- Ruth KJ, Neaton JD. Evaluation of two biological markers of tobacco exposure. MRFIT Research Group. Prev Med. 1991;20:574–89.
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88.
- Lu G, Ades AE. Combination of direct and indirect evidence in mixed treatment comparisons. Stat Med. 2004;23: 3105–24.
- Bucher HC, Guyatt GH, Griffith LE, Walter SD. The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. J Clin Epidemiol. 1997;50:683–91.
- Walter SD. Choice of effect measure for epidemiological data. J Clin Epidemiol. 2000;53:931–9.
- Perng RP, Hsieh WC, Chen YM, Lu CC, Chiang SJ. Randomized, double-blind, placebo-controlled study of transdermal nicotine patch for smoking cessation. J Formos Med Assoc. 1998;97:547–51.
- Mills EJ, Ghement I, O’Regan C, Thorlund K. Estimating the power of indirect comparisons: a simulation study. PLOS One. 2011;6:e16237.
- Mills EJ, Bansback N, Ghement I, Thorlund K, Kelly S, Puhan M, . Multiple treatment comparison meta-analyses: a step forward into complexity. Clin Epidemiol. 2011;3:1–10.
- O’Regan C, Ghement I, Eyawo O, Guyatt GH, Mills EJ. Incorporating multiple interventions in meta-analysis: an evaluation of the mixed treatment comparison with the adjusted indirect comparison. Trials. 2009;10:86.
- Song F, Harvey I, Lilford RAdjusted indirect comparison may be less biased than direct comparison for evaluating new pharmaceutical interventions. J Clin Epidemiol. 2008; 61:455–63.
- Stead LF, Perera R, Bullen C, Mant D, Lancaster T. Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev. 2008:CD000146.
- Ebbert JO, Hays JT, Hurt RD. Combination pharmacotherapy for stopping smoking: what advantages does it offer?Drugs. 2010;70:643–50.
- Ebbert JO, Burke MV, Hays JT, Hurt RDCombination treatment with varenicline and nicotine replacement therapy. Nicotine Tob Res. 2009;11:572–6.