Abstract
The prevalence of monoclonal gammopathy of undetermined significance (MGUS) is generally estimated at 3.4% in the general population over 50 years, and its incidence increases with age. MGUS represents a preneoplastic entity that can transform into multiple myeloma or other lymphoproliferative disorders. The risk of malignant transformation is estimated at 1% per year and persists over time. Predictors of malignant transformation have been identified such as the heavy chain isotype, The level of monoclonal proteins, increasing levels of the monoclonal component during the first years off follow-up, the percentage of bone marrow plasmocytosis, the dosage of serum free light chains, the presence of immunophenotypically abnormal plasma cells, aneuploidy, and the presence of circulating plasma cells. Prognostic scores that combine certain of these factors have been proposed and allow the identification of high-risk patients. Their use could assist in tailoring the care for each patient, based on his/her risk profile.
Key messages
Monoclonal gammopathies are frequent findings in daily clinical practice. This review tries to propose recommendations for the diagnosis and follow-up and to give concise information for referring physicians on MGUS, transformation risks, and predictive scores.
Introduction
Monoclonal gammopathy of undetermined significance (MGUS) is an asymptomatic, clonal expansion of B lineage cells (lymphocytes or plasma cells) that secrete a monoclonal immunoglobulin. In 1952, Waldenström introduced the term ‘essential hypergammaglobulinemia’ to characterize patients with a monoclonal gammopathy without any evidence for multiple myeloma (MM), Waldenström's macroglobulinemia (WM), amyloidosis, or a related pathology (Citation1). The denomination ‘monoclonal gammopathy of undetermined significance’ and its acronym ‘MGUS’ was first used by Kyle in 1978 (Citation2) and is currently widely accepted.
In this review, the members of the MM study group of the Belgian Hematological Society discussed the diagnostic and prognostic work-up of MGUS and its implication for referring physicians. The discussions were based on an extended review of the literature in order to propose consensus recommendations. These recommendations were initially circulated in draft form to each panel member, who had an opportunity to comment on them. The manuscript subsequently underwent rounds of revision until consensus was reached by all authors.
Definition
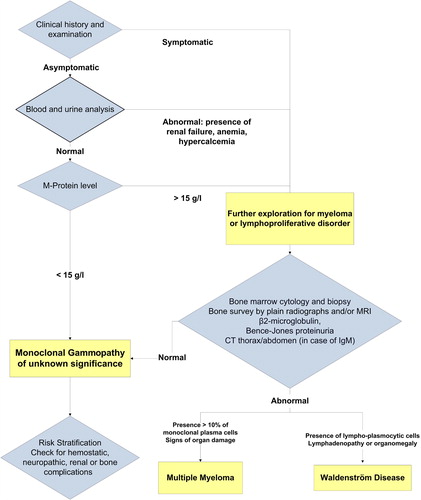
A MGUS is defined as a monoclonal gammopathy without any clinical signs of MM, WM, amyloidosis, or other lymphoproliferative disorders (Citation3). Diagnostic criteria are based on different laboratory and clinical features: level of monoclonal protein inferior to 30 g/L, normal renal function, calcemia and hemoglobin level, the percentage of bone marrow (BM) plasmocytosis below 10%, and absence of osteolytic lesions on skeletal imaging (Citation3).
There are three distinct clinical types of MGUS, each with a small risk of progressing to a malignant plasma cell dyscrasia or lymphoproliferative disorder. Non-IgM MGUS (IgG and IgA MGUS) are the most common subtypes of MGUS, while IgM MGUS accounts for approximately 15% of MGUS cases (Citation4). Monoclonal IgD is rarely associated with MGUS, and light chain MGUS is a unique subtype of MGUS in which the secreted monoclonal protein (M-protein) lacks the immunoglobulin heavy chain component. Light chain MGUS may progress to light chain MM, amyloid light chain amyloidosis, or light chain deposition disease (Citation5). Subjects with an abnormal free light chain ratio without expression of an immunoglobulin heavy chain or an increased concentration of the involved light chain on immunofixation are not considered to have light chain MGUS (these abnormal ratios likely reflect renal dysfunction or polyclonal activation).
