Abstract
Introduction. Prenatal and childhood growth influence the risk of developing the metabolic syndrome and type 2 diabetes. Both conditions are associated with non-alcoholic fatty liver disease (NAFLD). Our aim was to explore the associations between early growth and adult NAFLD.
Methods. We studied 1587 individuals from the Helsinki Birth Cohort Study (HBCS) born 1934–44 for whom birth, childhood, and adult clinical data were available. NAFLD was defined using the NAFLD liver fat score and equation. The score was converted into a dichotomous variable, with outcomes defined as either a positive or negative score. The equation predicts liver fat percentage.
Results. A positive score was found in 43% of men and 22.5% of women. Several measurements of birth and childhood body size were negatively associated with both NAFLD outcomes after adjustment for adult BMI. Those from the smallest BMI tertile at age 2 who were obese in adulthood had an OR of 18.5 for a positive score compared to those from the same group who were normal weight in adulthood.
Conclusions. A larger childhood body size was negatively associated with NAFLD outcomes. Individuals who are small during early childhood and obese as adults seem to be at the highest risk of developing NAFLD.
Key messages
A small body size during infancy and childhood is associated with a higher likelihood of adult non-alcoholic liver fatty disease (NAFLD) and a higher liver fat percentage, as defined by the NAFLD liver fat score and equation.
The most pronounced results were seen among those who were among the smallest at age 2 and subsequently obese in adulthood.
Introduction
The Developmental Origins of Health and Disease (DOHaD) hypothesis posits that many non-communicable diseases have their origins in utero and during early childhood. The intrauterine environment—influenced by maternal diet, hormonal factors, body composition, and placental function—affects the developing foetus via a number of pathways, among them hormonal and epigenetic signalling, resulting in either favourable or adverse adult health outcomes (Citation1). Cardiovascular disease, type 2 diabetes, stroke, hypertension, and the metabolic syndrome (MetS) are all associated with a small body size at birth—a sign of non-optimal intrauterine growth (Citation2–4). Childhood growth trajectories further modify these associations, where a small body size at birth and slow growth during infancy, followed by a more than adequate compensatory weight gain in childhood, is associated with higher incidences of coronary heart disease and type 2 diabetes (Citation5–8).
Non-alcoholic fatty liver disease (NAFLD) is defined by a liver fat content exceeding 5%–10% of liver mass in individuals without excessive alcohol intake or other liver disease (Citation9). The spectrum of disorders encompassed by NAFLD ranges from simple steatosis (SS) to non-alcoholic steatohepatitis (NASH) and cirrhosis (Citation10). NAFLD is one of the most common causes of chronic liver disease worldwide with a prevalence of 20%–30% in Western countries (Citation11). It is closely associated with MetS, type 2 diabetes, and obesity (Citation12,Citation13) and is often proposed as being the hepatic manifestation of MetS (Citation14,Citation15) with insulin resistance as the central pathogenic mechanism. The pathogenesis of NAFLD has been described elsewhere in detail (Citation10,Citation12,Citation14,Citation16).
To our knowledge, to date there have only been a handful of studies exploring the developmental origins of NAFLD. One study, conducted in a paediatric population, showed that intrauterine growth retardation (IUGR) was positively associated with NAFLD (Citation17). Another study reported a negative association between birth weight and serological markers of liver disease in adulthood (Citation18). Furthermore, animal studies have linked both suboptimal maternal nutrition (Citation19), as well as maternal high-fat feeding (Citation20,Citation21), with hepatic lipid accumulation. Insulin resistance is associated with both IUGR and early catch-up growth (Citation22), a mechanism through which NAFLD might have its origins during early life. The aim of this study was to explore the associations between birth size, early growth, and NAFLD in adulthood.
