Abstract
Background. Early diagnosis of acute coronary syndrome (ACS) is frequently a challenging task.
Aims. To assess the role of novel biomarkers to identify ACS.
Methods. Concentrations of lipids, lipoproteins, oxidized LDL (oxLDL), high-sensitivity C-reactive protein (hsCRP), paraoxonase-1 (PON1), secretory phospholipase A2 (sPLA2), and myeloperoxidase (MPO) were measured in 703 patients (mean age 65.5 ± 11.2 years; 422 men, 281 women) without diabetes mellitus assigned to coronary angiogram. The subjects were divided into three groups: ACS (n = 242), stable angina pectoris (SAP) (n = 242), and normal coronary artery (NCA) (n = 219).
Results. HDL-cholesterol (HDL-C) (P < 0.001) and apolipoproteinA-I concentrations (P < 0.0001) were lowest in subjects with ACS. LDL-C (P = 0.008) and non-HDL (P < 0.0001) were higher in the ACS group than in the SAP group. Leukocyte count (P < 0.0001), oxLDL (P < 0.05), hsCRP (P < 0.001), sPLA2 (P < 0.05), and MPO (P < 0.0001) were highest in the ACS group. In multivariate models, comprising all biomarkers, elevated level of MPO had the best discriminatory power to identify patients with ACS. Receiver-operating characteristic curve with and without MPO comparison differed significantly (P = 0.03 for both ACS versus NCA and ACS versus SAP).
Conclusion. Our study shows that ACS associates with low HDL-C and biomarkers of oxidative stress and inflammation. The addition of MPO in biomarker panels might improve diagnostic accuracy for ACS.
Key messages
Acute coronary syndrome associates with low high-density lipoprotein cholesterol and biomarkers of oxidative stress and inflammation.
The addition of myeloperoxidase in biomarker panels might improve diagnostic accuracy for acute coronary syndrome.
Introduction
Traditional risk factors, including low-density lipoprotein cholesterol (LDL-C), diabetes mellitus (DM), hypertension, and smoking, are strong risk factors for cardiovascular disease outcomes (Citation1,Citation2). However, atherosclerosis also develops in a significant number of individuals with relatively modest risk factor profiles, highlighting the need for novel biomarkers that improve the prediction of an adverse cardiovascular outcome (Citation3). In particular, early diagnostic assessment of patients suspected of having acute coronary syndrome (ACS) remains a challenge, especially when the electrocardiography is inconclusive. In addition, the prolonged release pattern of troponins and the limited sensitivity of the routine troponin assay make it difficult to diagnose ACS at an early stage.
Recent reports have indicated that increases in biomarkers upstream from biomarkers of necrosis (cTroponinT and cTroponinI), such as markers of inflammation (myeloperoxidase (MPO), high-sensitivity C-reactive protein (hsCRP)) (Citation4), specific lipid parameters (lipoprotein(a), secretory phospholipase A2 (sPLA2)) (Citation5,Citation6), and oxidative stress (oxidized LDL (oxLDL)) (Citation7), may facilitate an earlier assessment of overall risk and the identification and management of patients with symptoms suggestive of ACS before tissue necrosis.
The role of inflammation in the translation of disease to clinical events is supported by the observation that increased systemic concentrations of the inflammatory biomarker, hsCRP, predict prospective cardiovascular risk in the settings of both primary and secondary prevention. HsCRP, which is produced in the liver after cytokine stimulations, is one of the earliest and most non-specific acute-phase reactants. However, the true utility of hsCRP as an independent predictor for cardiovascular disease (CVD) events is hotly debated. Several lines of evidence suggest that hsCRP is a surrogate marker, but not a causal factor, for CVD events (Citation8).
MPO, a heme enzyme linked to both inflammation and oxidative stress, has been implicated in the development and subsequent instability of atherosclerotic plaques (Citation9). Recently, it has become clear that MPO is involved in rendering high-density lipoprotein cholesterol (HDL-C) dysfunctional (Citation10). Increasing evidence suggests that circulating concentrations of MPO predict the prospective risk of adverse cardiac events in cohorts of patients who are asymptomatic (Citation11), have stable coronary artery disease (CAD) (Citation12), or either present with ACS (Citation13) or to the emergency department for the assessment of chest pain of suspected cardiac origin (Citation14). Given these promising data, there has been increasing interest in developing a multi-biomarker approach that incorporates MPO. A recent study showed that the combined panel of leukocyte count, MPO concentration, and pregnancy-associated plasma protein-A concentration was able correctly to classify acute coronary events (Citation15). Although these prospective and retrospective studies have supported the prognostic utility of MPO both in patients with stable CAD and in patients with ACS, other studies have highlighted its potential diagnostic limitations (Citation16,Citation17).