The potential progression of MGUS to lymphoplasmocytic disorders was shown in 1978 by Kyle (Citation2) and subsequently confirmed in other studies (Citation4,Citation6–13). Based on these observations, MGUS should be considered as a preneoplastic state that needs regular and prolonged follow-up.
Diagnosis
A MGUS is typically detected as an incidental finding when patients undergo a protein electrophoresis as part of an evaluation for a wide variety of clinical symptoms (e.g. fatigue, neuropathy, arthritis) and disorders (e.g. anemia, elevated sedimentation rate, hyperproteinemia, hypercalcemia, renal failure). There are no scientific data that currently support systematic screening of asymptomatic patients for monoclonal gammopathy (Citation14).
Individuals with a suspected monoclonal gammopathy should undergo protein electrophoresis and immunofixation on both serum and urine (24-h urine collection). Conventional qualitative tests (chemical strips or ‘dipsticks’) for proteinuria do not detect Bence-Jones proteins and have no value in the diagnosis of MGUS. The size of the M-protein should be quantified both in the serum and urine by electrophoresis in order to assess a patient's risk for development of a B cell malignancy. Immunoglobulin levels should be determined in order to assess the size of the M-protein and the presence of depressed levels of uninvolved immunoglobulins. Moreover, the physician should order a complete blood count and routine blood chemistry tests in order to determine renal function and serum electrolytes including calcium.
The differential diagnosis of MGUS relies on the isotype of the monoclonal immunoglobulin. In case of an IgA or IgG isotype, the most frequent disease to exclude is MM. Following the guidelines of the International Myeloma Working Group, the diagnosis of symptomatic MM is based on a BM plasmocytosis superior to 10%, the presence of serum and/or urine M-protein, and evidence of end-organ damage defined as the ‘CRAB’ criteria (Citation3). This ‘CRAB’ acronym summarizes the most common clinical manifestations of MM: hypercalcemia (C); renal dysfunction (R); anemia (A); and lytic bone lesions (B). MGUS and smoldering MM (SMM) are both asymptomatic and, by defenition, are never associated with organ failure. The diagnosis of MGUS is retained when the level of the serum monoclonal component is below 30 g/L and the clonal BM plasmocytosis is below 10% (Citation3). In case of related organ dysfunction, the diagnosis of symptomatic MM can be made independently of the levels of M-protein or BM plasmocytosis. Generally, a radiological skeletal survey is recommended to screen for osteolytic lesions. Once performed, this skeletal survey does not need to be routinely repeated, except in case of new or worsening of existing complaints (Citation3). Computerized tomography, magnetic resonance imaging, and positron emission tomography are more sensitive than plain radiographs in detecting bone involvement by MM. We reserve computerized tomography or magnetic resonance imaging of the spine and pelvis for selected patients such as those with bone pain without any abnormality on skeletal survey.
When the isotype consists of an IgM immunoglobulin, WM and other lymphoproliferative disorders should be excluded by clinical and radiological evaluation of enlarged lymph nodes, organs, or BM infiltration by lymphoplasmocytic cells. M-proteins can also be found in different infectious diseases or auto-immune disorders. The different entities that remain to be excluded in case of a monoclonal gammopathy are listed in .
Table I. Causes of monoclonal gammopathies.
Recommendations on initial investigations before referral
Most cases of monoclonal gammopathies are diagnosed by general practitioners or specialists in internal medicine. In addition to a detailed medical history and physical examination, initial assessment requires M-protein typing and serum immunoglobulin level determination, qualitative urine test for urinary protein excretion, and further laboratory testing including full blood count, serum creatinine, urea, and electrolytes with calcium (complementary examinations are resumed in ).
Table II. Examinations to be performed before and after referral to a hematologist.