Subjects and methods
The Helsinki Birth Cohort Study comprises 8,760 individuals born as singletons between 1934 and 1944 at the Helsinki University Central Hospital who were still alive and resident in Finland in 1971 when every citizen received a unique personal identification number. For each individual, a detailed set of birth records are available, including data on birth weight, height, and head circumference, as well as mother's age, weight, parity, and last menstrual period. Childhood weight and height measurements between 2 and 11 years are available from child welfare and school records. An average of 18 height and weight measurements were recorded between birth and 12 years of age (Citation23). Birth and childhood records have been described elsewhere in detail (Citation5,Citation24). Between 2001 and 2004, a subsample of the cohort (n = 2,003) underwent a clinical study including anthropometry and laboratory sampling. Current social status, based on occupation, was derived from census data in 1980 whereas childhood social status was based on father's occupation, as recorded in child welfare clinic records (Citation23).
The study participants attended the clinic after an overnight fast. Weight was measured to the nearest 0.1 kg and height to the nearest 0.1 cm. BMI was calculated as kg/m2. Blood samples included a standard 75 g oral glucose tolerance test. Plasma glucose was measured by a hexokinase method (Citation25), and plasma insulin was determined by a two-site immunometric assay (Citation26). Serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT) concentrations were measured by using photometric International Federation of Clinical Chemistry (IFCC) methods. A team of three trained research nurses performed all measurements.
Information on medical history and current medication was obtained using standardized questionnaires at the clinic. In addition, information on educational attainment, smoking, and leisure-time physical activity was obtained from self-administered questionnaires. Subjects were defined as physically active if they exercised at least moderately three times a week or more. Moderate exercise was defined as brisk walking or comparable activity. A food frequency questionnaire (FFQ) was used to collect data on diet, alcohol intake, and total caloric intake. Diabetes diagnosis was based on current diabetes medication and/or the WHO 1999 criteria for diabetes (Citation27). Diagnosis of MetS was based on the 2009 harmonized criteria (Citation28).
In Finland, all hospital admissions are recorded in the national hospital discharge register and all deaths in the national mortality register. To identify subjects with current or previous liver disease, we performed a search for relevant ICD codes in both these registers (ICD 8–9: 070, 155, 291, 303, 570, 571, 572, 573; ICD 10: B15–19, C22, F10, G62, K70–77, K86), in total identifying and excluding 80 subjects, 71 with alcohol-related disease. No cases of explicit non-alcoholic steatosis or steatohepatitis were found in either register. Another 54 subjects were excluded based on American Association for the Study of Liver Disease (AASLD) guidelines on diagnosis and management of NAFLD due to medication associated with secondary hepatic steatosis (Citation29). A further 31 subjects were excluded due to morbid obesity (BMI > 40 kg/m2). Of the remaining 1,838 subjects, 1,592 had a reported alcohol intake < 20 g/day (FFQ data), and 1,587 of these had all required data available for calculating the NAFLD liver fat score and equation (Citation30). Characteristics of these individuals are presented in .
Table I. Characteristics of the study participants.
Detection of NAFLD
As no imaging studies or liver biopsy were performed during the clinical study, the NAFLD liver fat score and equation were utilized to detect NAFLD in the study population.
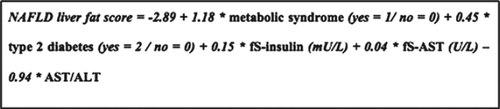
The NAFLD liver fat score
The NAFLD liver fat score () is based on five variables: presence of MetS, presence of type 2 diabetes, fasting serum (fS)-insulin, fS-AST, and AST/ALT ratio. Values greater than the optimal cut-off point of –0.640 predicted NAFLD with a sensitivity of 86% and a specificity of 71% against diagnosis by proton-magnetic resonance spectroscopy (MRS) in the original study population (Citation30). For the purpose of the current study, we defined values greater than –0.640 as a positive NAFLD score.
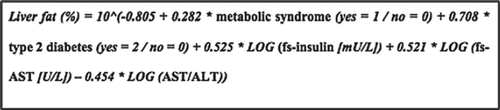
The NAFLD liver fat equation
The liver fat equation () includes the same variables as the liver fat score and allows prediction of liver fat percentage. The correlation coefficient between liver fat as predicted by the equation and measured by proton-MRS was 0.70 (P < 0.001) in the original study cohort (Citation30).
Ethical statement
The study was conducted according to the guidelines in the Declaration of Helsinki, and approved by the Ethics Committee of Hospital District of Helsinki and Uusimaa. Written informed consent was obtained from all subjects.
