Abstract
Disrupted circadian rhythms may lead to failures in the control of the cell division cycle and the subsequent malignant cell growth. In order to understand the pathogenesis of cancer more in detail, it is crucial to identify those mechanisms of action which contribute to the loss of control of the cell division cycle. This mini-review focuses on the recent findings concerning the links between the human circadian clock and cancer. Clinical implications concern not only feasible methods for the assessment of the circadian time of an individual or for the determination of the best time for administration of a drug of treatment, but also in the future genetic tests for screening and for planning treatment.
Key messages
Circadian clocks guide a number of cancer-related biological pathways.
Disruption of this timed regulation may predispose individuals to cancer.
The circadian clock and the cell division cycle are regulated by the same light- induced signaling pathway.
Long-term circadian misalignment, as it is induced e.g. by work at night, may be permissive to malignant cell growth and tumor development.
Introduction
Circadian clocks, the circadian clock genes and their encoded proteins, guide the organism to follow the natural signals of time and help in adaptation to the routine changes in environmental demands. The approximate 24-hour, i.e. circadian, rhythm is clearly present in the human physiological and behavioral processes. Circadian clock genes through their scheduled transcription modify the metabolic cycle and respiration of cells and are thus also important regulators of the cell division cycle. The circadian clock genes encode the proteins that mediate the information to the whole cell and to the network of cells (Citation1). Considering the whole individual, as much as 2% to 10% of all the mammalian genes (the exact proportion varying from tissue to tissue) are regulated by the circadian mechanism (Citation2–4).
From a physiological point of view, the night is an unnatural time to work, and night shift work is very likely to disrupt the circadian rhythms unless no attempt to protect from such disruption is made. In contrast, work at night on a regular basis only makes a difference in that it may not lead to disruption of the circadian rhythms but to a fixed alignment of the rhythms. On the basis of epidemiological and experimental studies, certain cancers are more common among those whose circadian rhythms are constantly disturbed by jet lag, shift work, or increased exposures to light at night (Citation5–15). More studies are still needed, as the human data are still scarce. Regarding e.g. the influence of jet lag on cancer, studies analyzing the risks for cancer are still far too much based on animal models rather than on humans. Thus far, the International Agency for Research on Cancer, being part of the World Health Organization, has recognized shift work that involves circadian disruption as a human carcinogen of group 2A (probably carcinogenic to humans), thereby being equal to e.g. diesel engine exhaust, inorganic lead compounds, or the human papillomavirus type 68. Even so, a substantial proportion of people of working age is engaged in irregular shift work schedules, for instance approximately 25% of the current employees in Finland (Citation16).
Exposure to work at night and to light at night desynchronizes the night-bound hormone production schedules, leading to the misalignment between the sleep phase of the sleep–wake cycle and the circadian rhythms as a whole as well as among the individual circadian rhythms. Therefore, light at night may induce malignant cell growth and tumor development in hormone-dependent cancers in particular (Citation17,Citation18), and this induction is likely through the actions of the proteins encoded by the circadian clock genes PER1 (Citation19) and PER2 (Citation20). Since circadian clock genes and their downstream clock-controlled genes are involved in a variety of cancer-related biological pathways, disrupted functions of these genes and their encoded proteins may enhance cancer development ( and ). In addition, people with certain clock gene variants might be more susceptible to cancers, especially when exposed to a condition involving disruption of the circadian rhythms such as to light exposure at night during shift work.
Figure 1. Association between circadian clock and cell cycle. CLOCK:BMAL1 complex regulates directly the WEE1 and MYC signaling pathway, influencing cell proliferation. CRY and PER1 participate in DNA damage repair mechanisms. CRY is directly involved in the ATR:CHK1 DNA damage repair complex by binding with TIM. PER1 on the other hand participates in the ATM:CHK2 DNA damage repair complex. DEC2 regulates VEGF, thus influencing angiogenesis (Citation38,Citation48,Citation49,Citation56,Citation63,Citation64).
