Abstract
Primary aldosteronism (PA) is the most common secondary form of arterial hypertension, with a particularly high prevalence among patients with resistant hypertension. Aldosterone has been found to be associated with cardiovascular toxicity. Prolonged aldosteronism leads to higher incidence of cardiac events, glomerular hyperfiltration, and potentially bone/metabolic sequels. The wider application of aldosterone/renin ratio as screening test has substantially contributed to increasing diagnosis of PA. Diagnosis of PA consists of two phases: screening and confirmatory testing. Adrenal imaging is often inaccurate for differentiation between an adenoma and hyperplasia, and adrenal venous sampling is essential for selecting the appropriate treatment modality. The etiologies of PA have two main subtypes: unilateral (aldosterone-producing adenoma) and bilateral (micro- or macronodular hyperplasia). Aldosterone-producing adenoma is typically managed with unilateral adrenalectomy, while bilateral adrenal hyperplasia is amenable to pharmacological approaches using mineralocorticoid antagonists. Short-term treatment outcome following surgery is determined by factors such as preoperative blood pressure level and hypertension duration, but evidence regarding long-term treatment outcome is still lacking. However, directed treatments comprising of unilateral adrenalectomy or mineralocorticoid antagonists still potentially reduce the toxicities of aldosterone. Utilizing a physician-centered approach, we intend to provide up-dated information on the etiology, diagnosis, and the management of PA.
Key words::
Key messages
The prevalence of primary aldosteronism in unselected patients with hypertension ranges between 3% and 20%, constituting an important cause of unrecognized secondary hypertension.
Primary aldosteronism can be divided into idiopathic adrenal hyperplasia, aldosterone-producing adenoma (rarely carcinoma), and rare familial forms. This classification facilitates the identification of potentially surgically curable patients.
Treatment of primary aldosteronism consists of unilateral adrenalectomy or pharmacologic mineralocorticoid antagonism, both of which reduce the detrimental effect of aldosterone on the cardiovascular system.
Introduction
There has been significant progress in the understanding of primary aldosteronism (PA), after Conn's initial description of PA more than 50 years ago (Citation1). Subsequent validation of laboratory renin and aldosterone assays in the 1960s and 1970s permitted more reliable PA diagnosis (including its differentiation from secondary forms of hyperaldosteronism). According to the Endocrine Society's guideline, PA is defined as a group of disorders in which ‘aldosterone production is inappropriately high, relatively autonomous from the renin-angiotensin system, and non-suppressible by sodium loading’ (Citation2). Hiramatsu first described the utility of plasma aldosterone concentration (PAC) to plasma renin activity (PRA) ratio in 1981 (Citation3). The wider application of the aldosterone/renin ratio (ARR) to both hypokalemic and normokalemic hypertensives further facilitated the screening for PA. This trend leads to the demonstration that PA is much more common than previously suspected. Wide variations exist in the estimate of PA prevalence, ranging from 3%–4% to nearly 20% (Citation4–7). The inconsistency of reported ARR largely stems from differences in the populations studied, diagnostic approaches, cut-off value of ARR, and in the use and selection of confirmatory tests.
What is the effect of excess aldosterone?
Aldosterone is produced in the zona glomerulosa of adrenal glands in response to renin-dependent angiotensin II stimulation. Plasma potassium (stimulatory) and other hormones such as adrenocorticotropic hormone (ACTH, stimulatory) and dopamine (inhibitory) are capable of modifying aldosterone secretion physiologically (Citation8). However, excess aldosterone secretion is associated with increased risk of cardiovascular diseases (CVD), including fatal stroke and sudden cardiac death, as indicated by the growing body of evidence (Citation9). Furthermore, patients with long-term exposure to increased aldosterone levels appear to develop more renal and metabolic sequelae (Citation10). These multi-organ untoward effects are largely independent of the blood pressure (BP) level. As reported, the odds ratio (OR) of non-fatal myocardial infarction was 6.5 in patients with PA compared with those with essential hypertension, while OR for atrial fibrillation and stroke are 12.1 and 4.2, respectively, independently of blood pressure levels (Citation11). Targeted management including optimal BP control and either adrenalectomy or medical treatment with mineralocorticoid antagonists is fundamental for the prevention of hypertension and cardiovascular events in patients with PA. Despite this, the awareness of PA remains low and clinicians rarely consider PA if their patients are free of hypokalemia. Consequently, it is important that physicians be familiar with the clinical spectrums of PA, so as to facilitate earlier diagnosis and prompt treatment.
Which symptoms/signs do patients with primary aldosteronism have, and why?
Detrimental effects from aldosterone excess are mediated through the activation of mineralocorticoid receptor (MR), which is widely expressed in epithelial cells (including renal, colonic, and salivary gland), smooth muscle cells, myocardiocytes, endothelial progenitor cells (Citation12), and neutrophils. Aldosterone exerts its actions on sodium and potassium handling through up-regulation of the activity of the distal tubule sodium epithelial channel. Aldosterone also increases oxidative stress and collagen remodeling, triggering endothelial dysfunction, delayed endothelial recovery (Citation12), and subsequent ventricular hypertrophy and myocardial fibrosis. Regarding kidney damage, the hyperfiltration phenomenon may mask impaired kidney function, leading to underestimation of the burden of chronic kidney disease in PA patients (Citation13). Even mild impairment of renal function may predict residual hypertension in treated PA patients. This masking effect may explain the intra-renal hemodynamic adaptation to aldosterone excess (Citation14).
The diagnosis of PA is usually made in patients who are in the third to sixth decade of life (Citation15). Hypertension and hypokalemia are the hallmarks of PA in most physicians’ minds, but, on the contrary, hypokalemia is not as common as was previously suspected in PA patients (Citation16). Patients with marked hypokalemia may present with muscle weakness and cramping, headaches, palpitations, polydipsia, polyuria, nocturia, or a combination of these. In fact, Omura et al. found that plasma potassium levels were less than 3.3 mEq/L in only 18% of all patients with confirmed PA (Citation17). In Taiwan, the TAIPAI group (TAIwan Primary Aldosteronism Investigation) identified hypokalemia in only 50% of cases (Citation18). In addition, hypokalemia can also be disguised by low dietary salt intake (Citation19). The polyuria and nocturia result from a hypokalemia-induced renal concentrating defect (Citation15), and the serum sodium concentration tends to be high-normal or slightly above the normal upper limit. Autonomous aldosterone secretion in PA patients might potentially antagonize parathyroid hormone (PTH) secretion and induce hypercalciuria with lowering of serum calcium (Citation20,Citation21). Occasionally, blood pressure may be normal in patients with well-documented PA (Citation22). In normotensive patients, low body mass index and green tea ingestion have been proposed as potential explanations for their relatively normal blood pressure (Citation23).