Statistical methods
The statistical analysis included tabulation of means, as well as multiple logistic and linear regression. The growth measurements for each individual were converted to a z score (SD score) representing the number of standard deviations the observation differed from the mean of the whole study group. The z score was obtained by interpolation as the children were not measured at exactly their birthday. The residuals from linear regression analysis, referred to as conditional growth, were used to measure how much size at any age differed from that predicted by the body size at an earlier age. For consistency with previous studies from this group, measurements at birth, as well as 2, 7, and 11 years, were used in the analysis. For the conditional analyses, the intervals of 0–2, 2–7, and 7–11 years were used. For childhood BMI, an additional variable for growth between 2 and 11 was calculated.
The NAFLD liver fat score was calculated for all 1,587 study participants and converted into a dichotomous variable, with values > –0.640 defined as a positive NAFLD score. The variable was entered into a series of multiple logistic regression models as the dependent variable. Age at clinical examination, gestational age, and sex were included as covariates, as well as one birth or childhood growth measurement per model. Data on all of the above-mentioned variables were available for 1,435 participants, making up the study population for the regression analyses. To account for adult body size, adult BMI was added to the models. A model including birth weight, sex, age, gestational age, and adult BMI was compared with a full model also including the product term of BMI and sex to control for statistical interaction. An omnibus test of model coefficients revealed a significance level of 0.04 for the full model. Significant interaction was found between sex and BMI (P for interaction 0.04). Accordingly, all models including BMI were stratified by sex. Additional adjustments for current and previous smoking, current and childhood social status, physical activity, and total caloric intake were explored but yielded no additional information compared to the BMI models. To control for the combined effects of childhood and adult body size, a model including BMI at 2 years of age and adult BMI was created (the former divided into tertiles, the latter into conventional groups (< 25, 25–30, > 30 kg/m2)). This model was adjusted by sex and age. Additional adjustment for gestational age was explored but yielded no additional information.
Liver fat per cent was calculated for all participants using the liver fat equation. Its distribution was skewed and therefore log-transformed and subsequently entered into a series of multiple linear regression models with adjustments as described for the logistic regressions (n = 1,435 included in linear regression). A model adjusting for sex, age, gestational age, birth weight, and BMI was compared with a full model also including the product term of BMI and sex to control for interaction. The significant F change between the reduced and the full model was 0.001 and the interaction term between sex and BMI 0.001. All BMI-adjusted models were stratified by sex, in accordance with the logistic regression models. As with the liver fat score analyses, no additional information was gained from adjusting for current or previous smoking or social status, physical activity, or total caloric intake.
For reasons of comparison, we also calculated the fatty liver index (FLI), an alternative algorithm test developed by Bedogni et al. (Citation31). FLI is calculated based on BMI, waist circumference, fasting triglycerides, and fasting gamma-glutamyl-transferase (GGT). A FLI > 60 predicted NAFLD with a specificity of 86% and a positive likelihood ratio of 4.3 in the original publication.
All statistical analyses were performed using SPSS version 21 software (SPSS, Inc., Chicago, IL).
Results
shows baseline characteristics of the study population, including childhood and adult anthropometrics as well as components of the NAFLD algorithms and MetS. The mean age of male subjects was 61.6 (SD 2.8) and of female subjects 61.6 (SD 3.0) years. Mean body mass index was 27.3 (SD 3.7) for men and 27.4 (SD 4.4) kg/m2 for women. A positive NAFLD score was found in 43% of men and 22.5% of women. The FLI produced even higher prevalence figures (50.4% among men, 32.6% among women, respectively). Median liver fat per cent, according to the fatty liver equation, was 4.2 (interquartile range 2.5; 6.9) among men and 3.0 (interquartile range 1.9; 5.3) among women. Diabetes was present in 17.6% of the men and 11.1% of the women. The metabolic syndrome was present in 56.7% of the men and 45.9% of the women.
Anthropometric measurements at birth and early childhood and NAFLD
Body weight at 2 years was negatively associated with the dichotomous NAFLD variable when adjusting for sex, age, and gestational age (model 1, ). After additional adjustment for adult BMI and stratification by sex, this association remained significant in both sexes. Additionally, each unit of BMI at 2 years was inversely associated with the dichotomous variable in both sexes (model 2, ).
