Abstract
Background. Teriparatide is a potent anabolic agent for severe osteoporosis.
Objectives. A primary objective of this retrospective study was to define the efficacy of teriparatide in terms of bone mineral density (BMD) changes and relief of back pain in clinical practice.
Methods. The patient population comprises 119 osteoporotic patients treated with teriparatide for median 539 (range 179–926) days.
Results. The mean BMD gain was 0.9% in the total hip (P = 0.0075), 2.1% in the femoral neck (P = 0.0006), and 8.5% in the lumbar spine (P = 0.0085). In the whole patient population age associated inversely with BMD changes in the total hip (P = 0.019) and in the femoral neck (P = 0.0036). A history of significant bisphosphonate pretreatment (n = 90) reduced BMD response in the total hip (P = 0.039). The total exposure of any prior bisphosphonate was negatively correlated with BMD response in the total hip (P = 0.0421). Half of the patients reported relief of back pain during the treatment. Leg pain, nausea, and dizziness were most frequent adverse concerns.
Conclusions. Teriparatide works in clinical practice as well as in clinical trials. Younger subjects benefited more than older patients from teriparatide in the total hip and in the femoral neck. Bisphosphonate pretreatment attenuated teriparatide-induced BMD gain.
Key messages
Teriparatide-induced femoral BMD gain was more pronounced in younger subjects.
Teriparatide works in clinical practice as well as in clinical trials, but prior bisphosphonate treatment diminishes the teriparatide effect on BMD.
Introduction
Teriparatide is a recombinant human parathyroid hormone (PTH)(1–34), which exhibits potent anabolic outcome on the bones when given by intermittent subcutaneous injections (Citation1). It was approved in 2002 by the US Food and Drug Administration (FDA) as the first anabolic agent for use in men and in postmenopausal women with osteoporosis. The treatment is recommended to be used at the dose of 20 μg subcutaneously daily for up to 2 years, but at the time of the present study the accepted duration of the treatment was 18 months in Europe.
Teriparatide improves skeletal architecture. Bone is gained, which is recognized for example as increased trabecular thickness and connectivity (Citation2,Citation3). Bone mineral density (BMD) increases in cancellous bone; in postmenopausal women the mean rise in the lumbar spine BMD has been 10%–14% and up to 5% in the femoral neck over 1–3 years (Citation4). Similar BMD changes have been seen also in men (Citation5) and in patients with glucocorticoid-induced osteoporosis (GIOP) (Citation6,Citation7). In 19 months’ study of postmenopausal women the risk of a new vertebral fracture and non-vertebral fragility fractures were reduced by 65% and 35%, respectively (Citation4). The prevention of vertebral fracture with teriparatide has also been shown in men and in GIOP in both sexes (Citation8,Citation9). Some controversy exists regarding the age dependency of the teriparatide-induced BMD response (Citation5,Citation10,Citation11). In a recent meta-analysis the BMD gain was significantly reduced by increasing age, in the spine but not in the hip (Citation12). Side-effects have been mild and generally do not require discontinuation of the therapy (Citation4). Dizziness and leg cramps have been the symptoms reported significantly more in the teriparatide group than in the placebo group and usually occur within a few hours of injection. About 3% of patients experience a significant increase in serum uric acid content. Transient hypercalcemia has been detected in about 11% of patients on teriparatide therapy. Only 3% of patients need a dose reduction (Citation4).
Antiresorptive bisphosphonates form the established first-line medication for osteoporosis. Teriparatide is considered as a second-line treatment after bisphosphonate therapy. However, this practice is shadowed by the existing controversy whether or not preceding antiresorptive treatment reduces the clinical efficacy of teriparatide. Previous alendronate treatment has impaired the anabolic effect of PTH(1–34) in some (Citation13–15) but not in all studies (Citation16,Citation17).