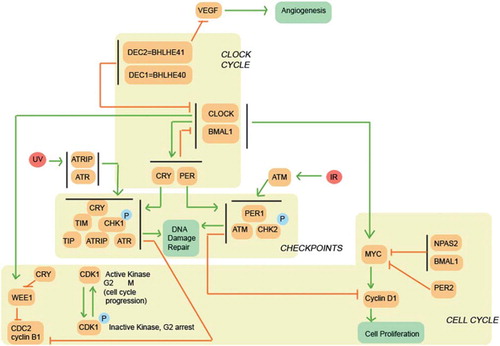
Table I. Human circadian clock gene variants associated with cancers. References are given in the brackets.
Circadian rhythm disruption and cancer development
Circadian clocks influence the cell division cycle through a complex regulatory pathway (). It is likely that a number of additional pathways contribute to the regulation of circadian rhythms, carcinogenesis, and progression of cancer, as e.g. the NONO (non-POU domain containing, octamer-binding) protein is involved in both transcriptional and post-transcriptional gene regulatory functions and DNA repair (Citation21–23). Thus, it is likely that there are several intersections of the cancer-related and circadian pathways. However, it is disruption of the circadian rhythms and the subsequent loss of synchronization in the regulation that is in common here. Therefore, the disruption is the key we think that can predispose individuals to the development of cancer. The mechanism by which the circadian clock is disrupted may make a difference: in particular, there is a common light-induced signaling pathway that regulates both the circadian rhythms and the cell division cycle (Citation24). The molecule of key importance may be different from tissue to tissue, e.g. ARNTL (BMAL1) in the skin (Citation25) and, hypothetically, PER2 or CRY2 in some of the remaining, and their disruption may then lead to a specific mode of cancer.
In recent epidemiological studies, circadian rhythm disruption has been indicated as a risk factor for breast cancer (Citation10,Citation12,Citation13). Long-term night shift work seems to associate with an increased risk for breast cancer (Citation26). However, studies in which the duration of shift work has been quantified demonstrate that robust elevations in risk are seen only after about 20 years of working night shifts, and it is unclear whether there is a risk after shorter durations. Heterogeneity of the exposure metrics and the study outcomes has been problematic in these studies and limited the usefulness of a meta-analysis as a conclusive tool.
Serum or saliva melatonin concentrations can be used as a reliable biomarker of the phase position of the circadian rhythms (Citation27). However, the current data concerning the actions of melatonin as a bioactive protein, the nocturnal synthesis of which is inhibited by exposure to light, in the pathogenesis of cancer are still conflicting (Citation28), albeit that melatonin induces CRY1 expression (Citation29,Citation30) and that melatonin levels, if reduced, are likely to affect the metabolic cascades of the cell, at least those in the liver, through the compromised actions of CRY1 and CRY2 (Citation31). It has been suggested that circadian rhythm disruption influences the regulation of estrogen levels, thereby increasing the risk for developing breast cancer (Citation18). Few epidemiological studies indicate a link between shift work and prostate cancer (Citation6,Citation7). Here, circadian rhythm disruption may also influence the levels of androgens and thereby increase the risk for prostate cancer.
Another line of evidence for the links between the circadian clock and cancer is based on findings which demonstrate that the long-term circadian misalignment, similar to that which occurs in circadian rhythm sleep disorders, reduces leptin levels throughout the day and night and thereby predisposes to weight gain (Citation32), known to be a risk factor for both breast and prostate cancers. However, further research is needed in order to elucidate whether these hypotheses are correct and, if correct, what the detailed mechanisms of action are.