Table II. Odds ratios and 95% confidence intervals for a positive NAFLD liver fat score per unit of birth and childhood anthropometric measurements.
Liver fat per cent was negatively associated with height and weight at 2 years in a model controlling for sex, age, and gestational age (model 1, ). After adding adult BMI to the regression and stratifying by sex, the association with weight at 2 years remained significant in both sexes, and several other associations with the dependant variable were revealed (model 2, ), for example with birth weight among female subjects. For a 1 kg increase in birth weight, liver fat per cent decreased (N. B.: relative decrease) 11.5% (95% CI –18.9; –3.2).
Table III. Relative per cent change of liver fat per cent per unit of birth and childhood anthropometric measurements.
Later childhood anthropometrics and NAFLD
shows the associations between later childhood anthropometrics and the dichotomous NAFLD variable. In the final model, weight and BMI at 7 and 11 years were all significantly negatively associated with the outcome variable among men, whereas no significant associations were seen in women. Liver fat per cent was negatively associated with several childhood anthropometrics in both sexes, most strongly with BMI at 7 years among men.
Conditional growth
and show the associations between the conditional growth variables and both NAFLD variables, respectively. Several significant negative associations were found for both outcomes in the final models. Of note is that the association between conditional weight growth from birth to 2 years of age and the dichotomous NAFLD is negative also in the model unadjusted for adult BMI.
Table IV. Odds ratios and 95% confidence intervals for a positive NAFLD liver fat score per SD of conditional growth.
Table V. Relative per cent change of liver fat per cent per SD of conditional growth.
BMI at 2 years versus adult BMI
shows the OR for a positive NAFLD score in a 3 × 3 table stratified by BMI at 2 years of age, adult BMI, and adjusted for sex and age. The reference was set as those who were leanest both at 2 years and in adulthood. Those who belonged to the group who were smallest at 2 years and subsequently largest in adulthood had an OR of 18.5 (95% CI 10.1; 33.6) for a positive NAFLD score compared to the reference group.
Table VI. Odds ratiosa and 95% confidence intervals for a positive NAFLD liver fat score per tertile of BMI at age 2 and conventional BMI groups in adulthood.
Discussion
We have explored the associations between early growth and NAFLD in adulthood, as defined by the NAFLD liver fat score and equation. When adjusting for adult body size, all significant associations between birth and childhood anthropometric measurements and the NAFLD outcomes were negative. For the early childhood anthropometrics, the associations with liver fat per cent were somewhat more pronounced among female subjects. A significant association with birth weight was only seen in women. One potential explanation could be the higher prevalence of both diabetes and MetS among the male subjects—something that could potentially ‘overshadow’ the impact of early growth and especially birth weight among the men. One might also note that the association between liver fat and birth weight among men, albeit not statistically significant at a 0.05 level, is still moving in the same direction and of the same magnitude as for the female subjects.
Previous studies conducted in our cohort have shown that growth patterns during childhood further affect the risk of adult disease. A small body size at birth followed by a later increase in body size is associated with coronary heart disease, and adiposity rebound at an early age is associated with an increased risk of diabetes mellitus (Citation8). In the current study, no such results were found. Instead, all significant associations between conditional growth variables and the NAFLD outcomes were negative in the final models. Theoretically this might be due to the fact that very few of the study participants were overweight during childhood. Based on this, one could assume that many of those with NAFLD developed it after the age of 11. To explore this further, we investigated the combined effect of BMI at 2 years and adult BMI, the former divided into tertiles, the latter into conventional groups, on the dichotomous NAFLD variable. An OR of 18.5 (95% CI 10.1; 33.6) for a positive NAFLD score was found among those who were leanest at 2 years of age and subsequently obese in adulthood. Indeed, more than two-thirds of this group had a positive score, indicating a very high prevalence of NAFLD. In comparison, those who were among the largest both at 2 years of age and in adulthood had an OR for a positive score of 8.8 (95% CI 5.0; 15.2) supporting the importance of not only adult obesity, but also childhood body size, and especially their mutual relation, in the development of NAFLD.