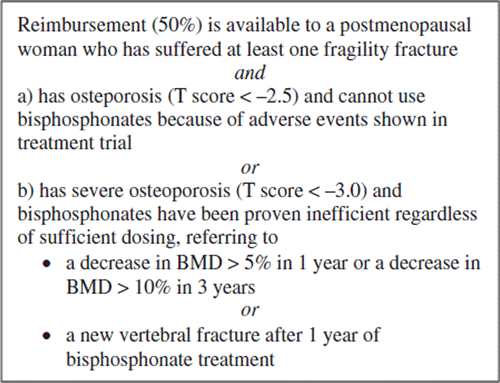
In this retrospective, observational study we examined the effectiveness and safety of teriparatide in clinical practice. A primary objective was to define the efficacy of teriparatide in terms of BMD changes and relief of back pain in Finnish patients with osteoporosis outside clinical trials. A secondary objective was to explore the side-effects of teriparatide. According to the reimbursement policy of the Social Insurance Institution of Finland, bisphosphonates are considered as a primary and teriparatide as a second-line therapy of osteoporosis ().
Patients and methods
Patients
The study population consists of 119 individuals (113 women and 6 men), who received teriparatide at a dosage of 20 μg per day on average for median (range) 539 (179–926) days. Mean age is 67.7 ± 12.9 years. In the cohort teriparatide was initiated from the year 2003 through 2007. During this time period 394 Finnish patients were qualified to receive the reimbursement of the costs of teriparatide therapy by the Social Insurance Institution of Finland (). The Social Insurance Institution did not accept our application to utilize register data on the patients qualified for the reimbursement. Thus the information requests were sent to specialists, mainly endocrinologists, treating patients with osteoporosis at university and central hospitals; replies on 51 patients were received (the so called non-Orton population). In addition, data were collected on 68 patients treated with teriparatide at a private clinic (Orton) in Helsinki (the Orton population). Patient selection was based on the reimbursement criteria (see ) in the non-Orton population. In the Orton population BMD at the level of osteoporosis (T score ≤ –2.5) or of osteopenia (–1 ≥ T score > –2.5) combined with a vertebral fracture was a prerequisite for teriparatide treatment. Most Orton patients had had bisphosphonate pretreatment, but it was not considered absolutely necessary as was the case for the non-Orton population, and a few patients used teriparatide treatment without the reimbursement by the state.
Clinically significant oral bisphosphonate therapy before teriparatide treatment was determined as follows: 2 to 24 weeks in the last 6 months, 8 to 48 weeks in the last 12 months, or more than 48 weeks in the last 24 months. For intravenous bisphosphonates an infusion during the past year was considered clinically relevant. For estrogen, raloxifene, strontium ranelate, and calcitonin, treatment for 1 month during the last 6 months preceding teriparatide treatment was labelled of clinical importance.
Primary efficacy measures consist of changes in BMD (lumbar spine, femoral neck, and total hip) and back pain before, during, and after the therapy.
All patients had BMD measurement by dual-energy X-ray absorptiometry (DXA) at baseline and a control examination after at least 12 months’ teriparatide treatment. The exact duration of teriparatide treatment between DXAs was median (range) 530 (179–784) days. Both Lunar Prodigy (GE-Lunar Inc. Madison, WI, USA) and Hologic (Hologic Inc. Bedford, MA, USA) densitometers were employed; control and baseline measurements were performed with the same machine. Serum N-terminal propeptide of type 1 procollagen (P1NP) was analysed with radioimmunoassay, and urinary N-terminal telopeptide of type I collagen (urinary NTx) was measured in spot samples with enzyme immunoassay in a commercial clinical laboratory using routine quality control procedures. Also serum alkaline phosphatase (AFOS) was occasionally measured. Symptoms of back pain were collected when documented in patient reports.
Safety measures include all adverse events documented by doctors taking care of teriparatide-treated patients. Special attention was paid to the occurrence of hypercalcemia, nephrolithiasis, gout, and osteosarcoma. The announcements of adverse reactions to the register of National Agency for Medicines were collected.
The study was approved by the ethical review board of Central Finland Health Care District, Jyväskylä, Finland. The permission of the Ministry of Social Affairs and Health was received for the study, and an announcement was sent to the office of the data protection ombudsman.
Statistical analysis
Baseline characteristics and BMD data were compared between the Orton and non-Orton patients with one-way analysis of variance or Wilcoxon rank sum test based on the distribution of the variable. The two study populations differed significantly by age. Consequently, inclusion of both age and study population (Orton versus non-Orton) as covariates into further analyses would have biased them, and thus we decided to exclude the population from further analyses.