On the other hand, circadian rhythm disruption that affects the immune response pathways might predispose to non-Hodgkin lymphoma (Citation33,Citation34). Here, the circadian clock gene PER2 in specific may be a key, because mice deficient in the mPer2 gene are prone to malignant lymphoma, having not only a substantial increase in tumor development and a reduced apoptosis in thymocytes after gamma irradiation but also spontaneously malignant lymphoma at younger age (Citation35). So far, only one study has investigated the association of night shift work with non-Hodgkin lymphoma, suggesting an increased risk for non-Hodgkin lymphoma as a result of shift work that involves night work and is therefore likely to cause circadian disruption (Citation9). Moreover, the leptin-guided signals may play a role, since leptin triggers an inflammatory response in tumor tissue by stimulating, e.g. in colorectal cancer, colonocytes to recruit T lymphocytes with a role in antitumor response in the tumor microenvironment (Citation36,Citation37). Currently, it is not known whether leptin has a similar role, if any, in other modes of cancer in humans.
Mechanisms of action
Current evidence suggests that the core circadian clock genes and their variants () are associated with non-Hodgkin lymphoma, prostate cancer, and breast cancer in humans (Citation38–45). However, the functional role of these gene variants and their clinical relevance are far from clear, as it is not known yet whether the identified gene variants change the transcription, thereby having an effect upstream or downstream in the pathways. Therefore, the gene variants or the expression profiles listed in cannot as yet be applied for disease screening or planning of treatment, respectively, in clinical practice.
A key to understanding the potential mechanisms of action lies in the function and the regulation of the ARNTL (BMAL1) gene (Citation46,Citation47), as it is essential to the circadian rhythm generation in the body. Its protein product ARNTL recruits either the CLOCK or NPAS2 protein to form a heterodimer of transcription factors with more than 100 target genes for each. On the one hand, the CLOCK:ARNTL complex regulates the cell division cycle directly through enhancing Wee1 and c-Myc transcription (Citation48,Citation49). On the other hand, the NPAS2:ARNTL complex suppresses c-Myc transcription (Citation48). The heightened expression levels of ARNTL, due to the reduced actions of PER1, PER2, and CRY2, have been demonstrated to associate with non-small-cell lung cancer (Citation50), epithelial ovarian cancer (Citation51), and colorectal cancer (Citation52), respectively, and their prognosis. Moreover, ARNTL is elementarily involved in the development of B lymphocytes (Citation53), and its disruption has been indicated in a range of hematologic malignancies as well (Citation54).
Reduced PER1 levels have been suggested to impair DNA damage repair and linked to both lung and breast cancer (Citation55,Citation56). PER1 has been shown to be part of the DNA damage repair pathway involving ATM and Chk2 by forming a complex with them that ensures apoptosis after DNA damage (Citation48,Citation49,Citation56). CRYs (CRY1 and CRY2) are part of the ATR-Chk1 DNA damage repair pathway and interact with TIMELESS protein directly (Citation49,Citation56). Recently, TIMELESS has been associated with the ATM-Chk2 DNA damage repair pathway, and mutations in the TIMELESS gene have been found in both breast and colorectal cancer (Citation57). Furthermore, reduced PER2 levels might be involved in the activation of the c-Myc signaling pathway, leading to cell proliferation (Citation38). On the other hand, the overexpression of PER1 (Citation55) and that of PER2 (Citation58) inhibits tumor growth, whereas mutations in the CLOCK gene favor tumor growth by having a downstream effect on CLOCK-binding elements near target DNA damage genes (Citation59).
In addition to the pathways referred to above, disruption of the circadian rhythms might increase the risk for cancer through angiogenesis (Citation60,Citation61). This is a fact because ARNTL controls cancer cell proliferation not only by generating the circadian rhythm, but also by timing DNA replication through thymidylate synthase activity, cell mitosis through WEE1 levels, and growth through vascular endothelial growth factor (VEGF) levels (Citation62). In line with the circadian-related pathogenesis are the findings that impaired sleep is associated with elevated serum VEGF concentrations (Citation63), and that the circadian clock protein BHLHE41 suppresses VEGF expression under hypoxic conditions (Citation64,Citation65).