In our cohort, prevalence of MetS was 56.7% in men and 49.5% in women, respectively. Although high, these prevalence figures are similar to those seen in another recent Finnish epidemiological study. In the 2007 FIN-D2D survey (Citation32) prevalence of MetS was 60.1% in men and 54.3% in women aged 45–74 years. A positive NAFLD liver fat score was found in 43% of the male subjects and 22.5% of the female. As previously mentioned, the prevalence of NAFLD in Western countries is 20%–30%, and one could assume the prevalence in Finland would be within this range or perhaps somewhat higher in the current age group. One reason for the even higher prevalence among men in our cohort could be co-morbidity with MetS.
In the current study, we employed the NAFLD liver fat score and equation to define NAFLD. The gold diagnostic standard, liver biopsy, is the only method able to separate SS from NASH through determination of grade of inflammation, necrosis, and fibrosis (Citation33,Citation34). Despite being a safe method, it is inconvenient for the patient and associated with a small degree of procedure-related morbidity and rarely even mortality (Citation35). Imaging techniques, such as ultrasonography (US), computed tomography (CT), magnetic resonance imaging (MRI), and magnetic resonance spectroscopy (MRS) offer non-invasive means of diagnosing NAFLD but are limited to the assessment of liver fat content (Citation36). Additional shortcomings include the operator dependability of US, the ionizing radiation of CT, and the inherent contraindications of MRI and MRS. Furthermore, all of the methods mentioned above are costly and time-consuming, making them less than ideal for large epidemiological studies. The algorithm tests that have appeared during recent years are based on readily available biochemical and clinical markers and aim at facilitating NAFLD diagnosis and thereby study of the condition in larger groups of individuals (Citation37,Citation38).
Although a number of alternative algorithm tests are available, we chose to employ the NAFLD liver fat score and equation as they were developed on a Finnish population. The algorithms have been validated in a study with 125 participants (40 non-diabetic patients with biopsy-proven NAFLD and 85 healthy controls) where the liver fat score's optimal cut-off point of –0.640 diagnosed NAFLD with a specificity of 0.93 and sensitivity of 0.80 compared to liver biopsy. In the same study, the liver fat equation correlated with degree of histological steatosis in both NASH and SS groups (rs > 0.66, P < 0.003) as well as with validated risk factors of cardiovascular disease both in healthy subjects and in those with NAFLD (Citation39). The fatty liver score has also been utilized in other contexts: in a prospective study with 3,812 non-diabetic participants and a nine-year follow-up, fatty liver score was able to predict incident diabetes (Citation40). We also calculated the FLI, which produced even higher NAFLD prevalence figures than the NAFLD liver fat score. However, for the purposes of this publication, no further analyses were conducted using the FLI.
To our knowledge, this is the first time adult NAFLD has been studied from a DOHaD-perspective in a large epidemiological setting. There have, however, previously been studies linking early growth to NAFLD. A case-control study revealed an association between intrauterine growth retardation and NAFLD in a paediatric population, where children who were born small for gestational age had an increased risk for biopsy-verified NAFLD (Citation17). There have also been studies indirectly linking early development with NAFLD. In one study, examining British women aged 60–79 years, a 1-SD increase in birth weight was associated with a 2% decrease in geometric mean of ALT and alkaline phosphatase (ALP) and a 4% decrease in GGT after adjustments for social class, smoking, alcohol consumption, and physical activity (Citation18). Associations with ALT and GGT, but not associations with ALP, were attenuated after additional adjustments for components of MetS.
In a study conducted on sheep, suboptimal maternal nutrition during early foetal development was shown to be positively associated with an increased hepatic lipid accumulation in obese offspring compared to obese sheep that had not been exposed to intrauterine nutrient restriction (Citation19). A study on mice showed, among other things, that offspring of obese dams developed a NAFLD phenotype. The same was true for offspring of lean dams who were suckled by obese dams (Citation21). Another mouse study showed similar results, linking maternal high-fat diet (HFD) during gestation and lactation with NAFLD (Citation20). To summarize, evidence from animal studies indicate that both maternal undernutrition and overnutrition are involved in the development of NAFLD.