Incidence and risk of progression
The incidence of MGUS is estimated at 3.4% in the general population over 50 years, and this incidence increases with age from 1.7% (50–59 years) to 6.6% beyond 80 years (Citation15). In a large observational study on 21,079 cases of monoclonal gammopathies, a diagnosis was retained in 62%, MM in 18%, amyloid light chain amyloidosis in 2.5%, WM in 2.5%, other lymphoproliferative diseases in 2%, and solitary plasmacytoma in 2% (Citation15).
Several hereditary, genetic, as well as environmental factors play a role in the development of MGUS, including age, race, gender, familial history, and obesity. The risk of MGUS increases with age. In Caucasians, it occurs in 3% above the age of 50 years, increasing to 5% above the age of 70, the highest incidence being over 80. MGUS is also more frequent in men (male/female ratio of 1.5) (Citation16). Prevalence of MGUS and MM differs strikingly across races. MGUS is less common in individuals of Asian descent, and 2- to 3-fold more frequent in Africans and African-Americans compared to Caucasians (Citation17). Few studies have indicated an increased occurrence of MGUS among relatives with a familial history of MM. A recent large Swedish population study identified a 2.8-fold increased risk of MGUS in first-degree relatives of patients with MGUS or MM (Citation18). Some studies have also shown a higher prevalence of MGUS among individuals exposed to pesticides (Citation19). These findings suggest that genetic susceptibility factors and/or shared environmental risk factors are involved in this phenomenon. Finally, MGUS was found to be twice as common among obese versus non-obese women. Obesity was being retained as an independent risk factor in a multivariate analysis (Citation20).
In 1978, Robert Kyle described the evolution of MGUS in a cohort of 241 patients over a 5-year follow-up period and observed a progression to a hematological malignancy in 11% of them (Citation2). On the last update, after a median follow-up of 13.7 years (range 0–39) (Citation21), 64 patients (27%) had finally progressed to a hematological malignancy: 44 patients developed MM, 8 patients developed amyloidosis, 7 developed WM, and 5 developed a lymphoproliferative disorder (chronic lymphocytic leukemia, malignant lymphoma). The risk of malignant transformation was estimated at 17% at 10 years, 34% at 20 years, and 39% at 25 years (Citation21). To confirm these data in a wider population, the same authors studied a cohort of 1384 MGUS patients diagnosed between 1960 and 1994, with a median age at diagnosis of 72 years (Citation4). After a median follow-up of 15.4 years, malignant transformation was observed in 115 cases (8%). The relative risk for developing MM was calculated at 25 (95% confidence interval 20–32), the relative risk for developing WM was 46 (confidence interval 19–95), and for developing amyloidosis or lymphoma 8.4 (confidence interval 4–16) and 2.4 (confidence interval 2–4), respectively. The actuarial risk of transformation in this population was estimated at 10% at 10 years, 21% at 20 years, and 26% at 25 years, or about 1% per year (Citation4).
Recently, Kristinsson and colleagues determined the mortality patterns and causes of death in MGUS patients in comparison to controls (Citation22). In a nation-wide cohort of 4259 MGUS patients, they demonstrated an elevated mortality in patients with MGUS that increased with longer follow-up. The authors extended this work by comparing the causes of death with those of the matched controls and found that MGUS patients had an increased risk of dying from a hematological malignancy, amyloidosis, cardiac disorders, bacterial infections, and liver or renal diseases (Citation22). Other groups reported earlier that the survival of MGUS patients was shorter when compared to the age- and sex-matched normal population (Citation23,Citation24).