Conclusions
Since the circadian rhythms and the cell division cycle share common regulatory elements, it is possible, or even likely, that disruption in functions of the circadian clock is a factor predisposing to cancer (Citation66–68). Night work, or any condition similar to it that disrupts circadian rhythms, challenges the circadian clock to the limit and may thereby cause a failure in the control of the cell division cycle, being permissive to malignant cell growth. In addition, circadian clock gene variants may modulate the physiological responses to such challenges, and de-novo mutations in circadian clock genes may contribute to tumor development. Thereby they both have a potential as biomarkers for those cancers known to be influenced by circadian factors or to have a circadian component in the pathogenesis. However, it is not only genetic variants or mutations that count, but environmental stimuli from wrongly scheduled time-givers also influence the development of certain cancers, and as they do so, preventive measures are available and can be taken forward.
Implications of the circadian systems biology in oncology are about to be introduced into clinical practice (Citation69). They include clinically feasible methods for the assessment of the individual's circadian time that are based on the analysis of levels of time-indicating molecules. Such molecules include metabolites derived from a single sample of venous blood and messenger RNAs captured directly from a tissue of interest through biopsy. In the former the assessment can be based on a range of methods, e.g. enzyme-linked immunosorbent assay or mass spectrometry using the approximate 20 time-indicating metabolites (Citation70), whereas in the latter it is based on DNA microarray using the approximate 60 time-indicating genes (Citation71).
Individuals of different circadian genotypes may have marked differences in their responses to toxic anticancer drug metabolites (Citation72). Therefore, methods for the determination of the best time, judging by both efficacy and toxicity, for administration of a drug in the treatment are well justified and welcome. Properties of the tumor such as the cell proliferation rate and the durations of the cell cycle phases may guide not only the drug of choice but also the timing of treatment schedules with the drug (Citation73). As exemplified with irinotecan in the treatment of colon cancer cells, the optimal scheme for any dose is 1) to start the administration between 2 h 10 min and 2 h 30 min circadian time, and 2) to administer it for 4–7 hours (Citation74). This scheme times the administration of irinotecan according to the levels of carboxylesterases and permits proteins involved in the elimination of irinotecan to protect healthy cells efficiently. However, despite these advances, there is still a lack of clinical verification and a gap of knowledge concerning the functional significance and the clinical relevance that needs to be explored, in order to have a better understanding of the link between circadian rhythm disruption and cancer disease.
To understand the pathogenesis of cancer in more detail, it is important to identify the details of those mechanisms that contribute to the loss of control of the cell division cycle in particular. All cancers where circadian rhythms are disrupted need to be identified, as some of the circadian-based options available for the treatment may prove to be clinically feasible. However, this step ahead needs to be based on experimental evidence and clinical trials. New potential preventive measures of these circadian-type cancers should then be targeted at large in order to avoid the long-term or recurrent circadian rhythm disruptions. Such actions can be achieved by making living and working circumstances more compatible with the circadian preference of an individual, which is driven by the timing of innate physiology.
Declaration of interest: The authors report no conflicts of interest.
References
- Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature. 2002;418:935–41.
- Le Minh N, Damiola F, Tronche F, Schutz G, Schibler U. Glucocorticoid hormones inhibit food-induced phase- shifting of peripheral circadian oscillators. EMBO J. 2001; 20:7128–36.
- Duffield GE, Best JD, Meurers BH, Bittner A, Loros JJ, Dunlap JC. Circadian programs of transcriptional activation, signaling, and protein turnover revealed by microarray analysis of mammalian cells. Curr Biol. 2002;12:551–7.
- Storch KF, Lipan O, Leykin I, Viswanathan N, Davis FC, Wong WH, . Extensive and divergent circadian gene expression in liver and heart. Nature. 2002;417:78–83.
- Schernhammer ES, Laden F, Speizer FE, Willett WC, Hunter DJ, Kawachi I, . Night-shift work and risk of colorectal cancer in the Nurses’ Health Study. J Natl Cancer Inst. 2003;95:825–8.