As mentioned previously, algorithm tests, compared to more objective diagnostic tools, are associated with a certain degree of diagnostic uncertainty. Still, in a large epidemiological study, employing liver biopsy or imaging techniques is not a viable option. However, our group is currently planning a follow-up study to this one on a small subset of the cohort, where liver fat will be assessed via MRS, at the same time validating the NAFLD liver fat score and equation using this imaging technique, something that has previously not been done.
We have removed individuals with alcohol-induced fatty liver from the analysis based on register linkage and reported alcohol intake in a FFQ. The FFQ used in our study has previously been validated against food records showing high correlation coefficients for alcohol consumption between methods (Citation41,Citation42). Despite this, there might still be a hidden alcohol overconsumption as underreporting often is an issue in interviews and questionnaires. Hence, there is a risk that NAFLD prevalence is over-estimated to some extent. It is, however, unlikely that this confounds the study's main findings of association between early growth and fatty liver, as there is no reason to suspect that veracity of reported alcohol intake would differ based on childhood body size or growth.
The cohort is restricted to individuals born in Helsinki. All of the subjects attended the voluntary child welfare clinics during childhood and might not represent the population living in Finland. However, social status at birth, as defined by father's highest attained occupational status, was similar to the population of Helsinki at the time (Citation43). Generalization of the findings might further be limited, as most of the subjects were born or grew up during the Second World War during which food shortages and other factors might have influenced the nutritional status of both mothers and offspring. Finally, survivor bias might have influenced the findings, as a small size at birth is associated with several chronic diseases as well as premature death (Citation43,Citation44), potentially leaving the least healthy of these individuals outside the study.
In conclusion, our results indicate that the development of NAFLD is in part influenced by early growth and that a larger body size during all stages of childhood lowers the risk of adult NAFLD. In contrast to previous results on diabetes and coronary heart disease, a larger than expected growth during childhood does not increase the risk of adult NAFLD. However, our results indicate that growth patterns after the age of 11 are of importance, as those who were lean in early childhood and subsequently obese in adulthood had an OR of 18.5 for having a positive NAFLD score. In other words, the impact of adult obesity on NAFLD risk was most pronounced in those who were small during early childhood, indicating that early obesity prevention might be especially important for these individuals. Our results support the concept of mismatch (Citation45) and the importance of a life-course approach when evaluating disease risk in adulthood.
Notice of Correction
The version of this article published online ahead of print on the 14th June 2013 contained errors in the abstract on page 1. The errors have been corrected for this version.
Declaration of interest: Supported by the Academy of Finland, the British Heart Foundation, EU FP7 (DORIAN) project number 278603, the Finnish Cultural Foundation, the Finnish Special Governmental Subsidy for Health Sciences, Finska Läkaresällskapet, the Juho Vainio Foundation, Liv och Hälsa, Samfundet Folkhälsan, the Signe and Ane Gyllenberg Foundation, and the Wilhelm and Else Stockmann Foundation. The authors declare no conflict of interest.
References
- Lillycrop K, Hanson M, Burdge G. Epigenetics and the influence of maternal diet. In: Newnham J, Ross M, editors. Early life origins of human health and disease. Basel: Karger; 2009. p. 11–20.
- Godfrey KM, Gluckman PD, Hanson MA. Developmental origins of metabolic disease: life course and intergenerational perspectives. Trends Endocrinol Metab. 2010;21:199–205.
- Whincup PH, Kaye SJ, Owen CG, Huxley R, Cook DG, Anazawa S, et al. Birth weight and risk of Type 2 diabetes. JAMA. 2008;300:2886–97.
- Andersen LG, Ängquist L, Eriksson JG, Forsen T, Gamborg M, Osmond C, et al. Birth weight, childhood body mass index and risk of coronary heart disease in adults: combined historical cohort studies. PLoS One. 2010;5:e14126.
- Eriksson J, Forsen T, Tuomilehto J, Winter P, Osmond C, Barker D. Catch-up growth in childhood and death from coronary heart disease: longitudinal study. BMJ. 1999;318:427–31.
- Barker DJP, Osmond C, Kajantie E, Eriksson JG. Growth and chronic disease: findings in the Helsinki Birth Cohort. Ann Hum Biol. 2009;36:445–58.