BMD changes over time (teriparatide effect) were examined by repeated measures analysis of variance, which included age, weight, corticosteroid intake, number of earlier fractures as covariates. Age × time interaction was included in the model to evaluate whether treatment effect differs by age. Furthermore, clinically significant (see above) previous use of any bisphosphonate was included in the model as well as bisphosphonate use × time interaction to study if treatment effect differed by bisphosphonate pretreatment. We also examined whether treatment effect has any interaction with corticosteroid intake, weight, and number of previous fractures. None of these interactions was statistically significant, and these effects were not included in the final model. The effect of alendronate pretreatment was evaluated by multiway analysis of variance, in a model in which alendronate use, age, weight, corticosteroid intake, and number of fractures were factors. Also Pearson correlation coefficients were calculated. All tests were performed as two-sided with a 0.05 significance level. The analyses were done with SASregister System (Version 9.2 for Windows).
Results
Baseline characteristics for the whole cohort and the Orton and non-Orton subpopulations separately are presented in . The Orton patients were older (P < 0.0001), and they started teriparatide sooner after stopping bisphosphonates than the non-Orton patients (P < 0.0001). On average all the patients used 800 IU vitamin D and 1 g calcium daily during teriparatide treatment. Ninety per cent of patients (n = 107) were reported to have 1–13 (median 4) fractures preceding teriparatide treatment. Eighty-two patients had suffered a vertebral fracture, and of them 64 patients had at least two fractured vertebrae. Thirteen patients had experienced hip fractures, two of them twice. Forty-seven patients had had one to four antebrachium fractures before teriparatide therapy. The Orton population had fewer fractures at baseline than the non-Orton subpopulation; for all fractures the difference was significant (P = 0.0021), but not for vertebral fractures (P = 0.2068).
Table I. Baseline characteristics (mean ± SD, or median (range)) for the whole study population and Orton and non-Orton-subpopulations separately.
Only 15 patients were bisphosphonate-naive, and 90 had received clinically relevant bisphosphonate therapy with at least one preparation prior to teriparatide; 60 patients alendronate, 27 risedronate, 9 zoledronic acid, 1 pamidronate. Nine patients had clinically significant raloxifene pretreatment, and 25 had used calcitonin. Only one patient had received strontium ranelate as pretreatment. The duration of pretreatment bisphosphonate exposure was median (range) 848 (2–5258) days. The lag time (time from stopping bisphosphonate to starting teriparatide) of any bisphosphonate was 0 (0–381) days. It was less than 1 month for 83.3%, from 1 to 6 months for 12.2%, and more than 6 months for 4.4% of the patients.
Follow-up time after teriparatide varied from few months to 2 years. No immediate post teriparatide antiresorptive treatment was initiated for 12% of the participants. Bisphosphonates were prescribed for 83 (alendronate 18, risedronate 28, ibandronate 5, zoledronic acid 32), strontium ranelate for 11, raloxifene for 8, and calcitonin for 2 patients after teriparatide.
The efficacy of teriparatide treatment
Bone mineral density
In the whole population the mean increases in BMD were 0.9% in the total hip (P = 0.0075) (n = 99), 2.1% in the femoral neck (P = 0.0006) (n = 107), and 8.5% in the lumbar spine (P = 0.0085) (n = 105) (). The percentage changes in the Orton and non-Orton subpopulations were 1.9% and –0.3% for the total hip, 3.0% and 0.9% in the femoral neck, and 8.6% and 8.3% for the spine, respectively. The baseline adjusted BMD changes were significantly different between the subpopulations except in the vertebral site ().
Table II. Baseline BMD and its changes during teriparatide in the whole study population and in the Orton- and non-Orton subpopulations separately.
In the whole study population there was no correlation between total hip or neck BMD and age, neither before nor after PTH treatment (). Instead, lumbar spine BMD was directly correlated to age; elderly subjects had higher bone density in the spine already at baseline.
Table III. Results of statistical analysis to explain teriparatide-induced BMD changes. The depicted analyses of age, weight, number of fractures, on-going glucocorticoid treatment, and any significant bisphosphonate treatment are associations to BMD levels. The impact of age and any significant bisphosphonate treatment are also shown as determinants of BMD changes during the teriparatide treatment.