- Kubo T, Ozasa K, Mikami K, Wakai K, Fujino Y, Watanabe Y, . Prospective cohort study of the risk of prostate cancer among rotating-shift workers: findings from the Japan collaborative cohort study. Am J Epidemiol. 2006;164: 549–55.
- Conlon M, Lightfoot N, Kreiger N. Rotating shift work and risk of prostate cancer. Epidemiology. 2007;18:182–3.
- Viswanathan AN, Hankinson SE, Schernhammer ES. Night shift work and the risk of endometrial cancer. Cancer Res. 2007;67:10618–22.
- Lahti TA, Partonen T, Kyyrönen P, Kauppinen T, Pukkala E. Night-time work predisposes to non-Hodgkin lymphoma. Int J Cancer. 2008;123:2148–51.
- Stevens RG. Working against our endogenous circadian clock: breast cancer and electric lighting in the modern world. Mutat Res. 2009;680:106–8.
- Blask DE, Hill SM, Dauchy RT, Xiang S, Yuan L, Duplessis T, . Circadian regulation of molecular, dietary, and metabolic signaling mechanisms of human breast cancer growth by the nocturnal melatonin signal and the consequences of its disruption by light at night. J Pineal Res. 2011;51:259–69.
- Hansen J, Stevens RG. Case-control study of shift-work and breast cancer risk in Danish nurses: impact of shift systems. Eur J Cancer. 2012;48:1722–9.
- Stevens RG, Hansen J, Costa G, Haus E, Kauppinen T, Aronson KJ, . Considerations of circadian impact for defining ‘shift work’ in cancer studies: IARC Working Group Report. Occup Environ Med. 2011;68:154–62.
- Logan RW, Zhang C, Murugan S, O’Connell S, Levitt D, Rosenwasser AM, . Chronic shift-lag alters the circadian clock of NK cells and promotes lung cancer growth in rats. J Immunol. 2012;188:2583–91.
- Wu M, Zeng J, Chen Y, Zeng Z, Zhang J, Cai Y, . Experimental chronic jet lag promotes growth and lung metastasis of Lewis lung carcinoma in C57BL/6 mice. Oncol Rep. 2012;27:1417–28.
- Sutela H. Working time in the European Union: Finland. Helsinki: Statistics Finland; 2009.
- Vinogradova IA, Anisimov VN, Bukalev AV, Ilyukha VA, Khizhkin EA, Lotosh TA, . Circadian disruption induced by light-at-night accelerates aging and promotes tumorigenesis in young but not in old rats. Aging (Albany NY). 2010;2:82–92.
- Kloog I, Portnov BA, Rennert HS, Haim A. Does the modern urbanized sleeping habitat pose a breast cancer risk?Chronobiol Int. 2011;28:76–80.
- Cao Q, Gery S, Dashti A, Yin D, Zhou Y, Gu J, . A role for the clock gene per1 in prostate cancer. Cancer Res. 2009;69:7619–25.
- Gery S, Virk RK, Chumakov K, Yu A, Koeffler HP. The clock gene Per2 links the circadian system to the estrogen receptor. Oncogene. 2007;26:7916–20.
- Shav-Tal Y, Zipori D. PSF and p54nrb/NonO – multi- functional nuclear proteins. FEBS Lett. 2002;531:109–14.
- Amelio AL, Miraglia LJ, Conkright JJ, Mercer BA, Batalov S, Cavett V, . A coactivator trap identifies NONO (p54nrb) as a component of the cAMP-signaling pathway. Proc Natl Acad Sci USA. 2007;104:20314–9.
- O’Neill JS, Maywood ES, Chesham JE, Takahashi JS, Hastings MH. cAMP-dependent signaling as a core component of the mammalian circadian pacemaker. Science. 2008;320:949–53.
- Uchida Y, Hirayama J, Nishina H. A common origin: signaling similarities in the regulation of the circadian clock and DNA damage responses. Biol Pharm Bull. 2010;33: 535–44.