Two large longitudinal studies have suggested that virtually all patients diagnosed with MM had a preceding MGUS, with 75% having a detectible M-protein more than 8 years prior to the diagnosis of MM (Citation25,Citation26). A prospective cancer screening trial, which banked serum samples from 75,000 individuals, identified 106 MM patients of whom 71 had serum samples available at least 2 years before diagnosis. Among them, more than 95% had MGUS at least 2 years prior to the diagnosis of MM (Citation25). The investigators analyzed M-protein levels and involved free light chain ratios over time in individual patients and identified two different patterns of evolution: approximately half the study population showed a slowly progressive increase of their M-protein prior to MM diagnosis, whereas the other half maintained largely stable M-protein levels up to the diagnosis of MM that suddenly manifested (Citation25). The second study analyzed medical files of MM patients who received an autologous transplantation at the Walter Reed Army Medical Center and whose serum was stored by the medical service of the US Army for medical surveillance purposes. For 30 out of 90 patients, serum samples could be retrieved, and, in 27 of them, the presence of MGUS was demonstrated at least 2 years prior to the diagnosis of MM. Of the three remaining patients, two had an IgD MM with prior serum samples available dating from 5 to 3.5 years before the diagnosis of MM. The very low levels of monoclonal immunoglobulins in this IgD isotype can hinder the recognition of a monoclonal peak after immunofixation on serum electrophoresis. The last patient had only one single pre-diagnostic serum sample available (Citation26).
Risk factors for progression
Large cohort studies with an extended follow-up tried to identify predictors of malignant transformation. Most importantly, these results were obtained by retrospective analyses and never confirmed in other, prospective cohorts.
Isotype as risk factor
In 1992, Bladé reported the predictive role of the immunoglobulin isotype (Citation6). In a cohort of 128 patients, he observed a transformation rate of 23.8% in patients with IgA MGUS compared to 7.5% in patients with IgG or IgM MGUS. A similar observation was made in a large Danish study on 1247 patients (Citation9). Kyle et al. observed an increased risk of progression of both IgA and IgM MGUS compared to IgG MGUS (Citation4). In an epidemiological study over 504 MGUS, Ogmundsdottir et al. assessed the relative risk of transformation for men and women harboring an IgA gammopathy to 27.8 and 62.1, respectively, compared to a relative risk of 6.59 and 16.5 in men and woman with an IgG MGUS (Citation27).
Monoclonal protein level
According to the Mayo Clinic study, the most discriminating prognostic factor was the M-protein level. The risk of transformation at 20 years was estimated at 49% when the initial peak was higher than 25 g/L compared to 14% when it was less than 5 g/L. This prognostic value was further confirmed in other studies, a concentration superior to 15 g/L being currently accepted to be associated with an increased risk for progression (Citation28). More recently, a Spanish study of 359 MGUS identified the evolution of serum M-protein as a predictor for malignant transformation, and progressive increase in M-protein levels during the first years of follow-up was one of the most important factors for disease progression (Citation11).
BM plasmocytosis
Current recommendations do not impose a BM aspiration in the absence of clinical or biological signs suggestive for a hematologic malignancy. In most published studies, a myelogram is only performed in a minority of patients, and BM plasmocytosis is rarely reported as a predictor of malignant transformation. In three studies, a BM plasmocytosis estimated above 5% was associated with an increased risk of transformation (relative risk of 1.44), but could only be retained in univariate analysis and not in multivariate analysis in the two Spanish studies (Citation10–12). Immunophenotyping of BM plasma cells by flow cytometry shows a different antigen expression pattern in monoclonal compared to normal plasma cells. Polyclonal normal plasma cells express CD38 and CD19, but do not express CD56. Conversely, malignant plasmocytes express monoclonal CD38 more weakly, do not express CD19, but express CD56. In MM, the majority of plasmocytes (> 95%) are abnormal, where normal and aberrant plasma cells coexist in MGUS. Pérez-Persona recently studied the phenotype of BM plasma cells in 407 MGUS patients (Citation12). The median percentage of aberrant plasmocytes as part of the whole BM plasma cell population was 72%. Increased levels (95% threshold) were found in 18% of the cases, which was associated with a transformation rate of 25% at 5 years. In multivariate analysis, the percentage of abnormal plasma cells and the existence of aneuploidy were both predictors of malignant transformation (Citation12).