The present study was undertaken to evaluate the independent diagnostic information of a comprehensive panel of several established and emerging biomarkers across the entire spectrum of patients with CAD.
Methods
Study population
Between July 2006 and March 2008 all consecutive patients (n = 5809) signed for coronary angiography were collected for prospective Corogene-registry in Helsinki University Central Hospital (Citation18). Of all Finnish patients 93% gave informed consent (n = 5295). Comprehensive information of patients, medical history, medication, and current condition were gathered from patient records, questionnaire, digital angiogram records, cardiac ultrasound, etc. Of these patients 2090 had ACS, 1799 had stable CAD, and 1202 had diagnostic angiograms without significant CAD.
We selected randomly 242 non-diabetic ACS patients. Subjects with stable angina pectoris (SAP) were matched to these ACS patients according to sex, age, body mass index, and lack of DM. Individuals with normal coronary artery (NCA) were matched according to age and lack of DM.
Written informed consent was obtained from all participants, and the study design was approved by the institutional Ethics Committee.
Diagnostic criteria
CAD was confirmed by the presence of coronary stenoses ≥ 50% lumen obstruction in at least one of the three main coronary arteries. Normal coronary arteries were defined as arterial wall irregularities of < 10% lumen narrowing (at most). ACS comprised subjects with non-ST segment elevation myocardial infarction (MI) or unstable angina class II to IIIB (according to the Braunwald classification) undergoing coronary angiography within 72 hours from admission or with a diagnosis of ST segment elevation MI undergoing primary percutaneous coronary intervention within 12 hours of symptom onset.
Hypertension was defined as current use of antihypertensive drugs. Smoking status was recorded as those who were current smokers and those who had quit smoking or never smoked.
Biochemical investigations
Blood samples were collected in acute phase at the beginning of the coronary angiography. Serum and ethylenediamine tetra- acetic plasma were separated by centrifugation and stored at –80°C until different biomarkers were analyzed in 2009 and 2010.
Total serum cholesterol, triglycerides, HDL-C, LDL-C, concentrations of lipoprotein(a), apolipoproteinA-I, apolipoproteinB, and hsCRP were automatically analyzed by Konelab analyzer 60i (Thermo Fisher Scientific, Vantaa, Finland) with Konelab TM kits. LDL peak particle diameter (LDL size) was determined on a non-denaturing polyacrylamide gel with electrophoresis. Non-HDL-C was calculated by subtracting HDL-C from the total cholesterol. A monoclonal antibody 4E6-based competition enzyme-linked immunosorbent assay was used for measuring plasma levels of oxLDL (Mercodia, Uppsala, Sweden). An ELISA kit (Uscn Life Science Inc., Wuhan, China) was used to measure concentrations of sPLA2 and paraoxonase-1 (PON1), respectively.
The plasma concentration of MPO was determined with an enzyme immunoassay (Hycult Biotech HK324, Uden, The Netherlands). The immunoassay technique is based on the sandwich technique in which two monoclonal antibodies are directed against separate antigenic determinants on the MPO molecule. The absorbance at 450 nm is measured spectrophotometrically and the respective concentration calculated by using Tecan Magellan software. The result is accepted if the absorbance of two parallel wells of one sample does not vary from each other more than 15%.
Leukocyte counts and measurement of TroponinT were performed using standard laboratory techniques.
Statistical analyses
All statistical analyses were performed with SPSS 17.0 for Windows (SPSS, Inc., Chicago, IL, USA) and R 2.12.2. Between-group differences were assessed by the t test, the Mann–Whitney test, and the chi-square test, as appropriate.