- Geyfman M, Kumar V, Liu Q, Ruiz R, Gordon W, Espitia F, . Brain and muscle Arnt-like protein-1 (BMAL1) controls circadian cell proliferation and susceptibility to UVB-induced DNA damage in the epidermis. Proc Natl Acad Sci USA. 2012;109:11758–63.
- Lie JA, Kjuus H, Zienolddiny S, Haugen A, Stevens RG, Kjærheim K. Night work and breast cancer risk among Norwegian nurses: assessment by different exposure metrics. Am J Epidemiol. 2011;173:1272–79.
- Lewy AJ, Wehr TA, Goodwin FK, Newsome DA, Markey SP. Light suppresses melatonin secretion in humans. Science. 1980;210:1267–9.
- Hill SM, Blask DE, Xiang S, Yuan L, Mao L, Dauchy RT, . Melatonin and associated signaling pathways that control normal breast epithelium and breast cancer. J Mammary Gland Biol Neoplasia. 2011;16:235–45.
- Johnston JD, Tournier BB, Andersson H, Masson-Pévet M, Lincoln GA, Hazlerigg DG. Multiple effects of melatonin on rhythmic clock gene expression in the mammalian pars tuberalis. Endocrinology. 2006;147:959–65.
- Wagner GC, Johnston JD, Tournier BB, Ebling FJ, Hazlerigg DG. Melatonin induces gene-specific effects on rhythmic mRNA expression in the pars tuberalis of the Siberian hamster (Phodopus sungorus). Eur J Neurosci. 2007;25: 485–90.
- Zhang EE, Liu Y, Dentin R, Pongsawakul PY, Liu AC, Hirota T, . Cryptochrome mediates circadian regulation of cAMP signaling and hepatic gluconeogenesis. Nat Med. 2010;16:1152–6.
- Nguyen J, Wright KPJr.Influence of weeks of circadian misalignment on leptin levels. Nat Sci Sleep. 2009;2010: 9–18.
- Zhu Y, Leaderer D, Guss C, Brown HN, Zhang Y, Boyle P, . Ala394Thr polymorphism in the clock gene NPAS2: a circadian modifier for the risk of non-Hodgkin's lymphoma. Int J Cancer. 2007;120:432–5.
- Hoffman AE, Zheng T, Stevens RG, Ba Y, Zhang Y, Leaderer D, . Clock-cancer connection in non-Hodgkin's lymphoma: a genetic association study and pathway analysis of the circadian gene cryptochrome 2. Cancer Res. 2009;69: 3605–13.
- Fu L, Pelicano H, Liu J, Huang P, Lee C. The circadian gene Period2 plays an important role in tumor suppression and DNA damage response in vivo. Cell. 2002;111:41–50.
- Abolhassani M, Aloulou N, Chaumette MT, Aparicio T, Martin-Garcia N, Mansour H, . Leptin receptor-related immune response in colorectal tumors: the role of colonocytes and interleukin-8. Cancer Res. 2008;68:9423–32.
- Aloulou N, Bastuji-Garin S, Le Gouvello S, Abolhassani M, Chaumette MT, Charachon A, . Involvement of the leptin receptor in the immune response in intestinal cancer. Cancer Res. 2008;68:9413–22.
- Zhu Y, Brown HN, Zhang Y, Stevens RG, Zheng T. Period3 structural variation: a circadian biomarker associated with breast cancer in young women. Cancer Epidemiol Biomarkers Prev. 2005;14:268–70.
- Chu LW, Zhu Y, Yu K, Zheng T, Yu H, Zhang Y, . Variants in circadian genes and prostate cancer risk: a population-based study in China. Prostate Cancer Prostatic Dis. 2008;11:342–8.
- Zhu Y, Stevens RG, Leaderer D, Hoffman A, Holford T, Zhang Y, . Non-synonymous polymorphisms in the circadian gene NPAS2 and breast cancer risk. Breast Cancer Res Treat. 2008;107:421–5.