Free light chain
Rajkumar et al. studied the contribution of the serum free light chain assay in the risk assessment of malignant transformation in a population of 1148 MGUS patients (Citation29). The kappa or lambda free light chain concentrations were increased in 64% of the patients, while the kappa/lambda ratio was abnormal in 33% of them. This abnormal kappa/lambda ratio was associated with an increased risk of malignant transformation (16.7% at 10 years and 35% at 20 years, compared to 5.3% at 10 years and 12.6% at 20 years in case of a normal kappa/lambda ratio). When this free light chain ratio was very abnormal (< 0.125, or > 8), this risk further increased to 60.5% at 20 years.
Recommendations for further investigations
Once detected, a series of staging investigations are undertaken, depending on the M-protein isotype, age of the patient, and results of the initial blood profile.
Three studies indicated an increased risk of transformation for IgA gammopathies, while studies at the Mayo Clinic described an increased risk for non-IgG gammopathies. Both IgA and IgM entities require special attention at diagnosis and follow-up (certainly if associated with an abnormal free light chain ratio). In contrast, IgG MGUS without alarming symptoms and normal free light chain ratio can be cautiously followed with laboratory and clinical observation. In cases of doubtful results (such as anemia, renal impairment), in young patients, or increased M-protein levels, the complete staging procedure should be carried out, including a skeletal survey and BM examination.
In the case of an IgM M-protein, imaging investigations such abdominal ultrasound, chest X-ray and/or computerized tomography scans should be undertaken to exclude lymphadenopathy or hepatosplenomegaly.
Predictive scores
The individual risk assessment of MGUS progression is currently based on a combination of the above-cited risk factors. Each of these predictors alone cannot correctly classify patients. In 1996, Baldini et al. reported that patients with a M-protein level below 15 g/L, BM plasmocytosis less than 5%, normal other immunoglobulin levels, and the absence of Bence-Jones proteinuria had a very low risk of progression (Citation7). Cesana et al. proposed a prognostic index taking into account four factors: sedimentation rate, immunoglobulin levels, presence of Bence-Jones proteinuria, and medullary plasmocytosis (Citation10). The risk of malignant transformation was well correlated with the calculated risk score.
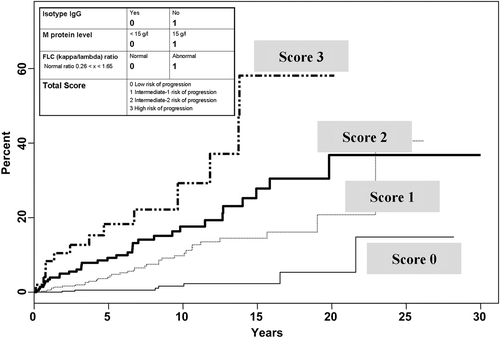
Rajkumar et al. proposed a predictive score that was only based on laboratory parameters, taking into account the isotype (IgG or non-IgG), the level of M-protein (less or more than 15 g/L), and the kappa/lambda ratio measured by the free light chain assay (normal or abnormal) (Citation29), and defined four different groups at risk of malignant transformation. Patients without a risk factor (449 patients or 39.1% of the cohort) had an estimated risk of transformation of 5% at 20 years. Patients with three risk factors (53 patients or 4.6% of the cohort) had an estimated risk of progression of 58% at 20 years and a relative risk of conversion estimated at 20.8 compared to the group without risk factors. The cumulative progression rates of these patients are illustrated in .
Figure 2. Risk stratification according to Rajkumar et al., based on free light chain ratio, level of M-protein, and isotype.
As described earlier, the immunophenotypic analysis of bone marrow plasma cells of MGUS patients can identify two distinct plasma cell subpopulations: normal polyclonal plasma cells and abnormal clonal plasma cells (Citation30). Pérez-Persona et al. introduced the percentage of abnormal plasma cells within the total bone marrow plasma cell population in a predictive score that furthermore included the ploidy status of plasma cells (using a double-staining for nuclear DNA with propidium iodide and plasma cell markers) (Citation12). Multivariate analysis identified a high percentage (> 95%) of immunophenotypically aberrant plasma cells and the presence of aneuploidy (hypo- and hyperdiploidy) as major risk factors. This score requires not only a BM aspiration, but also the ability to perform a six-color flow cytometry, which is not available everywhere.