In addition to electrolyte and blood pressure disturbance, aldosteronism elevates risk of metabolic syndrome and bone loss, possibly at least in part due to increased calciuria and magnesiuria (Citation20,Citation24). Blockade of mineralocorticoid receptors significantly ameliorates the sodium volume retention and the inflammatory reaction. This effect is at least partly independent of blood pressure reduction per se (Citation25). Furthermore, patients with PA have a greater risk of developing left ventricular hypertrophy, myocardial fibrosis, and diastolic dysfunction than patients with essential hypertension. PA patients also demonstrate increasing arterial stiffness and greater carotid intima-media thickness (Citation26). Recently, Sonino and co-workers demonstrated that psychiatric illnesses, including generalized anxiety disorders, occurred more often in patients with primary aldosteronism than in those with essential hypertension (Citation27).
Is all primary aldosteronism the same or are there different subtypes?
The most common forms of PA are idiopathic bilateral hyperplasia (IHA) and aldosterone-producing adenoma (APA), accounting for more than 90% of clinical cases () (Citation28,Citation29). Unilateral adrenal hyperplasia is less commonly identified, but this diagnosis should be pursued if lateralization on adrenal venous sampling (AVS) is found without radiological evidence of adenoma. Approximately 1% of patients have aldosterone-producing carcinoma, and 10% of these patients manifest metastasis at diagnosis (Citation30). Patients with aldosterone-producing adrenal carcinoma often manifest rapid development of signs of aldosteronism (Citation30).
Table I. Subtypes of primary aldosteronism.a
Familial forms of primary aldosteronism include familial hyperaldosteronism type I (glucocorticoid-remediable aldosteronism, GRA), type II (familial occurrence of adenoma or hyperplasia, FH-II), and type III (). Features of each form are displayed in . GRA is characterized by early-onset, severe hypertension, refractory to conventional antihypertensive medications. The underlying mutation is a chimeric gene duplication resulting from unequal crossing-over between the promoter sequences of 11β hydroxylase (CYP11B1), which is responsive to corticotrophin stimulation, and coding sequences of aldosterone synthase (CYP11B2), both on chromosome 8. An autosomal dominantly inherited disorder, this leads to ACTH-sensitive aldosterone overproduction. The main features consist of increased aldosterone and hybrid steroid products (18-OH-cortisol and 18-oxo-cortisol), which are suppressible with exogenous corticosteroid (Citation31).
Table II. Clinical, genetic, and biochemical features of different forms of familial hyperaldosteronism (FH).
FH type II is an autosomal dominantly inherited, genetically heterogeneous disorder. FH type II families can present clinically with APA or IHA, and are not distinguishable from patients with non-familial PA (Citation2). The exact prevalence of FH type II is not known, but there are case series reporting a high percentage of affected patients (7%) (Citation32). FH type II is more common than GRA, and is reportedly linked to chromosome 7p22 (Citation33).
FH type III is a newly discovered clinical entity. First described by Geller and co-workers, it is a particularly aggressive form of hyperaldosteronism, with medication-resistant hypertension (Citation34). The genetic cause of this syndrome appears to be inherited germline mutations in KCNJ5, which encodes the potassium channel Kir 3.4 (Citation35). Somatic mutations of KCNJ5 have also been reported in approximately 34% of APAs removed from patients with apparently non-familial PA (Citation36). These germline and somatic mutations result in loss of channel selectivity for potassium and increased sodium conductance, leading to depolarization of zona glomerulosa cells. This depolarization activates voltage-gated Ca2+ channels with intracellular Ca2+ rising, inducing aldosterone production (Citation36). Chronic Ca2+ stimulation also promotes zona glomerulosa cellular proliferation (Citation37). Clinically patients with FH type III present with severe hypertension and variable hypokalemia, with radiological bilateral massive adrenal hyperplasia (Citation34).
Diagnosis and clinical reasoning: step by step
Step 1: When should we consider screening patients for primary aldosteronism?
The optimal timing to test for PA is still an unresolved issue. Some experts suggest routine screening in all hypertensive patients owing to its high prevalence and cost-effective curable potential. A consensus endorsed by the Endocrine Society (Citation2) indicates that testing should be performed in patients with the following features: 1) Joint National Commission stage 2 (systolic/diastolic > 160–179/100–109 mmHg) and stage 3 (> 180/110 mmHg) hypertension; 2) drug-resistant hypertension; 3) hypertension with spontaneous hypokalemia or diuretic-induced hypokalemia; 4) hypertension with adrenal incidentaloma; 5) hypertension and a family history of early-onset hypertension, or cerebrovascular accident at a young age (< 40-year-old); and 6) patients with first-degree relatives diagnosed with PA.
Step 2: How can we achieve the diagnosis of primary aldosteronism?
Screening test: aldosterone/renin ratio (ARR)
The ARR is currently the most reliable method for screening for PA. ARR is superior to serum potassium or plasma aldosterone concentration alone because of higher sensitivity and to plasma renin assay alone because of higher specificity (Citation38). Assay should be performed in the morning with pre-test normokalemia achieved by potassium supplementation where necessary (hypokalemia might impair aldosterone production). Plasma potassium assay should be measured in blood collected carefully without stasis or hemolysis. Patients should be instructed to liberalize their salt intake during the diagnostic studies.
ARR is calculated typically by dividing PAC by PRA or direct renin concentration (DRC), each with different proponents. If PRA is unmeasurably low (as sometimes found in PA patients), the duration of the assay incubation phase should be prolonged to improve sensitivity (Citation2,Citation39). While PRA is measured using production of angiotensin I from endogenous substrate, DRC measures serum renin concentrations immunoradiometrically (Citation40). An interconversion factor between PRA and DRC ranges from 8.2 to 12 (Citation2). There are studies claiming the superiority of DRC to PRA, but most are limited owing to variations between hormone assays and study populations (Citation40,Citation41).
Tests such as 24-hour urine aldosterone levels during high-salt diet, tetrahydroaldosterone (THA) levels, and urine aldosterone level/PRA ratio have been utilized as an alternative approach for screening PA patients (Citation42–44). Abdelhamid et al. found that 24-hour urine THA is more sensitive for PA diagnosis than ARR, with equal specificity (Citation42). Urine collection over 24 hours could address the confounding effect of diurnal aldosterone variation more effectively than single plasma PAC measurement (Citation43). A drawback, however, is that this approach relies upon the accuracy of the 24-hour urine collection.