Multivariate logistic regression analysis with disease statuses as outcomes was used to evaluate the significance of biological markers (total cholesterol, LDL-, HDL-, and non-HDL-C, non-HDL-C/HDL, triglycerides, hsCRP, oxLDL, apolipoproteinA-I, apolipoproteinB, apolipoproteinB/apolipoproteinA-I, lipoprotein(a), LDL size, MPO, leukocyte count, PON1, and sPLA2). When comparing ACS and NCA groups, models were adjusted for sex, body mass index, hypertension, smoking, and the use of β-blockers, aspirin, and statins. When ACS cases were compared to SAP subjects, the models were adjusted for hypertension, smoking, previous MI, and the use of β-blockers, aspirin, and statins. We tested the significance of each biological marker individually in logistic regression models and simultaneously in the same multivariate model. The relevance of these markers was further examined with receiver-operating characteristic curve statistics. A P value < 0.05 was considered statistically significant.
Results
Demographic characteristics of the three patient groups are shown in . There were more women in the NCA group than in the two other groups. Body mass index was higher in the NCA group than in the ACS group. Smoking habits of patients in the NCA group differed from those in the two other groups: there were more never-smokers in the NCA group. Two-thirds of subjects in the SAP group and one-third in the ACS group had previous MI. None in the NCA group had suffered any such attacks. Use of β-blockers was less frequent, and the use of aspirin was more frequent in the ACS group than in the two other groups. Therefore, we adjusted for all these background variables in the analyses presented below.
Table I. Clinical characteristics of the study groups.
Mean levels of the biomarkers and their confidence intervals in each patient group along with between-group comparisons are presented in . The total cholesterol level was lowest in SAP patients. LDL-C was highest in the ACS group, and HDL-C was highest in the NCA group, gradually lowering from SAP to ACS groups. ApolipoproteinA-I levels followed the pattern of HDL-C. Non-HDL-C, as a marker of all triglyceride-rich lipoproteins, and oxLDL were highest in the ACS group. On the contrary, lipoprotein(a) was highest in the SAP group. Inflammatory parameters showed similar patterns across the three groups: hsCRP, oxLDL, MPO, and leukocyte count were all highest in the ACS group as compared to SAP and NCA groups.
Table II. Biochemical characteristics of the study groups.
Next we tested each biomarker individually with logistic regression models adjusting for background variables (sex, body mass index, hypertension, previous MI, smoking, and the use of β-blockers, aspirin, and statins). HDL-C, hsCRP, apolipoproteinA-I, MPO, and leukocyte count were all strongly associated with ACS status when compared to either SAP or NCA groups. Additionally, apolipoproteinB/apolipoproteinA-I and non-HDL-C/HDL-C were associated with ACS when compared to NCA groups, and apolipoproteinB and lipoprotein(a) were significant in ACS versus SAP analyses ().
Table III. Association of biomarkers with acute coronary syndrome (ACS) when compared to subjects with normal coronary artery (NCA) and stable angina pectoris (SAP).a.
Because of the strong correlations between the measured biomarkers (data not shown) we also tested all the biomarkers in one multivariate model. As expected, apolipoproteinA-I, apolipoproteinB/apolipoproteinA-I, and leukocyte count, as well as LDL-C were significant in NCA versus ACS analyses (apolipoproteinA-I: P = 0.007; apolipoproteinB/apolipoproteinA-I: P = 0.04; leukocyte count: P = 0.04; LDL-C: P = 0.002), and apolipoproteinB remained significant when ACS was compared with SAP patients (P < 0.0001). Also, LDL-C (P<0.00001) and triglycerides (P < 0.001) were significant in this group. Interestingly, only hsCRP and MPO remained statistically significant in both groups when all biomarkers were tested simultaneously in the same model (ACS versus NCA: hsCRP P < 0.001, MPO P < 0.00001; ACS versus SAP: hsCRP P < 0.001, MPO P < 0.001). These results indicate that MPO and hsCRP have strong associations with the ACS status, and they seem to be mostly independent of other biomarkers.