- Forsén T, Eriksson J, Tuomilehto J, Reunanen A, Osmond C, Barker D. The fetal and childhood growth of persons who develop type 2 diabetes. Ann Intern Med. 2000;133:176–82.
- Eriksson JG. Early growth and coronary heart disease and type 2 diabetes: findings from the Helsinki Birth Cohort Study (HBCS). Am J Clin Nutr. 2011;94:1799S–802S.
- Puri P, Sanyal AJ. Nonalcoholic fatty liver disease: definitions, risk factors, and workup. Clinical Liver Disease. 2012;1:98–102.
- Levene AP, Goldin RD. The epidemiology, pathogenesis and histopathology of fatty liver disease. Histopathology. 2012;61:141–52.
- Bellentani S, Scaglioni F, Marino M, Bedogni G. Epidemiology of non-alcoholic fatty liver disease. Dig Dis. 2010;28:155–61.
- Musso G, Gambino R, Cassader M, Pagano G. Meta-analysis: natural history of non-alcoholic fatty liver disease (NAFLD) and diagnostic accuracy of non-invasive tests for liver disease severity. Ann Med. 2011;43:617–49.
- Nakao H, Yoneda M. The intertwisted correlations among non-alcoholic fatty liver disease, atherosclerosis, and metabolic syndrome. J Gastroenterol. 2009;44:1162–4.
- Vanni E, Bugianesi E, Kotronen A, De Minicis S, Yki-Järvinen H, Svegliati-Baroni G. From the metabolic syndrome to NAFLD or vice versa?Dig Liver Dis. 2010;42:320–30.
- Marchesini G, Brizi M, Bianchi G, Tomassetti S, Bugianesi E, Lenzi M, et al. Nonalcoholic fatty liver disease a feature of the metabolic syndrome. Diabetes. 2001;50:1844–50.
- Lewis JR, Mohanty SR. Nonalcoholic fatty liver disease: a review and update. Dig Dis Sci. 2010;55:560–78.
- Nobili V, Marcellini M, Marchesini G, Vanni E, Manco M, Villani A, et al. Intrauterine growth retardation, insulin resistance, and nonalcoholic fatty liver disease in children. Diabetes Care. 2007;30:2638–40.
- Fraser A, Ebrahim S, Davey Smith G, Lawlor DA. The associations between birthweight and adult markers of liver damage and function. Paediatr Perinat Epidemiol. 2007;22:12–21.
- Hyatt M, Gardner D, Sebert S, Wilson V, Davidson N, Nigmatullina Y, et al. Suboptimal maternal nutrition, during early fetal liver development, promotes lipid accumulation in the liver of obese offspring. Reproduction. 2011;141:119–26.
- Bruce KD, Cagampang FR, Argenton M, Zhang J, Ethirajan PL, Burdge GC, et al. Maternal high‐fat feeding primes steatohepatitis in adult mice offspring, involving mitochondrial dysfunction and altered lipogenesis gene expression. Hepatology. 2009;50:1796–808.
- Oben JA, Mouralidarane A, Samuelsson AM, Matthews PJ, Morgan ML, Mckee C, et al. Maternal obesity during pregnancy and lactation programs the development of offspring non-alcoholic fatty liver disease in mice. J Hepatol. 2010;52:913–20.
- Morrison JL, Duffield JA, Muhlhausler BS, Gentili S, McMillen IC. Fetal growth restriction, catch-up growth and the early origins of insulin resistance and visceral obesity. Pediatr Nephrol. 2010;25: 669–77.
- Barker DJP, Forsen T, Eriksson JG, Osmond C. Growth and living conditions in childhood and hypertension in adult life: a longitudinal study. J Hypertens. 2002;20:1951–6.
- Forsen T, Eriksson J, Tuomilehto J, Teramo K, Osmond C, Barker D. Mother's weight in pregnancy and coronary heart disease in a cohort of Finnish men: follow up study. BMJ. 1997;315:837–40.
- Kunst A, Draeger B, Ziegenhorn J. UV-methods with hexokinase and glucose-6-phosphate dehydrogenase. Methods of Enzymatic Analysis. 1983;6:163–72.