The introduction of liquid chromatography–tandem mass spectrometry (LC-MS/MS) for determining plasma or urine aldosterone levels, compared with antibody-based methods, prominently enhances the diagnostic accuracy (Citation45,Citation46). This advanced assay was once limited by the lack of calibrators, but isotope-labeled aldosterone as internal standard is now available (Citation47). The feasibility of semi-automated quantification further enhances the applicability of LC-MS/MS in contemporary aldosterone measurements (Citation47,Citation48).
Preparation for ARR test: are there issues that clinicians should know before checking ARR?
Before interpreting the paired PRA and PAC values, several points should be considered. First, several medications could interfere with ARR values (). Most notably, diuretics, including mineralocorticoid receptor antagonists (spironolactone, eplerenone) and amiloride, significantly compromise the test results (false negativity from stimulating renin secretion more than aldosterone) and therefore should be discontinued at least 6 weeks before testing (Citation49). Angiotensin-converting enzyme (ACE) inhibitors and angiotensin-receptor blockers (ARB) could also result in a falsely negative ARR value from suppressing aldosterone secretion and raising renin levels.
Table III. Medications potentially affecting aldosterone/renin ratio interpretation.
Therefore, in a patient treated with ACE inhibitor or ARB, the finding of low ARR does not exclude the diagnosis of PA. Direct renin inhibitor (aliskiren) also reportedly skews ARR (lowered PRA or elevated direct renin concentration (DRC), leading to false positive or false negative ARRs, respectively) (Citation2). Other antihypertensive medications including β-adrenergic blockers and central α2-agonists (clonidine and methyldopa) can lower renin level more than aldosterone, resulting in a false positive ARR. Dihydropyridine calcium channel blockers (DHP-CCB) also cause elevated renin levels, thus a falsely low ARR value (Citation50). It would be ideal to discontinue these medications for 2–4 weeks (4–6 weeks for diuretics) before arranging ARR testing, but this is frequently difficult in real-world settings, because of the need to maintain BP control. Moreover, in patients using ARR-lowering medications (such as ACE inhibitors), a high ARR value could by itself confirm the presence of aldosteronism. An optimized testing scenario without drug wash-out, in this circumstance, would also be acceptable. Consequently, we suggest that it is ideal to use neutral antihypertensive drugs such as verapamil (slow-release form), hydralazine (combined with verapamil to avoid reflex tachycardia), and α1-antagonists (prazosin, doxazosin, terazosin, etc.) in order to maintain adequate BP control (Citation2). If this is not feasible, per Endocrine Society's guideline, K-sparing and K-wasting diuretics should be discontinued at least for 4 weeks prior to ARR test. If subsequent ARR is non-diagnostic, less ARR-interfering medications (ACE inhibitors, ARB, DHP-CCB) should also be withheld, with ARR repeated (Citation2).
In addition to antihypertensives, several other drugs also potentially introduce interference to ARR results, such as products derived from licorice (e.g. chewing tobacco), non-steroidal anti-inflammatory agents (NSAID), and oral contraceptives, especially drospirenone (17-α spironolactone) (Citation2). Drospirenone can cause falsely positive ARR values (Citation51). While it is usually feasible to withhold confounding agents before patients receive diagnostic tests, oral contraceptives should not be ceased unless careful alternative strategies are put in place to prevent unwanted pregnancy.
After obtaining ARR values, how should clinicians interpret test results?
A lack of uniform assay methods and ARR cut-off values has created a high variability in threshold values among various investigation groups. For PAC, 1 ng/dL converts to 27.7 pmol/L in Systeme International (SI) units (measured by radioimmunoassay (RIA) method); while for PRA, 1 ng/mL/h converts to 12.8 pmol/L/min in SI units (Citation52). Threshold values for the ARR (calculated by PAC/PRA) are reported to range between 20 and 40 (conventional units) or 60 and 80 (SI units) (Citation2). If calculated by PAC/DRC (in mU/L or ng/L), the values range from 2.4 to 7.7 (conventional units) or from 91 to 192 (SI units) () (Citation2). As the methods for measurements of renin levels (by DRC) evolve, so are the cut-off thresholds for ARR (Citation45,Citation46).
Table IV. Recommended threshold of aldosterone/renin ratio for screening of primary aldosteronism in different units.
Although PA patients often have a PAC level above the normal limit or within the upper half of the reference range (Citation53), an extremely low PRA level could, on the contrary, result in falsely positive ARR despite apparently low PAC level (Citation54). Some researchers have proposed that an additional criterion of PAC > 15 ng/dL (415 pmol/L) could avoid false positivity conferred by extremely low renin levels (Citation15), but no consensus currently exists. The variability of assay methodology between laboratories still constitutes a major obstacle to the standardization of ARR interpretation. Others have proposed post-captopril ARR as a sensitive method for screening, but firm evidence is also lacking (Citation55). The Endocrine Society suggests that the most commonly adopted ARR cut-off is 30 (conventional units) or 750 (SI units; pmol/L by ng/mL/h) (Citation2). From TAIPAI experience, we propose that a cut-off value of ARR of 35 (conventional units), or 750 (SI units), based on PRA and PAC (both measured by RIA method), could be considered for both research and for clinical screening purposes, with high sensitivity and specificity (Citation18). Nonetheless, the judgment of the most suitable ARR threshold for PA case detection is still at the discretion of each institution’s local laboratory.
Step 3: Confirming the diagnosis after positive screening testing—what tests can be used for PA confirmation? ()
An increased ARR is not diagnostic by itself but indicates that further testing is warranted. An accurate, negative confirmatory test can spare the patient from subsequent invasive and costly examinations and, in particular, adrenal venous sampling. However, reference standards for PA confirmation have not been established to date. Currently available tests include oral salt loading (OST) or intravenous salt infusion testing (SIT), fludrocortisone stimulation testing (FST), and captopril or losartan challenge testing (CST/LST) (Citation2), each with their proponents. In OST, the sensitivity and specificity are around 96% and 93%, respectively, with the main limitation being the potential for inaccurate 24-hour urine collection (Citation15). The accuracy of urine aldosterone assay is another concern, but should be overcome with the introduction of new mass-spectrometric techniques (Citation2). SIT is currently the most popular confirmatory test for PA, since patients can complete the test procedure during one clinic visit (Citation52). However, both the OST and SIT should be avoided in patients with heart failure or renal insufficiency because of the risk of fluid overload, and in those with severe hypokalemia (Citation2). FST is performed in-hospital mainly due to the requirement of frequent blood sampling to monitor plasma potassium levels, but the risk of fluid overload is relatively lower. Finally, CST/LST is utilized in limited centers only, and false negative or non-diagnostic results are not uncommon (Citation56,Citation57). However, the advantage of CST/LST is that it avoids the risk of fluid overload (Citation55).