In a statistical model with multiple relevant factors as covariates (age, weight, glucocorticoid intake, number of earlier fractures), age and weight were significantly associated with BMD levels. Regarding BMD changes over teriparatide treatment in the whole cohort, age associated inversely with BMD changes in the total hip and the femoral neck, but not in the lumbar spine (Repeated measures analysis of variance ANOVA P = 0.019, P = 0.0036, and P = 0.48; ). Thus, younger subjects benefited more from teriparatide in the total hip and the femoral neck than older patients. In the lumbar region a similar effect was seen for only the non-Orton population; the younger the patient the greater the BMD increase. In the whole cohort weight was expectedly positively correlated with BMD at the three sites (P <0.0001 in the total hip and the femoral neck, P = 0.0003 in the lumbar spine) but did not show any association with BMD change.
Neither on-going glucocorticoid treatment (14 patients) nor the number of earlier fractures had any significant association with BMD levels or teriparatide-induced BMD change at any site. Each vertebral fracture was calculated separately in the total number of fractures.
Per cent BMD gain correlated negatively to baseline BMD at all the measurement sites (P = 0.0011 for the lumbar spine, P = 0.084 for the femoral neck, P = 0.0041 for the total hip). Thus, patients with lower baseline BMD had a higher percentage response to teriparatide. Absolute BMD changes were not statistically significantly associated to basal BMD.
Fractures
The number of fractures during teriparatide treatment was very low. Six patients of the whole study population had 11 osteoporotic fractures (2 hip, 7 vertebral, and 2 ankle fractures) while on teriparatide treatment.
Markers of bone turnover
Bone turnover markers were measured in a few patients and in the non-Orton subpopulation only. Paired samples at the beginning of the treatment and during the follow-up of the treatment were available for 34 patients for AFOS, 12 patients for P1NP, and 13 patients for urinary NTx. Median values (with range) for paired samples before and during the teriparatide treatment were as follows: 76 (29–242) U/L and 74 (35–188) U/L for AFOS, 24.65 (13.2–53.7) μg/L and 89.15 (40.10–305.20) μg/L for serum P1NP, 26 (12–117) nmol/mmol creatinine and 69 (40–354) nmol/mmol creatinine for urinary NTx.
Back pain
Back pain was commonly reported at the start of treatment. Information of back pain was accessed for 106 patients at baseline; 85 of those reported back-ache. Fifty-seven patients described pain relief while on teriparatide. At the end of the treatment only 15% of patients (n = 91) reported back pain.
The impact of the former osteoporosis therapy for the effectiveness of teriparatide
Any clinically significant bisphosphonate pretreatment reduced BMD response in the total hip (P = 0.039); a similar trend was seen for lumbar spine BMD response (P = 0.091) (, ). The length of any prior bisphosphonate treatment was negatively correlated with BMD response in the total hip (P = 0.0421), but not in the other BMD sites.
Table IV. The effect of any significant bisphosphonate pretreatment on baseline BMD levels and their changes during teriparatide treatment (mean (SE)).
Alendronate pretreatment was also a negative determinant of basal BMD at the femoral sites (P = 0.0283 for the total hip, P = 0.0694 for the femoral neck) when adjusted for age, body weight, corticosteroid treatment, and the number of fractures.
The safety of teriparatide treatment
Transient hypercalciuria was detected in 8% and temporary hypercalcemia in 18% of the patients in the non-Orton population. In the Orton population these parameters were not regularly measured. Hypercalcemia was not considered clinically important in any case and did not lead to dose adjustments or interruptions of teriparatide treatment; calcium and vitamin D substitution was reduced in three patients. In the Orton subgroup only one case with an adverse event (costal pain) was reported. In the non-Orton population adverse events were reported for 15 patients. Injection site irritation, transient urticaria, arthralgia, hypotension, and atrial fibrillation were all reported once. Leg pain was reported by six subjects. In addition to leg pain one patient had truncal myalgia, which led to discontinuation of treatment after 1 year. This case was the only one reported to the National Agency for Medicines. The patient recovered fully after stopping teriparatide. Three patients had nausea and dizziness; one of these patients was withdrawn from teriparatide after 17 months. In one case hypertension and nausea led to an early interruption of teriparatide therapy.