- Sobey WJ, Beer S, Carrington CA, Clark P, Frank B, Gray I, et al. Sensitive and specific two-site immunoradiometric assays for human insulin, proinsulin, 65–66 split and 32–33 split proinsulins. Biochem J. 1989;260:535–41.
- Consultation W. Definition, diagnosis and classification of diabetes mellitus and its complications. Geneva, Switzerland: World Health Organization; 1999. p. 31–3.
- Alberti K, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the metabolic syndrome. A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–5.
- Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, et al. The diagnosis and management of non‐alcoholic fatty liver disease: Practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012; 55:2005–23.
- Kotronen A, Peltonen M, Hakkarainen A, Sevastianova K, Bergholm R, Johansson LM, et al. Prediction of non-alcoholic fatty liver disease and liver fat using metabolic and genetic factors. Gastroenterology. 2009; 137:865–72.
- Bedogni G, Bellentani S, Miglioli L, Masutti F, Passalacqua M, Castiglione A, et al. The Fatty Liver Index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006;6:33.
- Pajunen P, Kotronen A, Korpi-Hyövälti E, Keinänen-Kiukaanniemi S, Oksa H, Niskanen L, et al. Metabolically healthy and unhealthy obesity phenotypes in the general population: the FIN-D2D Survey. BMC Public Health. 2011;11:754.
- Palekar NA, Naus R, Larson SP, Ward J, Harrison SA. Clinical model for distinguishing nonalcoholic steatohepatitis from simple steatosis in patients with nonalcoholic fatty liver disease. Liver Int. 2006;26: 151–6.
- Wieckowska A, Feldstein AE. Diagnosis of nonalcoholic fatty liver disease: invasive versus noninvasive. Semin Liver Dis. 2008;28: 386–95.
- Weigand K, Weigand K. Percutaneous liver biopsy: retrospective study over 15 years comparing 287 inpatients with 428 outpatients. J Gastroenterol Hepatol. 2009;24:792–9.
- Roldán-Valadez E, Favila R, Martínez-López M, Uribe M, Méndez-Sánchez N. Imaging techniques for assessing hepatic fat content in nonalcoholic fatty liver disease. Ann Hepatol. 2008;7:212–20.
- Miller MH, Ferguson MAJ, Dillon JF. Systematic review of performance of non‐invasive biomarkers in the evaluation of non‐alcoholic fatty liver disease. Liver Int. 2011;31:461–73.
- Patel K. Noninvasive tools to assess liver disease. Curr Opin Gastroenterol. 2010;26:227–33.
- Musso G, Gambino R, Durazzo M, Cassader M. Noninvasive assessment of liver disease severity with liver fat score and CK‐18 in NAFLD: prognostic value of liver fat equation goes beyond hepatic fat estimation. Hepatology. 2010;51:715–17.
- Balkau B, Lange C, Vol S, Fumeron F, Bonnet F. Nine-year incident diabetes is predicted by fatty liver indices: the French D.E.S.I.R. study. BMC Gastroenterol. 2010;10:56.
- Paalanen L, Männistö S, Virtanen MJ, Knekt P, Räsänen L, Montonen J, et al. Validity of a food frequency questionnaire varied by age and body mass index. J Clin Epidemiol. 2006;59:994–1001.
- Männistö S, Virtanen M, Mikkonen T, Pietinen P. Reproducibility and validity of a food frequency questionnaire in a case-control study on breast cancer. J Clin Epidemiol. 1996;49:401–9.
- Eriksson JG, Forsen T, Tuomilehto J, Osmond C, Barker DJP. Early growth and coronary heart disease in later life: longitudinal study. BMJ. 2001;322:949–53.
- Kajantie E, Osmond C, Barker DJ, Forsén T, Phillips DI, Eriksson JG. Size at birth as a predictor of mortality in adulthood: a follow-up of 350 000 person-years. Int J Epidemiol. 2005;34:655–63.
- Gluckman PD, Hanson MA, Beedle AS. Early life events and their consequences for later disease: a life history and evolutionary perspective. Am J Hum Biol. 2007;19:1–19.