Table V. Available confirmatory tests for diagnosis of primary aldosteronism.
Step 4: Which PA subtypes do these patients have?
After confirmation of autonomous aldosterone secretion (by means of positive ARR and confirmatory tests), further testing is required to determine the subtypes. Major subtypes of PA are listed in . The critical next step is the arrangement of an abdominal computed tomography (CT) without contrast enhancement and thin-sliced (as low as 1 mm) for adrenal glands, to enable better detection of adrenal tumors. Adrenal CT is very important for the subsequent treatment strategies, since the optimal treatment for each subtype of PA differs ().
Figure 1. The diagnostic and treatment protocol. (*The age threshold (40 years) used is arbitrary, according to Mayo group's experience (Young Jr WF, et al. Surgery 2004). # Patients < 40 years with adrenal lesion ≥ 1 cm can also receive AVS if clinically indicated.) (ARR = aldosterone/renin ratio; AVS = adrenal venous sampling; CT = computed tomography.).
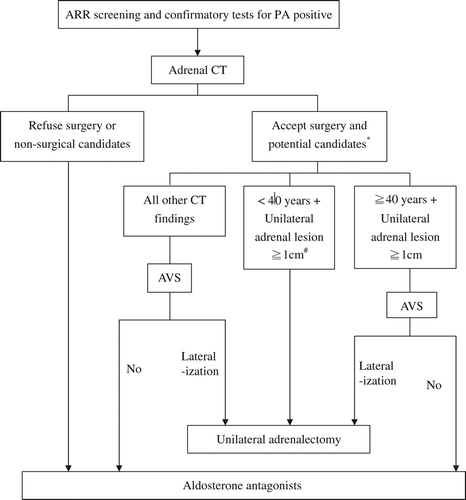
Step 5: Is there lateralization in this patient with PA?
To establish whether there is ‘lateralization’ is a critical step for guiding further treatment. Lateralization refers to localization of the aldosterone secretion to one adrenal gland (rather than both). Distinguishing between unilateral and bilateral PA permits reliable selection of patients likely to benefit from unilateral adrenalectomy in terms of normalization of hypokalemia and hypertension. Patients with bilateral lesions (IHA) are usually managed medically (Citation58).
Determination of lateralization is not always straightforward. Imaging often fails to visualize microadenomas, or to provide definite clues for lateralization even if a nodule is visualized. The probability of identifying an adrenal mass in the general population by CT is not low, but most adrenal nodules are non-functional and benign. Findings on adrenal CT in PA include normal-appearing adrenal glands, unilateral macroadenoma/microadenoma (> 1 cm or ≤ 1 cm), minimal unilateral adrenal limb thickening, or bilateral adenoma (Citation2). The limitations include misinterpretation of small APAs as IHA (bilateral nodularity) or normal adrenals, and inadvertent recognition of hyperplastic areas as microadenoma (Citation15). Magnetic resonance imaging is an alternative modality to detect adrenal tumors, but most studies do not demonstrate better sensitivity or specificity than CT.
In one study, the detection rate of adrenal CT for single nodules in patients with unilateral PA reached only 50%, and decreased further to less than 25% if APAs were smaller than 1 cm in diameter (Citation2). In addition, CT may be misleading if the image reveals a unilateral non-functioning nodule in patients with bilateral disease, resulting in inappropriate surgery. Because of these considerations, adrenal venous sampling (AVS) is the procedure of choice for differentiation between unilateral and bilateral forms of PA, despite its expense and invasiveness. Some investigators have suggested that a combination of typical image findings and biochemical features can be highly specific for lateralization, thus replacing the role of AVS before surgery at least in some patients (Citation59). At the Mayo clinic, patients are spared AVS if they are younger than 40-year-old with solitary unilateral apparent adenoma on CT, as unilateral adrenalectomy in this population still has been associated with high biochemical and clinical cure rates even in the absence of pre-operative AVS confirmation of unilateral PA. Most group, however, recommend AVS in all patients who are surgical candidates and are willing to receive surgery. AVS can obviously be omitted in patients refusing surgery or who are not appropriate surgical candidates. The sensitivity and specificity of successful AVS (95% and 100%) for detecting unilateral disease is significantly better than adrenal CT (Citation60).
AVS is a difficult procedure and usually requires dedicated staff with experienced hands, since cannulating the smaller right adrenal vein is technically demanding. According to a systematic review of 47 reports, the success rate was 74%, but rose to 90%–96% if angiographers were highly experienced (Citation61). Intraprocedural measurement of adrenal vein cortisol further enhances the success rate of correct catheter placement. Nonetheless, it should be noted that AVS service is not available in many institutes, and the procedure is technically demanding.
The various reported protocols for AVS can be divided into several types: unstimulated sequential or simultaneous bilateral AVS, unstimulated sequential or simultaneous bilateral AVS followed by bolus cosyntropin-stimulated sequential or simultaneous bilateral AVS, and continuous cosyntropin infusion with sequential bilateral AVS. The aim of continuous cosyntropin infusion during AVS is to minimize stress-induced fluctuations in aldosterone secretion during sequential AVS, maximize the gradient of cortisol between adrenal vein and inferior vena cava (IVC) (to ensure optimal cannulation), and to stimulate the secretion of aldosterone from APAs (Citation60). Dividing the right and left adrenal vein PAC by their respective cortisol levels can neutralize the diluting influence of the inferior phrenic vein or IVC, contributing to samples obtained from the left and right adrenal vein, respectively. In the absence of cosyntropin infusion, a cortisol-corrected aldosterone lateralization ratio of more than 2:1 has been used to confirm unilateral disease (Citation62), although other groups use higher ratios of 3–4 Some groups also compare adrenal vein aldosterone/cortisol ratios to those in simultaneously collected peripheral venous samples (cubital) or IVC. The acceptable cut-off points in this setting are an adrenal/peripheral venous ratio of more than 2.5:1 on the hypersecreting side, coupled with contralateral suppression (ratio on the other side no higher than 1:1).
Other than AVS, several radionuclide imaging modalities have also been investigated for their potential for determining lateralization, since they are non-invasive and avoid the adverse effects of contrast agents used in CT/magnetic resonance. The utility of adrenocortical scintigraphy (NP-59, I-131-6-beta-iodomethyl-19-norcholesterol and NP59-SPECT/CT) following dexamethasone suppression has been proposed (Citation63), but its application is limited geographically and lacks reliability. Recently, Burton et al. used 11C-metomidate positron-emission tomography computed tomography (PET-CT) with dexamethasone suppression to identify lateralization in PA patients (Citation64). They achieved a sensitivity of 76% and specificity of 87% for differentiating between APAs and adrenal incidentaloma. This radionuclide imaging is promising, despite lower patient numbers in the report, and further validation studies are still awaited.