One case of pulmonary adenocarcinoma was diagnosed while the patient was on the teriparatide treatment. Eight months after fulfilling teriparatide therapy one patient presented with multiple myeloma (Citation18). No case of osteosarcoma was reported.
Discussion
In this retrospective study of 119 patients with osteoporosis treated in everyday clinical practice, teriparatide treatment increased BMD 8.5% in the lumbar spine, 2.1% in the femoral neck, and 0.9% in the total hip. These figures are virtually identical to teriparatide-induced BMD changes initially published by Neer et al. (Citation4). The response was more pronounced in younger versus older patients, especially in the femoral neck and the total hip. Any clinically relevant bisphosphonate treatment attenuated teriparatide-induced BMD gain in the total hip. The BMD response at the femoral sites (but not in the lumbar spine) was lower, the longer the pretreatment exposure to bisphosphonates.
The most important limitation of the present study is the heterogeneous structure of the patient cohort with the two subgroups. In the first place this originates from the very strict reimbursement policy in Finland that only patients with severe osteoporosis can use teriparatide with low costs. On the other hand, we have patients who are ready to pay more for the drug and start using it with less strict indications. In addition, the Social Insurance Institution did not allow us access to register data on the patients who qualified for the reimbursement. Consequently, the present cohort represents at most one-third of the patients who used teriparatide in Finland during the study years.
In the whole study population age was inversely correlated to the BMD gain in the hip and also to that in the lumbar spine in the non-Orton subpopulation.
In contrast to our finding, Marcus and co-workers (Citation10) found that teriparatide increased vertebral BMD more in an older age group (women over 65 years) than in younger postmenopausal women (younger than 65 years), but this was not translated to a reduction in vertebral fracture incidence. The mean age in that study was 70 years, and all participants were at least 5 years beyond menopause (mean 21 years). In the Fracture Prevention Trial women aged 75 and older had similar teriparatide-induced BMD effect in the femoral neck to that of younger women. In the spine, despite similar percentage changes in the BMD, the treatment effect was diminished in the older patient group because of greater BMD increase in the placebo group (Citation11). In men the effect of teriparatide has been reported to be independent of age (Citation5). Interestingly, in a recent meta-analysis increasing age attenuated the PTH-induced BMD gain in the spine but had no effect in the hip (Citation12). Both teriparatide and PTH(1-84)-treated patients were included in that analysis. In our study, increasing age attenuated the teriparatide response also in the femoral sites. The mean age in our study was 68 years, but the age range was wide (23–92 years), which may at least partly explain the inverse relationship between age and the skeletal response to teriparatide. We did not see age dependency of teriparatide response in the spine in the Orton population, in which most patients irrespectively of age received a good BMD response in the lumbar spine. Of 15 bisphosphonate-naive patients in our cohort, 14 were in the Orton group, which may have contributed to different lumbar spine BMD gains between the groups. In terms of overall disease history (not shown) the subjects at the private clinic Orton were older but healthier than patients in the non-Orton group, which may also have contributed to different teriparatide responses in the spine. It is possible that the skeletal effect of teriparatide is affected not only by age but also by overall health status of the patient. Finally, excluded is not the possibility that the higher age of the Orton patients resulted in more frequent degenerative changes in the spine artificially increasing BMD and consequently masking the inverse relationship between age and BMD change.
In the present study previous fracture history did not have a significant impact on the response to teriparatide treatment. Instead, patients with lower versus higher baseline BMD benefited more from teriparatide treatment in terms of per cent BMD gain. However, absolute BMD changes were not significantly associated to the basal BMD. Marcus and co-workers have reported a consistent finding of an inverse correlation between baseline vertebral BMD and percentage but not absolute gain in BMD (Citation10).