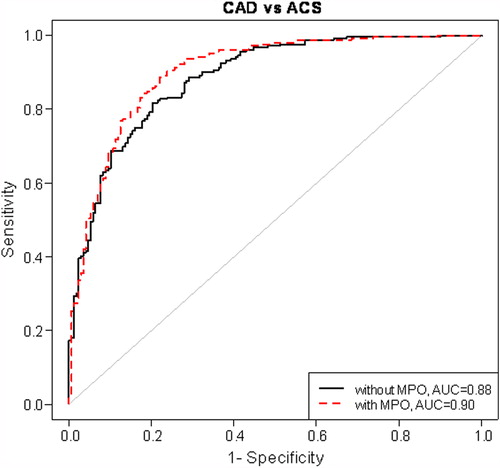
To follow up this finding, we next estimated the areas under the receiver-operating characteristics curves for each individually associated biomarker. MPO had the largest area under curve (ACS versus NCA: AUC = 0.87; ACS versus SAP: AUC = 0.85). When multivariate models consisting of all biomarkers and with and without MPO were compared, AUC increased from 0.92 to 0.94 (P = 0.03) () and from 0.88 to 0.90 (P = 0.03) (), in ACS versus NCA and ACS versus SAP analyses, respectively.
Figure 1. Risk discrimination of acute coronary syndrome (ACS) by receiver-operating characteristic curve. Models have been adjusted for sex, body mass index, hypertension, smoking, and the use of β-blockers, aspirin, and statins. CAD = coronary artery disease; MPO = myeloperoxidase; AUC = area under curve.
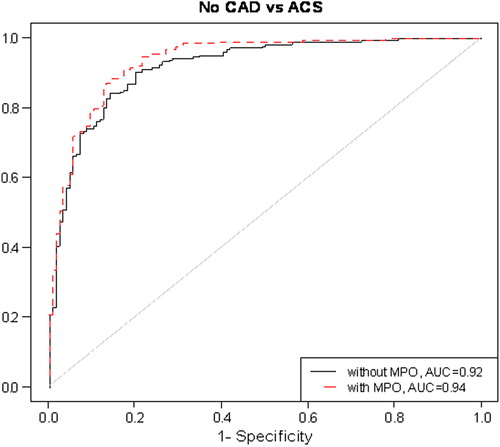
Figure 2. Risk discrimination of acute coronary syndrome (ACS) by receiver-operating characteristic curve. Models have been adjusted for hypertension, smoking, previous myocardial infarction and the use of β-blockers, aspirin, and statins. CAD = coronary artery disease; MPO = myeloperoxidase; AUC = area under curve.
Discussion
Our study aimed to evaluate the potential diagnostic information contributed by a comprehensive spectrum of different biomarkers representing lipid metabolism, inflammation, oxidative stress, plaque destabilization, plaque rupture, and myocardial necrosis in a consecutive series of case and control subjects in whom the presence of CAD was defined by coronary angiographic examination. The main findings of the study are as follows: 1) HDL-C and apolipoproteinA-I were significantly reduced in ACS patients as compared with SAP and subjects with NCA; 2) Biomarkers of oxidative stress and inflammation (leukocyte count, MPO, oxLDL, and hsCRP) were significantly increased as compared with SAP and subjects with NCA; 3) Elevated level of MPO had the best discriminatory power to identify patients with ACS.
The role of LDL-C in atherosclerotic cardiovascular disease is indisputable. Lowering LDL-C with statins in large placebo-controlled trials in both primary and secondary prevention has been successful to prevent cardiovascular disease outcomes (Citation19). Despite the overwhelming benefit of lowering LDL-C, there is a considerable residual risk for cardiovascular events (Citation20). Our study, comprising a high rate of statin users, shows that ACS associates with low HDL-C and biomarkers of oxidative stress and inflammation. Therefore additional therapy to relieve the burden of the residual risk should focus on potential risk factors beyond LDL-C.
Cardiac troponins owing to their superior sensitivity and specificity for detecting myocardial necrosis are, so far, the biomarker of choice and are critical to the universal definition of MI. Unfortunately, these biomarkers are not consistently elevated within the first few (< 6) hours after onset of symptoms, and therefore a challenge is to identify patients at risk during an earlier stage of the disease. In this setting inflammatory biomarkers have been extensively assessed given the fact that vascular inflammation is acknowledged as a key factor leading to plaque rupture and clinical events.