Special issues: when should we test for familial forms of PA?
Genetic testing for familial forms of PA can be considered in the following settings: patients with a family history of PA, patients with onset of PA at a younger age (< 20 years old), and patients with PA who have a family history of young stroke (Citation65).
Test for familial hyperaldosteronism type I (GRA)
To diagnose this rare disorder, several methods are available: measurement of urinary 18-hydroxycortisol or 18-oxo-cortisol, dexamethasone suppression testing (to demonstrate glucocorticoid suppressibility of aldosterone), and genetic testing for the chimeric gene. Genetic testing is the most highly sensitive and specific, but in light of its rarity, most investigators agree that not all patients with PA require genetic testing for GRA (Citation65).
Test for familial hyperaldosteronism type II
The molecular basis of this syndrome is unknown, but chromosomal region 7p22 has been implicated as an associated area. Hence, genetic testing is not clinically available to date.
Test for familial hyperaldosteronism type III
FH type III is a very new disease entity, and an ion channel (KCNJ5) has been implicated as one potential origin (Citation35). There is no other available confirmatory test for this subtype of FH currently. Treatment with bilateral adrenalectomy has been clinically beneficial in some cases (Citation35).
Treatment of PA
Treatment of PA is aimed at the prevention of morbidity and mortality stemming from hypertension, hypokalemia, and direct aldosterone-associated organ damage. Blood pressure reduction should not be the only goal in managing patients with PA, since MRs have been found in a variety of tissues including myocardium, brain, and blood vessels. The optimal treatment strategy depends upon the subtype of PA. The general principle is that patients with unilateral PA (i.e. APA or unilateral adrenal hyperplasia) should be offered unilateral laparoscopic adrenalectomy (Citation2). If patients are unwilling or unable to receive surgery, medical treatment with aldosterone antagonist should be attempted. For patients with PA from bilateral adrenal disease, aldosterone antagonists are the first-line choice (Citation2). An algorithm of case detection, confirmation, differential subtyping testing, and management of PA based on TAIPAI group experience is summarized ().
Patients should be well-informed of the potential benefits and the risks of operation. Well-corrected hypertension and hypokalemia preoperatively (e.g. with mineralocorticoid antagonist agents) decreases surgical risk (Citation2). Laparoscopic adrenalectomy is the preferred surgical approach, associated with shorter hospitalization and less morbidity compared with conventional open approaches. The surgical approach usually involves removal of the entire gland since nodules are commonly small and multiple in patients with unilateral PA, but laparoscopic partial adrenalectomy or enucleation has also been utilized, with shorter operative time but more blood loss (Citation66).
Unilateral adrenalectomy for APA and unilateral hyperplasia
Unilateral adrenalectomy in patients with unilateral PA can effectively improve hypertension and reverse hypokalemia in these patients. Hypertension is cured (defined as medication-free and blood pressure < 140/90 mmHg) in around 50%–80% of the operated patients with APA. In the TAIPAI registry, we found that patients with unilateral hyperaldosteronism receiving unilateral adrenalectomy displayed reversal of myocardial structure change (as evidenced in cardiac ultrasonographic findings) and reduction in carotid intimal thickness (Citation67). The temporal changes of glomerular filtration rate and proteinuria provide convincing evidence that the vasculotoxic effect of hyperaldosteronism can be mitigated after adrenalectomy more than through MR antagonist use (Citation68). However, whether this BP reduction combined with end-organ protection can be translated into improved survival is still yet unknown, although there is one report describing better long-term BP control and less health care spending with adrenalectomy compared with lifelong spironolactone use (Citation69).
Factors associated with resolution of hypertension postoperatively include having no first-degree relative with hypertension, preoperative use of less than three types of antihypertensive agents, lower preoperative serum creatinine level, and shorter duration of hypertension (Citation70). The most common reasons for persistent postoperative hypertension are advanced age, hypertension of unknown etiology, and longer preoperative hypertension (Citation70). Blood pressure typically normalizes or improves within 1–6 months after unilateral adrenalectomy, but some reports indicate that this improvement can lag up to 1 year or more (Citation2).
Mineralocorticoid receptor (MR) antagonists
Bilateral adrenal disease (IHA, bilateral APAs, and GRA) are often managed medically with aldosterone antagonists. However, no randomized placebo-controlled trials have evaluated the efficacy of each drug in the treatment of PA.
Spironolactone
This drug has been the treatment of choice for PA for more than four decades. The dosage is 12.5 mg to 25 mg per day initially and titrated upward to 50–100 mg per day if necessary to achieve normokalemia without potassium supplementation. However, side-effects may occur in high doses. The response of hypokalemia usually occurs promptly, but maximal BP response may take months to occur (Citation15). Observational studies in patients with IHA have reported a mean reduction in systolic BP of 25% and diastolic BP of 22% (Citation71). Most patients require as little as 25–50 mg spironolactone per day after several months of therapy. Serum potassium and creatinine should be monitored frequently during the first 4–6 weeks of treatment, especially in patients with abnormal renal function.
Side-effects of spironolactone include gynecomastia, decreased libido, and erectile dysfunction in men, or menstrual abnormality in women, an effect originating from concomitant activity on sex steroid receptors (Citation28). Gynecomastia is dose-related, with reported incidence of 5%–10% at spironolactone doses of 25–50 mg per day for 6 months, but increasing to 50% if patients receive more than 150 mg per day (Citation72). Hyperkalemia is a potentially life-threatening adverse effect of spironolactone, and is more likely in patients with clinically significant renal insufficiency or who are receiving other drugs which promote potassium retention including ACE inhibitors, ARBs or non-steroidal anti-inflammatory agents. In addition, spironolactone can increase the half-life of digoxin, and digoxin dosage should be adjusted when used simultaneously (Citation15). Salicylate also interferes with tubular secretion of the active metabolite of spironolactone, decreasing its efficacy (Citation15).
Eplerenone
Eplerenone is a more selective MR antagonist, with minimal anti-androgen and progesterone agonist effects and lower incidence of adverse effect (Citation2). The main structural modification leading to low androgenic and progesterone effect resides in the 9,11-epoxide group. It is currently approved for treating heart failure after myocardial infarction and, in selected countries, for treating essential hypertension (Citation73). Although side-effects may be reduced, its potency is lower than spironolactone by 25%–50%. It is reasonable to start with a dosage of 25 mg twice daily (owing to the short half-life of eplerenone), and titrating slowly upwards to achieve normokalemia and for greater blood pressure-lowering effect. The maximal dosage for hypertension is 100 mg twice daily.