Bisphosphonates inhibit the teriparatide-induced increment in bone turnover and consequently the augmentation of BMD (Citation19). Theoretically the suppression of the function of osteoclasts might diminish the anabolic effect of PTH due to impairment of the remodelling cycle. One-year alendronate pretreatment postponed the effect of teriparatide on biochemical markers and BMD by 6 months in postmenopausal women (Citation15). Even 6 months’ pretreatment with alendronate blunted the teriparatide-induced increase in bone turnover markers in men (Citation20). However, in a retrospective study of 52 patients from UK, no reduction in the teriparatide-induced BMD gain was observed in patients with versus without prior exposure to bisphosphonates, even though P1NP increases were slightly suppressed (Citation16). In an open-label, prospective study of 39 patients no influence of prior alendronate or risedronate was seen in teriparatide-induced gains in BMD or in rises of bone turnover markers over 12 months (Citation17). Some investigations indicate that bisphosphonates have dissimilar influences on the anabolic effect of teriparatide (Citation21), but the observations have not been constant (Citation22). In the Eurofors study the duration of bisphosphonate pretreatment did not affect the BMD response (Citation22). In the present study the duration of pretreatment exposure to bisphosphonates contributed to the BMD change at the femoral sites, but not significantly at the lumbar spine.
Teriparatide has been shown to reduce back pain (Citation23). In this clinical patient material 67% of patients with back pain at the start of teriparatide treatment reported pain relief during therapy; in the absence of the control group the significance of this finding remains, however, open.
Persisting hypercalciuria has been reported in 3% of teriparatide-treated patients, and less than 1% demanded dose adjustments regarding either calcium or teriparatide (Citation24). In this patient series the dosage of calcium or vitamin D was reduced in 2.5% of patients. Three patients interrupted teriparatide treatment, one for myalgia and leg pain and two for nausea.
The non-homogeneous patient material in this clinical study comprises a limitation, which is emphasized by two different subgroups. Without access to the registry information we had to collect patient data directly from clinicians. Consequently, our cohort comprises at maximum 30% of the Finnish patients treated with teriparatide from 2003 through 2007. Because of the strict Finnish reimbursement criteria, most patients had severe osteoporosis and thus a really justified need for anabolic treatment of osteoporosis. Our findings on the response-lessening effect of high age and prior exposure to bisphosphonates indicate that the potent anabolic therapy with teriparatide should be considered for patients with significant osteoporosis at an earlier phase of the disease.
Conclusions
In the present clinical patient material teriparatide treatment increased bone mineral density to the same extent as in previous randomized studies. The BMD response in the total hip and in the femoral neck was higher in younger than older patients. Bisphosphonate pretreatment reduced teriparatide-induced BMD increases. No unexpected safety trouble was perceived.
Acknowledgements
We thank doctors Heikki Valleala, Juhani Koski, and Anja Orvola for additional help in collecting patients for the study.
Declaration of interest: The study costs were covered by a grant from Eli Lilly.
References
- Reeve J, Meunier PJ, Parsons JA, Bernat M, Bijvoet OL, Courpron P, et al. Anabolic effect of human parathyroid hormone fragment on trabecular bone in involutional osteoporosis: a multicentre trial. Br Med J. 1980;280:1340–4.
- Bradbeer JN, Arlot ME, Meunier PJ, Reeve J. Treatment of osteoporosis with parathyroid peptide (hPTH 1-34) and oestrogen: increase in volumetric density of iliac cancellous bone may depend on reduced trabecular spacing as well as increased thickness of packets of newly formed bone. Clin Endocrinol (Oxf). 1992;37:282–9.
- Jiang Y, Zhao JJ, Mitlak BH, Wang O, Genant HK, Eriksen EF. Recombinant human parathyroid hormone (1-34) [teriparatide] improves both cortical and cancellous bone structure. J Bone Miner Res. 2003;18:1932–41.
- Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Reginster JY, et al. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 2001;344:1434–41.
- Orwoll ES, Scheele WH, Paul S, Adami S, Syversen U, Diez-Perez A, et al. The effect of teriparatide [human parathyroid hormone (1-34)] therapy on bone density in men with osteoporosis. J Bone Miner Res. 2003;18:9–17.
- Lane NE, Sanchez S, Modin GW, Genant HK, Pierini E, Arnaud CD. Parathyroid hormone treatment can reverse corticosteroid-induced osteoporosis. Results of a randomized controlled clinical trial. J Clin Invest. 1998;102:1627–33.
- Saag KG, Shane E, Boonen S, Marin F, Donley DW, Taylor KA, et al. Teriparatide or alendronate in glucocorticoid-induced osteoporosis. N Engl J Med. 2007;357:2028–39.