- Zhu Y, Stevens RG, Hoffman AE, Fitzgerald LM, Kwon EM, Ostrander EA, . Testing the circadian gene hypothesis in prostate cancer: a population-based case-control study. Cancer Res. 2009;69:9315–22.
- Hoffman AE, Yi CH, Zheng T, Stevens RG, Leaderer D, Zhang Y, . CLOCK in breast tumorigenesis: genetic, epigenetic, and transcriptional profiling analyses. Cancer Res. 2010;70:1459–68.
- Hoffman AE, Zheng T, Yi CH, Stevens RG, Ba Y, Zhang Y, . The core circadian gene Cryptochrome 2 influences breast cancer risk, possibly by mediating hormone signaling. Cancer Prev Res (Phila). 2010;3:539–48.
- Yi C, Mu L, de la Longrais IA, Sochirca O, Arisio R, Yu H, . The circadian gene NPAS2 is a novel prognostic biomarker for breast cancer. Breast Cancer Res Treat. 2010;120:663–9.
- Dai H, Zhang L, Cao M, Song F, Zheng H, Zhu X, . The role of polymorphisms in circadian pathway genes in breast tumorigenesis. Breast Cancer Res Treat. 2011;127: 531–40.
- Bunger MK, Wilsbacher LD, Moran SM, Clendenin C, Radcliffe LA, Hogenesch JB, . Mop3 is an essential component of the master circadian pacemaker in mammals. Cell. 2000;103:1009–17.
- Zeng ZL, Wu MW, Sun J, Sun YL, Cai YC, Huang YJ, . Effects of the biological clock gene Bmal1 on tumour growth and anti-cancer drug activity. J Biochem. 2010;148: 319–26.
- Sahar S, Sassone-Corsi P. Metabolism and cancer: the circadian clock connection. Nat Rev Cancer. 2009;9:886–96.
- Reddy AB, O’Neill JS. Healthy clocks, healthy body, healthy mind. Trends Cell Biol. 2010;20:36–44.
- Gery S, Komatsu N, Kawamata N, Miller CW, Desmond J, Virk RK, . Epigenetic silencing of the candidate tumor suppressor gene Per1 in non-small cell lung cancer. Clin Cancer Res. 2007;13:1399–404.
- Tokunaga H, Takebayashi Y, Utsunomiya H, Akahira J, Higashimoto M, Mashiko M, . Clinicopathological significance of circadian rhythm-related gene expression levels in patients with epithelial ovarian cancer. Acta Obstet Gynecol Scand. 2008;87:1060–70.
- Oshima T, Takenoshita S, Akaike M, Kunisaki C, Fujii S, Nozaki A, . Expression of circadian genes correlates with liver metastasis and outcomes in colorectal cancer. Oncol Rep. 2011;25:1439–46.
- Sun Y, Yang Z, Niu Z, Peng J, Li Q, Xiong W, . MOP3, a component of the molecular clock, regulates the development of B cells. Immunology. 2006;119:451–60.
- Taniguchi H, Fernández AF, Setién F, Ropero S, Ballestar E, Villanueva A, . Epigenetic inactivation of the circadian clock gene BMAL1 in hematologic malignancies. Cancer Res. 2009;69:8447–54.
- Gery S, Komatsu N, Baldjyan L, Yu A, Koo D, Koeffler HP. The circadian gene per1 plays an important role in cell growth and DNA damage control in human cancer cells. Mol Cell. 2006;22:375–82.
- Sancar A, Lindsey-Boltz LA, Kang TH, Reardon JT, Lee JH, Ozturk N. Circadian clock control of the cellular response to DNA damage. FEBS Lett. 2010;584:2618–25.
- Yang MY, Yang WC, Lin PM, Hsu JF, Hsiao HH, Liu YC, . Altered expression of circadian clock genes in human chronic myeloid leukemia. J Biol Rhythms. 2011;26: 136–48.