Recently, in a randomized trial comparing eplerenone and spironolactone in treating patients with PA, Parthasarathy et al. found that spironolactone outperformed eplerenone with regard to antihypertensive efficacy (Citation74). Furthermore, eplerenone is more costly than spironolactone, and is not available in several countries for treatment of PA. Consequently, the clinical judgment weighing side-effects against effectiveness and availability still needs scrutinization. The precautions for eplerenone are similar to spironolactone, including hyperkalemia (serum potassium > 5.5 mEq/L) and clinically significant renal insufficiency (serum creatinine > 2.0 mg/dL (176 μmol/L) in men; and > 1.8 mg/dL (158 μmol/L) in women). Side-effects include dizziness, headache, fatigue, diarrhea, abnormal liver enzymes, and hypertriglyceridemia (Citation15).
Other pharmacologic agents
Amiloride and triamterene are distal sodium epithelial channel antagonists, and amiloride in particular has been studied most extensively in patients with PA. Amiloride can ameliorate both hypertension and hypokalemia, and is well-tolerated, without the notorious side-effects of spironolactone. However, it appears to lack the beneficial effects on endothelial function (Citation75).
Conclusion
Once considered a rare disorder with very low prevalence among unselected hypertensive patients, it is now realized that PA may constitute up to 5–15% of patients with hypertension. More importantly, the harm incurred by aldosterone to a variety of tissues may go far beyond hypertension. Early treatment, whether through surgical (adrenalectomy) or medical (aldosterone antagonists) modalities, can effectively ameliorate these adverse effects. It is important that physicians recognize this disorder early and arrange appropriate diagnostic testing.
Acknowledgements
Taiwan Primary Aldosteronism Investigation (TAIPAI) Study Group: Vin-Cent Wu, MD, Yen-Hung Lin, MD, Yi-Luwn Ho, MD, PhD, Hung-Wei Chang, MD, PhD, Lian-Yu Lin, Kao-Lang Liu, MD, Shuo-Meng Wang, MD, Kuo-How Huang, MD, Yung-Ming Chen , MD, Chin-Chi Kuo, MD, Chin-Chen Chang, MD, Shih-Chieh Chueh, MD, PhD, Ching-Chu Lu, MD , Shih-Cheng Liao, MD, Ruoh-Fang Yen , MD, PhD, Wei-Chou Lin, MD, PhD, Bor-Sen Hsieh , MD, PhD, and Kwan-Dun Wu, MD, PhD.
Declaration of interest: The authors report no conflicts of interest. This study was supported by The Ta-Tung Kidney Foundation, Taiwan National Science Council (grant NSC 96-2314-B-002-164, grant NSC 96-2314-B-002-033- MY3, and grant NSC 97-2314-B-002-155-MY2), 98-N1177, 99-N1408, 100-N1776 from NTUH, and NTUH-TVGH Joint Research Program (VN9803, VN9906 and VN10009).
References
- Conn J. Presidential address. Part II. Primary aldosteronism, a new clinical syndrome. J Lab Clin Med. 1955;45:6–17.
- Funder JW, Carey RM, Fardella C, Gomez-Sanchez CE, Mantero F, Stowasser M, et al. Case detection, diagnosis, and treatment of patients with primary aldosteronism: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2008;93:3266–81.
- Hiramatsu K, Yamada T, Yukimura Y. A screening test to identify aldosterone-producing adenoma by measuring plasma renin activity: results in hypertensive patients. Arch Intern Med. 1981;141:1589–93.
- Williams JS, Williams GH, Raji A, Jeunemaitre X, Brown NJ, Hopkins PN, et al. Prevalence of primary hyperaldosteronism in mild to moderate hypertension without hypokalaemia. J Hum Hypertens. 2005;20:129–36.
- Amar L, Plouin P-F, Steichen O. Aldosterone-producing adenoma and other surgically correctable forms of primary aldosteronism. Orphanet J Rare Dis. 2010;5:9.
- Hannemann A, Wallaschofski H. Prevalence of primary aldosteronism in patient’s cohorts and in population-based studies - a review of the current literature. Horm Metab Res. 2012;44:157–62.
- Rossi GP, Bernini G, Caliumi C, Desideri G, Fabris B, Ferri C, et al. A prospective study of the prevalence of primary aldosteronism in 1,125 hypertensive patients. J Am Coll Cardiol. 2006;48:2293–300.
- Chang HW, Chu TS, Huang HY, Chueh SC, Wu VC, Chen YM, et al. Down-regulation of D2 dopamine receptor and increased protein kinase Cmu phosphorylation in aldosterone-producing adenoma play roles in aldosterone overproduction. J Clin Endocrinol Metab. 2007;92:1863–70.
- Tomaschitz A, Pilz S, Ritz E, Meinitzer A, Boehm BO, Marz W. Plasma aldosterone levels are associated with increased cardiovascular mortality: the Ludwigshafen Risk and Cardiovascular Health (LURIC) study. Eur Heart J. 2010;31:1237–47.
- Rocha R, Funder JW. The pathophysiology of aldosterone in the cardiovascular system. Ann N Y Acad Sci. 2002;970:89–100.
- Milliez P, Girerd X, Plouin P-F, Blacher J, Safar ME, Mourad J-J. Evidence for an increased rate of cardiovascular events in patients with primary aldosteronism. J Am Coll Cardiol. 2005;45:1243–8.
- Wu VC, Lo SC, Chen YL, Huang PH, Tsai CT, Liang CJ, et al. Endothelial progenitor cells in primary aldosteronism: a biomarker of severity for aldosterone vasculopathy and prognosis. J Clin Endocrinol Metab. 2011;96:3175–83.
- Kuo CC, Wu VC, Tsai CW, Wu KD. Relative kidney hyperfiltration in primary aldosteronism: a meta-analysis. J Renin Angiotensin Aldosterone Syst. 2011;12:113–22.
- Sechi LA, Di Fabio A, Bazzocchi M, Uzzau A, Catena C. Intrarenal hemodynamics in primary aldosteronism before and after treatment. J Clin Endocrinol Metab. 2009;94:1191–7.
- Young WF. Primary aldosteronism: renaissance of a syndrome. Clin Endocrinol (Oxf). 2007;66:607–18.
- Lee P-H, Wu C-J, Chen Y-C, Chen H-H. Aldosterone-producing adenoma: clinical presentation, diagnosis and outcomes of surgery in Northern Taiwan. Acta Nephrol. 2009;23:143–8.
- Omura M, Sasano H, Saito J, Yamaguchi K, Kakuta Y, Nishikawa T. Clinical characteristics of aldosterone-producing microadenoma, macroadenoma, and idiopathic hyperaldosteronism in 93 patients with primary aldosteronism. Hypertens Res. 2006;29:883–9.