Recommendations for prognostic stratification
Two important predictive models have been developed based on two large retrospective studies. However, the proposed scores still require further validation. The Mayo Clinic score is based on laboratory parameters, while the Salamanca score includes immunophenotypic abnormalities of BM plasma cells. There is currently no scientific evidence that one model determines the outcome of an individual patient more accurately. BM examinations are nowadays not routinely performed in MGUS patients but remain indicated in individual cases (at the physician's discretion). Because the included tests are universally available and the results are validated in a large cohort of patients (n = 1148), we recommend using the Mayo Clinic risk stratification model that is based on the concentration of the serum M-protein, the type of immunoglobulin, and the free light chain ratio. This model is able to identify low-risk patients for whom further examinations can be postponed.
MGUS and bone disease
A few studies have investigated the presence of bone disease in MGUS. Incidence of bone fractures, bone mineral density, and bone turnover markers were studied in 65 postmenopausal MGUS patients and compared to 130 matched controls (Citation31). Thirteen patients had a normal bone density, while 35 were osteopenic (–1 < T-score < –2.5) and 17 osteoporotic (T-score < –2.5). Fifty-two percent of these patients had at least one vertebral fracture compared to 23.8% in the control population. Patients who presented a fracture were in general older and had a longer MGUS history compared to patients without fracture signs. These results were confirmed in another study that followed 488 MGUS patients over 7 years and observed a relative risk of 2.7 for vertebral fractures (Citation32). These two studies suggest that MGUS is associated with bone loss, and long-term persistence of MGUS was identified as a risk factor for developing a fracture. A recent population-based study confirmed that MGUS is associated with vertebral and hip fractures and osteoporosis (Citation33,Citation34). The risk of fractures was independent of the risk of progression to MM, suggesting that bone alterations occur early in the pathogenesis of MM.
There are only two studies available regarding the use of bisphosphonates in MGUS patients. Pepe et al. studied the effects of 70 mg alendronate once weekly, in addition to calcium and vitamin D intakes in a group of 30 MGUS patients (Citation35). After 18 months of treatment, the mean bone mineral density measured in the lumbar spine and total femur increased by 6.1% and 1.5%, respectively. This increase was associated with a significant reduction in serum bone alkaline phosphatase, osteocalcin, and C-terminal collagen cross-links. Berenson et al. studied the effect of three infusions of 4 mg zoledronic acid repeated every six months in 54 MGUS patients with signs of at least osteopenia (Citation36). At the end of the study, the lumbar and femoral bone mineral density increased by 15% and 6%, respectively. No randomized studies comparing bisphosphonates to placebo have been completed in MGUS patients.
Recommendations
Physicians should be aware that MGUS is associated with a higher risk of bone loss and fractures and that screening, prevention, and treatment of bone-related problems are important in these patients. High-risk patients (postmenopausal women, history of vertebral fractures) could benefit from a bone density scan in order to assess accurately the bone mineral density and determine if they may be appropriate candidates for antiresorptive therapies. Serum vitamin D levels should be assessed, and adequate calcium intake (dietary, plus supplemental if necessary) should be preconized.
MGUS and coagulation disorders
MGUS patients are also more likely to experience thromboembolic events, such as deep vein thromboses. In two population-based studies (United States and Sweden), the relative risk of venous thrombosis for MGUS patients was calculated at 3.3 and 2.1, respectively (Citation37,Citation38). Thromboses most frequently occurred during the first year after diagnosis, predominantly in IgG and IgA MGUS, but not IgM MGUS. A recent study based on 1610 MGUS patients observed an increased relative risk of venous thromboembolic events, which was estimated at 1.37 (Citation39).