- Miyazaki K, Wakabayashi M, Hara Y, Ishida N. Tumor growth suppression in vivo by overexpression of the circadian component, PER2. Genes Cells. 2010;15:351–8.
- Alhopuro P, Björklund M, Sammalkorpi H, Turunen M, Tuupanen S, Biström M, . Mutations in the circadian gene CLOCK in colorectal cancer. Mol Cancer Res. 2010;8:952–60.
- Saharinen P, Eklund L, Pulkki K, Bono P, Alitalo K. VEGF and angiopoietin signaling in tumor angiogenesis and metastasis. Trends Mol Med. 2011;17:347–62.
- Weis SM, Cheresh DA. Tumor angiogenesis: molecular pathways and therapeutic targets. Nat Med. 2011;17: 1359–70.
- Wood PA, Du-Quiton J, You S, Hrushesky WJ. Circadian clock coordinates cancer cell cycle progression, thymidylate synthase, and 5-fluorouracil therapeutic index. Mol Cancer Ther. 2006;5:2023–33.
- Guess J, Burch JB, Ogoussan K, Armstead CA, Zhang H, Wagner S, . Circadian disruption, Per3, and human cytokine secretion. Integr Cancer Ther. 2009;8:329–36.
- Sato F, Bhawal UK, Kawamoto T, Fujimoto K, Imaizumi T, Imanaka T, . Basic-helix-loop-helix (bHLH) transcription factor DEC2 negatively regulates vascular endothelial growth factor expression. Genes Cells. 2008;13:131–44.
- Hamaguchi H, Fujimoto K, Kawamoto T, Noshiro M, Maemura K, Takeda N, . Expression of the gene for Dec2, a basic helix-loop-helix transcription factor, is regulated by a molecular clock system. Biochem J. 2004;382:43–50.
- Harris NL, Jaffe ES, Diebold J, Flandrin G, Muller- Hermelink HK, Vardiman J, . The World Health Organization classification of neoplastic diseases of the hematopoietic and lymphoid tissues. Report of the Clinical Advisory Committee meeting, Airlie House, Virginia, November, 1997. Ann Oncol. 1999;10:1419–32.
- Khapre RV, Samsa WE, Kondratov RV. Circadian regulation of cell cycle: molecular connections between aging and the circadian clock. Ann Med. 2010;42:404–15.
- Yu EA, Weaver DR. Disrupting the circadian clock: gene-specific effects on aging, cancer, and other phenotypes. Aging (Albany NY). 2011;3:479–93.
- Innominato PF, Lévi FA, Bjarnason GA. Chronotherapy and the molecular clock: clinical implications in oncology. Adv Drug Deliv Rev. 2010;62:979–1001.
- Minami Y, Kasukawa T, Kakazu Y, Iigo M, Sugimoto M, Ikeda S, . Measurement of internal body time by blood metabolomics. Proc Natl Acad Sci U S A. 2009;106:9890–5.
- Ueda HR, Chen W, Minami Y, Honma S, Honma K, Iino M, . Molecular-timetable methods for detection of body time and rhythm disorders from single-time-point genome-wide expression profiles. Proc Natl Acad Sci U S A. 2004;101:11227–32.
- Gorbacheva VY, Kondratov RV, Zhang R, Cherukuri S, Gudkov AV, Takahashi JS, . Circadian sensitivity to the chemotherapeutic agent cyclophosphamide depends on the functional status of the CLOCK/BMAL1 transactivation complex. Proc Natl Acad Sci U S A. 2005;102:3407–12.
- Bernard S, Čajavec Bernard B, Lévi F, Herzel H. Tumor growth rate determines the timing of optimal chronomodulated treatment schedules. PLoS Comput Biol. 2010;6: e1000712.
- Ballesta A, Dulong S, Abbara C, Cohen B, Okyar A, Clairambault J, . A combined experimental and mathematical approach for molecular-based optimization of irinotecan circadian delivery. PLoS Comput Biol. 2011;7:e1002143.