- Kuo C-C, Wu V-C, Huang K-H, Wang S-M, Chang C-C, Lu C-C, et al. Verification and evaluation of aldosteronism demographics in the Taiwan Primary Aldosteronism Investigation Group (TAIPAI Group). J Renin Angiotensin Aldosterone Syst. 2011;12:348–57.
- Sato A, Saruta T. Aldosterone-induced organ damage: plasma aldosterone level and inappropriate salt status. Hypertens Res. 2004;27: 303–10.
- Chhokar VS, Sun Y, Bhattacharya SK, Ahokas RA, Myers LK, Xing Z, et al. Hyperparathyroidism and the calcium paradox of aldosteronism. Circulation. 2005;111:871–8.
- Rossi GP. Hyperparathyroidism, arterial hypertension and aortic stiffness: a possible bidirectional link between the adrenal cortex and the parathyroid glands that causes vascular damage? Hypertens Res. 2011;34:286–8.
- Ito Y, Takeda R, Takeda Y. Subclinical primary aldosteronism. Best Pract Res Clin Endocrinol Metab. 2012;26:485–95.
- Médeau V, Moreau F, Trinquart L, Clemessy M, Wémeau J-L, Vantyghem MC, et al. Clinical and biochemical characteristics of normotensive patients with primary aldosteronism: a comparison with hypertensive cases. Clin Endocrinol. 2008;69:20–8.
- Fallo F, Pilon C, Urbanet R. Primary aldosteronism and metabolic syndrome. Horm Metab Res. 2012;44:208–14.
- Rocha R, Rudolph AE, Frierdich GE, Nachowiak DA, Kekec BK, Blomme EAG, et al. Aldosterone induces a vascular inflammatory phenotype in the rat heart. Am J Physiol Heart Circ Physiol. 2002; 283:H1802–10.
- Bernini G, Galetta F, Franzoni F, Bardini M, Taurino C, Bernardini M, et al. Arterial stiffness, intima-media thickness and carotid artery fibrosis in patients with primary aldosteronism. J Hypertens. 2008;26: 2399–405.
- Sonino N, Tomba E, Genesia ML, Bertello C, Mulatero P, Veglio F, et al. Psychological assessment of primary aldosteronism: a controlled study. J Clin Endocrinol Metab. 2011;96:E878–83.
- Mattsson C, Young WF. Primary aldosteronism: diagnostic and treatment strategies. Nat Clin Pract Nephrol. 2006;2:198–208.
- Rayner B. Primary aldosteronism and aldosterone-associated hypertension. J Clin Pathol. 2008;61:825–31.
- Seccia TM, Fassina A, Nussdorfer GG, Pessina AC, Rossi GP. Aldosterone-producing adrenocortical carcinoma: an unusual cause of Conn's syndrome with an ominous clinical course. Endocrine Relat Cancer. 2005;12:149–59.
- McMahon GT, Dluhy RG. Glucocorticoid-remediable aldosteronism. Cardiol Rev. 2004;12:44–8.
- Stowasser M, Gordon RD. Primary aldosteronism: from genesis to genetics. Trend Endocrinol Metab. 2003;14:310–7.
- So A, Duffy DL, Gordon RD, Jeske YW, Lin-Su K, New MI, et al. Familial hyperaldosteronism type II is linked to the chromosome 7p22 region but also shows predicted heterogeneity. J Hypertens. 2005;23: 1477–84.
- Geller DS, Zhang J, Wisgerhof MV, Shackleton C, Kashgarian M, Lifton RP. A novel form of human mendelian hypertension featuring nonglucocorticoid-remediable aldosteronism. J Clin Endocrinol Metab. 2008;93:3117–23.
- Mulatero P, Tauber P, Zennaro M-C, Monticone S, Lang K, Beuschlein F, et al. KCNJ5 Mutations in European families with nonglucocorticoid remediable familial hyperaldosteronism. Hypertension. 2012;59:235–40.
- Choi M, Scholl UI, Yue P, Björklund P, Zhao B, Nelson-Williams C, et al. K+ channel mutations in adrenal aldosterone-producing adenomas and hereditary hypertension. Science.Science. 2011;331:768–72.
- McEwan PE, Lindop GB, Kenyon CJ. Control of cell proliferation in the rat adrenal gland in vivo by the renin-angiotensin system. Am J Physiol Endocrinol Metab. 1996;271:E192–8.
- Stowasser M, Gordon RD, Gunasekera TG, Cowley DC, Ward G, Archibald C, et al. High rate of detection of primary aldosteronism, including surgically treatable forms, after ‘non-selective’ screening of hypertensive patients. J Hypertens. 2003;21:2149–57.
- Sealey JE, Laragh JH. Radioimmunoassay of plasma renin activity. Semin Nucl Med. 1975;5:189–202.
- Ferrari P, Shaw SG, Nicod J, Saner E, Nussberger J. Active renin versus plasma renin activity to define aldosterone-to-renin ratio for primary aldosteronism. J Hypertens. 2004;22:377–81.
- Olivieri O, Ciacciarelli A, Signorelli D, Pizzolo F, Guarini P, Pavan C, et al. Aldosterone to renin ratio in a primary care setting: the Bussolengo Study. J Clin Endocrinol Metab. 2004;89:4221–6.
- Abdelhamid S, Blomer R, Hommel G, Haack D, Lewicka S, Fiegel P, et al. Urinary tetrahydroaldosterone as a screening method for primary aldosteronism: a comparative study. Am J Hypertens. 2003;16:522–30.
- Mourad J-J, Girerd X, Milliez P, Lopez-Sublet M, Lejeune S, Safar ME. Urinary aldosterone-to-active-renin ratio: a useful tool for predicting resolution of hypertension after adrenalectomy in patients with aldosterone-producing adenomas. Am J Hypertens. 2008;21:742–47.
- Bernini G, Moretti A, Orlandini C, Berti P, Miccoli P, Bardini M, et al. Plasma and urine aldosterone to plasma renin activity ratio in the diagnosis of primary aldosteronism. J Hypertens. 2008;26:981–8.
- Yamashita K, Okuyama M, Nakagawa R, Honma S, Satoh F, Morimoto R, et al. Development of sensitive derivatization method for aldosterone in liquid chromatography–electrospray ionization tandem mass spectrometry of corticosteroids. J Chromatogr A. 2008;1200:114–21.
- Chappell DL, McAvoy T, Weiss B, Weiner R, Laterza OF. Development and validation of an ultra-sensitive method for the measurement of plasma renin activity in human plasma via LC–MS/MS. Bioanalysis. 2012;4:2843–50.