MPO, reflecting both inflammatory and oxidative processes distinct from those detected by markers of necrosis, has come up as a reliable early candidate biomarker for ACS associated with unfavorable clinical outcome (Citation11–15). Baldus et al. (Citation13) found that, in 1090 patients with ACS, the MPO above a cut-off of 350 μg/L was associated with an adjusted hazard ratio for the 6-month incidence of death and acute MI of 2.25% (95% CI 1.32–3.82). Notably, the prognostic value of MPO in this study was independent of biomarkers such as cardiac troponin, CRP, and soluble CD40 ligand. Furthermore, Brennan et al. (Citation14) showed that elevated concentrations of MPO predicted 30-day risk of acute MI and other major adverse coronary events, even among patients whose initial cardiac troponin levels were undetectable. However, available studies have given conflicting results, and the place of MPO in the clinical work-up of patients with CAD is still disputable (Citation16,Citation17). In a post hoc analysis of the MERLIN-TIMI 36 trial including 4300 patients with ACS, Scirica et al. (Citation16) found that only N-terminal proB-type natriuretic peptide and cardiac troponin were independently associated with cardiovascular death, but not MPO or CRP. Recently, Schaub et al. (Citation17) found that concentrations of MPO, myeloid-related protein 8/14, pregnancy-associated plasma protein-A, and CRP were significantly elevated in patients with acute MI, but the diagnostic accuracy of these markers was low and inferior compared to troponin. However, Schaub et al. (Citation17) showed that the concentrations of these biomarkers were higher in non-survivors than in survivors and predicted all-cause mortality with moderate accuracy.
A potential limitation of using MPO in early risk stratification may be related to drug treatment. Recent limited studies reveals that acute use of unfractionated heparin induces pre-analytical bias and error in MPO measurements and may thus influence MPO level interpretation (Citation21,Citation22). However, no data exist on the repeated injections of heparin and its effects on MPO levels.
Experimental and clinical studies provide support for a potential impact of therapy with statins on tissue and plasma MPO levels (Citation23–26). In clinical studies, statins reduced circulating levels of MPO in patients with ACS (Citation26), congestive heart failure (Citation23), and patients with DM on dialysis (Citation25). Recently, Ndrepepa et al. (Citation27) found that pre-admission therapy with statins, β-blockers, or angiotensin-converting enzyme inhibitors associated with reduced MPO levels in patients with ACS. Our observations provide further evidence linking inflammatory cascades that promote MPO release from activated leukocytes, MPO activity, and acute ischemic events. Notably, the MPO values in ACS were 2-fold higher than in SAP and NCA without overlap of confidence limits. In addition, our study shows that MPO has a strong association with ACS independently of pre-admission therapy. To the best of our knowledge this is the first study to report the superiority of MPO to predict ACS in patients assessed for a comprehensive panel of different established and novel biomarkers.
Based on the receiver-operating characteristic curve analyses, our study reveals that elevated MPO level had the best and a stronger discriminatory power than hsCRP to predict ACS. This finding is supported in the study by Ndrepepa et al. (Citation12) who suggested that MPO may have a more direct involvement in the plaque destabilization and thus a stronger link with ACS. Increased numbers of MPO expressing macrophages have been observed in human atherosclerotic plaques with vulnerable features, as compared with fatty streak lesions, which show little or no MPO (Citation9). Studies by Brennan et al. (Citation14) and Baldus et al. (Citation13) both revealed the potential utility of circulating MPO levels as a predictor of plaque vulnerability in subjects at risk for incident major adverse cardiac events, even in the absence of detectable levels of myocardial necrosis.
In recent years, accumulated evidence has shown that elevated sPLA2 activity is a strong independent risk for CAD (Citation6), correlates with the degree of atheromatosis (Citation28), and predicts recurrent events in patients with severe ACS (Citation29). Similarly, we found a progressive increase in the sPLA2 activity with the increase in severity of CAD. However, when sPLA2 was tested with a logistic regression model adjusting for background variables the association of sPLA2 with ACS was insignificant. Similarly, we could not find an association between PON1, a major contributor to the antioxidant activity of HDL-C, and ACS (). The reason for this remains unknown.
Some important points should be noted. This study has by nature a cross-sectional design, which limits our ability to infer causality. We did not assess the time-dependency of the biomarkers, and this represents a limitation regarding a potential role of these markers as predictors of risk over time. However, the data obtained in ACS subjects are of clinical relevance as our patients are representative of contemporary management strategies, and, more importantly, the use of medication was taken into account in multivariate analyses. Additionally, inflammatory marker levels might have been influenced by acute infections or inflammatory states, conditions that were not used as exclusion criteria in our study. Furthermore, some of our patients had received enoxaparin before collection of blood samples. Nevertheless, all blood samples were taken before coronary angiography and ethylenediamine tetra-acetic plasma samples were used for MPO analyses.