- Kaufman JM, Orwoll E, Goemaere S, San Martin J, Hossain A, Dalsky GP, et al. Teriparatide effects on vertebral fractures and bone mineral density in men with osteoporosis: treatment and discontinuation of therapy. Osteoporos Int. 2005;16:510–6.
- Saag KG, Zanchetta JR, Devogelaer JP, Adler RA, Eastell R, See K, et al. Effects of teriparatide versus alendronate for treating glucocorticoid-induced osteoporosis: thirty-six-month results of a randomized, double-blind, controlled trial. Arthritis Rheum. 2009;60:3346–55.
- Marcus R, Wang O, Satterwhite J, Mitlak B. The skeletal response to teriparatide is largely independent of age, initial bone mineral density, and prevalent vertebral fractures in postmenopausal women with osteoporosis. J Bone Miner Res. 2003;18:18–23.
- Boonen S, Marin F, Mellstrom D, Xie L, Desaiah D, Krege JH, et al. Safety and efficacy of teriparatide in elderly women with established osteoporosis: bone anabolic therapy from a geriatric perspective. J Am Geriatr Soc. 2006;54:782–9.
- Schwarz P, Jorgensen NR, Mosekilde L, Vestergaard P. Effects of increasing age, dosage, and duration of PTH treatment on BMD increase—a meta-analysis. Calcif Tissue Int. 2012;90:165–73.
- Finkelstein JS, Hayes A, Hunzelman JL, Wyland JJ, Lee H, Neer RM. The effects of parathyroid hormone, alendronate, or both in men with osteoporosis. N Engl J Med. 2003;349:1216–26.
- Finkelstein JS, Wyland JJ, Lee H, Neer RM. Effects of teriparatide, alendronate, or both in women with postmenopausal osteoporosis. J Clin Endocrinol Metab. 2010;95:1838–45.
- Ettinger B, San Martin J, Crans G, Pavo I. Differential effects of teriparatide on BMD after treatment with raloxifene or alendronate. J Bone Miner Res. 2004;19:745–51.
- Middleton ET, Steel SA, Doherty SM. The effect of prior bisphosphonate exposure on the treatment response to teriparatide in clinical practice. Calcif Tissue Int. 2007;81:335–40.
- Keel C, Kraenzlin ME, Kraenzlin CA, Muller B, Meier C. Impact of bisphosphonate wash-out prior to teriparatide therapy in clinical practice. J Bone Miner Metab. 2010;28:68–76.
- Koski AM, Sikiö A, Forslund T. Teriparatide treatment complicated by malignant myeloma. BMJ Case Rep. 2010; doi:10.1136/bcr.01. 2010.2681.
- Obermayer-Pietsch BM, Marin F, McCloskey EV, Hadji P, Farrerons J, Boonen S, et al. Effects of two years of daily teriparatide treatment on BMD in postmenopausal women with severe osteoporosis with and without prior antiresorptive treatment. J Bone Miner Res. 2008;23: 1591–600.
- Finkelstein JS, Leder BZ, Burnett SM, Wyland JJ, Lee H, de la Paz AV, et al. Effects of teriparatide, alendronate, or both on bone turnover in osteoporotic men. J Clin Endocrinol Metab. 2006;91:2882–7.
- Miller PD, Delmas PD, Lindsay R, Watts NB, Luckey M, Adachi J, et al. Early responsiveness of women with osteoporosis to teriparatide after therapy with alendronate or risedronate. J Clin Endocrinol Metab. 2008;93:3785–93.
- Boonen S, Marin F, Obermayer-Pietsch B, Simoes ME, Barker C, Glass EV, et al. Effects of previous antiresorptive therapy on the bone mineral density response to two years of teriparatide treatment in postmenopausal women with osteoporosis. J Clin Endocrinol Metab. 2008;93:852–60.
- Miller PD, Shergy WJ, Body JJ, Chen P, Rohe ME, Krege JH. Longterm reduction of back pain risk in women with osteoporosis treated with teriparatide compared with alendronate. J Rheumatol. 2005;32: 1556–62.
- Miller PD, Bilezikian JP, Diaz-Curiel M, Chen P, Marin F, Krege JH, et al. Occurrence of hypercalciuria in patients with osteoporosis treated with teriparatide. J Clin Endocrinol Metab. 2007;92:3535–41.