- Taylor PJ, Gordon RD, Stowasser M. Calibrators for measuring aldosterone by liquid chromatography-tandem mass spectrometry. Clin Chim Acta. 2012;413:346–7.
- Taylor PJ, Cooper DP, Gordon RD, Stowasser M. Measurement of aldosterone in human plasma by semiautomated HPLC–tandem mass spectrometry. Clin Chem. 2009;55:1155–62.
- Seifarth C, Trenkel S, Schobel H, Hahn EG, Hensen J. Influence of antihypertensive medication on aldosterone and renin concentration in the differential diagnosis of essential hypertension and primary aldosteronism. Clin Endocrinol. 2002;57:457–65.
- Grasko JM, Nguyen HH, Glendenning P. Delayed diagnosis of primary hyperaldosteronism. BMJ. 2010;340:c2461.
- Pizzolo F, Pavan C, Corrocher R, Olivieri O. Laboratory diagnosis of primary aldosteronism, and drospirenone-ethinylestradiol therapy. Am J Hypertens. 2007;20:1334–7.
- Mulatero P, Stowasser M, Loh K-C, Fardella CE, Gordon RD, Mosso L, et al. Increased diagnosis of primary aldosteronism, including surgically correctable forms, in centers from five continents. J Clin Endocrinol Metab. 2004;89:1045–50.
- Hannemann A, Friedrich N, Lüdemann J, Völzke H, Rettig R, Peters J, et al. Reference intervals for aldosterone, renin, and the aldosterone-to-renin ratio in the population-based Study of Health in Pomerania (SHIP-1). Horm Metab Res. 2010;42:392–9.
- Montori VM, Schwartz GL, Chapman AB, Boerwinkle E, Turner ST. Validity of the aldosterone-renin ratio used to screen for primary aldosteronism. Mayo Clin Proc. 2001;76:877–82.
- Agharazii M, Douville P, Grose JH, Lebel M. Captopril suppression versus salt loading in confirming primary aldosteronism. Hypertension. 2001;37:1440–3.
- Mulatero P, Bertello C, Garrone C, Rossato D, Mengozzi G, Verhovez A, et al. Captopril test can give misleading results in patients with suspect primary aldosteronism. Hypertension. 2007;50:e26–7.
- Hambling C, Jung RT, Gunn A, Browning MC, Bartlett WA. Re-evaluation of the captopril test for the diagnosis of primary hyperaldosteronism. Clin Endocrinol (Oxf). 1992;36:499–503.
- Wu VC, Chueh SC, Chang HW, Lin WC, Liu KL, Li HY, et al. Bilateral aldosterone-producing adenomas: differentiation from bilateral adrenal hyperplasia. QJM. 2008;101:13–22.
- Küpers EM, Amar L, Raynaud A, Plouin P-F, Steichen O. A clinical prediction score to diagnose unilateral primary aldosteronism. J Clin Endocrinol Metab. 2012;97:3530–7.
- Young Jr WF, Stanson AW, Thompson GB, Grant CS, Farley DR, van Heerden JA. Role for adrenal venous sampling in primary aldosteronism. Surgery. 2004;136:1227–35.
- Daunt N. Adrenal vein sampling: how to make it quick, easy, and successful. Radiographics. 2005;25:S143–58.
- Rossi GP, Sacchetto A, Chiesura-Corona M, De Toni R, Gallina M, Feltrin GP, et al. Identification of the etiology of primary aldosteronism with adrenal vein sampling in patients with equivocal computed tomography and magnetic resonance findings: results in 104 consecutive cases. J Clin Endocrinol Metab. 2001;86:1083–90.
- Yen RF, Wu VC, Liu KL, Cheng MF, Wu YW, Chueh SC, et al. 131I-6beta-iodomethyl-19-norcholesterol SPECT/CT for primary aldosteronism patients with inconclusive adrenal venous sampling and CT results. J Nucl Med. 2009;50:1631–7.
- Burton TJ, Mackenzie IS, Balan K, Koo B, Bird N, Soloviev DV, et al. Evaluation of the sensitivity and specificity of 11C-metomidate positron emission tomography (PET)-CT for lateralizing aldosterone secretion by Conn's adenomas. J Clin Endocrinol Metab. 2012;97:100–9.
- Gates LJ, Benjamin N, Haites NE, MacConnachie AA, McLay JS. Is random screening of value in detecting glucocorticoid-remediable aldosteronism within a hypertensive population? J Hum Hypertens. 2001;15:173–6.
- Fu B, Zhang X, Wang G-X, Lang B, Ma X, Li H-Z, et al. Long-term results of a prospective, randomized trial comparing retroperitoneoscopic partial versus total adrenalectomy for aldosterone producing adenoma. J Urol. 2011;185:1578–82.
- Lin Y-H, Lee H-H, Liu K-L, Lee J-K, Shih S-R, Chueh S-C, et al. Reversal of myocardial fibrosis in patients with unilateral hyperaldosteronism receiving adrenalectomy. Surgery. 2011;150:526–33.
- Wu V-C, Kuo C-C, Wang S-M, Liu K-L, Huang K-H, Lin Y-H, et al. Primary aldosteronism: changes in cystatin C-based kidney filtration, proteinuria, and renal duplex indices with treatment. J Hypertens. 2011;29:1778–86.
- Sywak M, Pasieka JL. Long-term follow-up and cost benefit of adrenalectomy in patients with primary hyperaldosteronism. Br J Surg. 2002;89:1587–93.
- Sawka AM, Young WF, Thompson GB, Grant CS, Farley DR, Leibson C, et al. Primary aldosteronism: factors associated with normalization of blood pressure after surgery. Ann Intern Med. 2001;135: 258–61.
- Kater CE, Biglieri EG, Schambelan M, Arteaga E. Studies of impaired aldosterone response to spironolactone-induced renin and potassium elevations in adenomatous but not hyperplastic primary aldosteronism. Hypertension. 1983;5:V115–21.
- Jeunemaitre X, Chatellier G, Kreft-Jais C, Charru A, Devries C, Plouin P-F, et al. Efficacy and tolerance of spironolactone in essential hypertension. Am J Cardiol. 1987;60:820–5.
- Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B, et al. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003;348:1309–21.
- Parthasarathy HK, Ménard J, White WB, Young WFJ, Williams GH, Williams B, et al. A double-blind, randomized study comparing the antihypertensive effect of eplerenone and spironolactone in patients with hypertension and evidence of primary aldosteronism. J Hypertens. 2011;29:980–90.
- Farquharson CAJ, Struthers AD. Spironolactone Increases nitric oxide bioactivity, improves endothelial vasodilator dysfunction, and suppresses vascular angiotensin I/angiotensin II conversion in patients with chronic heart failure. Circulation. 2000;101:594–7.