In conclusion, our data highlight the role of antiatherogenic lipids, oxidative stress, and inflammation in processes leading to plaque rupture and ACS. Moreover, our results indicate that, in the context of a conventional troponin, the addition of MPO to the biomarker profile might improve diagnostic accuracy for acute ACS in clinical practice.
Acknowledgements
The authors gratefully acknowledge the excellent technical assistance by Hannele Hildén, Virve Naatti, and Helinä Perttunen-Nio.
Declaration of interest: The authors report no conflicts of interest. M.G and E.T. contributed equally to the work. This work was supported by grants from Helsinki University Central Hospital Research Foundation, the Finnish Foundation for Cardiovascular Research, the Aarne Koskelo foundation, and the Sigrid Juselius Foundation. S.R. was supported by the Academy of Finland Centre of Excellence in Complex Disease Genetics (grant #213506 and #129680) and Academy of Finland (#251217).
References
- Stamler J, Daviglus ML, Garside DB, Dyer AR, Greenland P, Neaton JD. Relationship of baseline serum cholesterol levels in 3 large cohorts of younger men to long-term coronary, cardiovascular, and all-cause mortality and to longevity. JAMA. 2000;284:311–8.
- Neaton JD, Wentworth D. Serum cholesterol, blood pressure, cigarette smoking, and death from coronary heart disease: overall findings and differences by age for 316 099 white men: Multiple Risk Factor Intervention Trial Research Group. Arch Intern Med. 1992;152:56–64.
- Law MR, Wald NJ. Risk factor thresholds: their existence under scrutiny. BMJ. 2002;324:1570–6.
- Morrow DA, Cannon CP, Jesse RL, Newby LK, Ravkilde J, Storrow AB, et al. National Academy of Clinical Biochemistry Laboratory Medicine Practice Guidelines: clinical characteristics and utilization of biochemical markers in acute coronary syndromes. Circulation. 2007;115: e356–75.
- ; The Emerging Risk Factors CollaborationErqou S, Kaptoge S, Perry PL, Di Angelantonio E, Thompson A, White IR, et al. Lipoprotein(a) concentration and the risk of coronary heart disease, stroke and nonvascular mortality. JAMA. 2009;302:412–23.
- ; The Lp-PLA2 Studies CollaborationThompson A, Gao P, Orfei L, Watson S, Di Angelantonio E, Kaptoge S, et al. Lipoprotein-associated phospholipase A2 and risk of coronary disease, stroke, and mortality: collaborative analysis of 32 prospective studies. Lancet. 2010;375: 1536–44.
- Meisinger C, Baumert J, Khuseyinova N, Loewel H, Koenig W. Plasma oxidized low-density lipoprotein, a strong predictor for acute coronary heart disease events in apparently healthy, middle-aged men from the general population. Circulation. 2005;112:651–7.
- Lorgeril M, Salen P, Abramson J, Dodin S, Hamazaki T, Kostucki W, et al. Cholesterol lowering, cardiovascular diseases, and the Rosuvastatin-JUPITER controversy. Arch Intern Med. 2010;170:1032–6.
- Sugiyama S, Okada Y, Sukhova GK, Virmani R, Heinecke JW, Libby P. Macrophage myeloperoxidase regulation by granulocyte macrophage colony-stimulating factor in human atherosclerosis and implications in acute coronary syndromes. Am J Pathol. 2001;158:879–91.
- Shao B, Oda MN, Oram JF, Heinece JW. Myeloperoxidase: an inflammatory enzyme for generating dysfunctional high-density lipoprotein. Curr Opin Cardiol. 2006;21:322–8.
- Meuwese MC, Stores ES, Hazen SL, van Miert JN, Kuivenhoven JA, Schaub RG, et al. Serum myeloperoxidase levels are associated with the future risk of coronary artery disease in apparently healthy individuals: the EPIC-Norfolk Prospective Population Study. J Am Coll Cardiol. 2007;50;159–65.
- Ndrepepa G, Braun S, Mehilli J, von Beckerath N, Schomig A, Kastrati A. Myeloperoxidase level in patients with stable coronary artery disease and acute coronary syndromes. Eur J Clin Invest. 2008;38:90–6.
- Baldus S, Heeschen C, Meinertz T, Zeiher AM, Eiserich JP, Munzel T, et al. Myeloperoxidase serum levels predicts risk in patients with acute coronary syndromes. Circulation. 2003;108:1440–5.
- Brennan ML, Penn MS, Van Lente F, Nambi V, Shishehbor MH, Aviles RJ, et al. Prognostic value of myeloperoxidase in patients with chest pain. N Engl J Med. 2003;349:1595–604.
- Lobbes MI, Kooi ME, Lutgens E, Ruiters AW, Lima Passos V, Braat SH, et al. Leukocyte counts, myeloperoxidase, and pregnancy-associated plasma protein A as biomarkers for cardiovascular disease: towards a multi-biomarker approach. Int J Vasc Med. 2010;43:240–5.
- Scirica BM, Sabatine MS, Jarolim P, Murphy SA, de Lemos JL, Braunwald E, et al. Assessment of multiple cardiac biomarkers in non-ST-segment elevation acute coronary syndromes: observations from the MERLIN-TIMI 36 trial. Eur Heart J. 2011;32:697–705.
- Schaub N, Reichlin T, Meune C, Twerenbold R, Haaf P, Hochholzer W, et al. Markers of plaque instability in the early diagnosis and risk stratification of acute myocardial infarction. Clin Chem. 2012;58:246–56.
- Vaara S, Nieminen MS, Lokki ML, Perola M, Pussinen PJ, Allonen J, et al. Cohort profile: the Corogene study. Int J Epidemiol. 2012;41: 1265–71.
- Baigent C, Keech A, Kearney PM, Blackwell L, Buck G, Pollicino C, et al. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90 056 participants in 14 randomised trials of statins. Lancet. 2005;366:1267–78.
- Bayturan O, Kapadia S, Nicholls SJ, Tuzcu EM, Shao M, Uno K, et al. Clinical predictors of plaque progression despite very low levels of low-density lipoprotein cholesterol. J Am Coll Cardiol. 2010;55:2737–42.
- Baldus S, Rudolph V, Roiss M, Ito WD, Rudolph TK, Eiserich JP, et al. Heparins increase endothelial nitric oxide bioavailability by liberating vessel-immobilized myeloperoxidase. Circulation. 2006;113:1871–8.
- Shih J, Datwyler SA, Hsu SC, Matias MS, Pacenti DP, Lueders C, et al. Effect of collection tube type and preanalytical handling on myeloperoxidase concentrations. Clin Chem. 2008;54:1076–9.
- Andreou I, Tousoulis D, Miliou A, Tentolouris C, Zisimos K, Gounari P, et al. Effects of rosuvastatin on myeloperoxidase levels in patients with chronic heart failure: a randomized placebo-controlled study. Atherosclerosis. 2010;210:194–8.
- Meuwese MC, Trip MD, van Wissen S, van Miert JN, Kastelein JJ, Stroes ES. Myeloperoxidase levels are not associated with carotid atherosclerosis progression in patients with familial hypercholesterolemia. Atherosclerosis. 2008;197:916–21.
- Stenvinkel P, Rodriques-Ayala E, Massy ZA, Quereshi AR, Barany B, Fellström B, et al. Statin treatment and diabetes affect myeloperoxidase activity in maintenance hemodialysis patients. Clin J Am Soc Nephrol. 2006;1:281–7.
- Zhou T, Zhou SH, Qi SS, Shen XQ, Zeng GF, Zhou HN. The effect of atorvastatin on serum myeloperoxidase and CRP levels in patients with acute coronary syndrome. Clin Chim Acta. 2006;368:168–72.
- Ndrepepa G, Braun S, Schömig A, Kastrati A. Impact of therapy with statins, beta-blockers and angiotensin-converting enzyme inhibitors on plasma myeloperoxidase in patients with coronary artery disease. Clin Res Cardiol. 2011;100:327–33.
- Lima LM, Carvalho MG, Neto CP, Garcia JC, Sousa MO. Secretory phospholipase A2 in patients with coronary artery disease. J Thromb Thrombolysis. 2010;29:276–81.
- Mallat Z, Steg G, Benessiano J, Tanguy ML, Fox KA, Collet JP, et al. Circulating secretory phospholipase A2 activity predicts recurrent events in patients with severe acute coronary syndromes. J Am Coll Cardiol. 2005;46:1249–57.