In a prospective single-center study based on 310 MGUS patients, 19 (6.1%) patients were diagnosed with venous thromboembolism over 44 months of follow-up. M-protein levels superior to 16 g/L at diagnosis and progression to a lymphoproliferative disease were described as risk factors for deep vein thrombosis (Citation40). Three retrospective studies confirmed an incidence of venous thromboses of 7.5%–8% (Citation41–43). In a univariate analysis, prior personal or family history of venous thromboembolism, immobility, low serum albumin level, and high leukocyte count were significant risk factors (Citation41). In addition, the Swedish population study also reported an increased risk for arterial thrombosis (hazard ratio of 1.5 at 10 years of follow-up) with an increased incidence of coronary artery disease and cerebrovascular events (Citation37).
MGUS can also be complicated by some bleeding disorders. An acquired deficiency of von Willebrand factor related to an inhibitory antibody has been reported in association with monoclonal gammopathies (Citation44). In the treatment of this bleeding complication, intravenous immunoglobulins are more efficient than von Willebrand factor concentrates, because their administration can result in a more sustained improvement of the bleeding complications (Citation45).
Recommendation concerning thromboembolic disease
Physicians should be aware at MGUS is associated with an increased risk of thromboembolic events compared to non-MGUS individuals, and the presence of a MGUS should be considered as an additional risk factor for thromboembolic disease. However, at the present time, thrombo-prophylaxis strategies for MGUS patients are the same as for the general population, and routine anticoagulation for MGUS patients is not recommended.
MGUS and neuropathy
MGUS is associated with neuropathy with a prevalence estimated at 5% for IgG, 5%–10% for IgA, and 30%–50% for IgM M-proteins. Before proposing the diagnosis of a MGUS-related neuropathy, a POEMS syndrome (defined by the presence of a peripheral neuropathy (P), associated with a monoclonal plasma cell disorder (M), and other paraneoplastic features including organomegaly (O), endocrinopathy (E), and skin changes (S)), an amyloid light chain amyloidosis, or cryoglobulinemia should be excluded (Citation46).
Based on clinical and electrophysiological findings, several variants of MGUS-related neuropathy have been described. In almost 50% of IgM-associated neuropathies, the paraprotein reacts with myelin-associated glycoprotein and cross-reactive glycoconjugates (Citation47). Anti-myelin-associated glycoprotein neuropathies usually have a benign course, resulting in a slowly progressive, distal, and asymmetric demyelinizing polyneuropathy. Patients generally present with distal sensory symptoms followed by a distal weakness and gait ataxia (Citation48). Neuropathies related to IgA, IgG, or IgM paraproteins without anti-myelin activity are more heterogeneous and sometimes indistinguishable from chronic inflammatory demyelinating polyneuropathies with symptoms of symmetrical, proximal, and distal weakness of the four limbs, sensory involvement, and areflexia. A few patients can present with an axonal neuropathy which is slowly progressive and does often not require any treatment, although some patients may benefit from anti-neuropathic pain agents (Citation49).
In anti-myelin associated glycoprotein (MAG) neuropathies, plasma exchanges are temporarily effective in about 50% of patients (Citation50). However, a prospective analysis comparing plasmapheresis and chlorambucil versus chlorambucil alone could not show any benefit of plasmapheresis (Citation51). Intravenous immunoglobulins or steroids used in combination with other immunosuppressive drugs may have a limited effect (Citation50). Two randomized trials compared the administration of four weekly perfusions of rituximab with a placebo and failed to show a consistent improvement in the primary outcome (an amelioration in a disability scale score or in a sensory score), but, based on a self-evaluation scales, 20% to 30% of the patients reported some improvement with time (Citation52,Citation53).
Recommendations
In the presence of symptoms of polyneuropathy, an electromyography and referral to a hematologist and a neurologist for cerebrospinal fluid analysis or nerve biopsy are suggested. Before deciding on a MGUS-related neuropathy, light chain amyloidosis and POEMS syndrome should be excluded.
In the presence of an IgM gammopathy, determination of anti-myelin-associated glycoprotein antibodies can be helpful to distinguish an anti-MAG neuropathy. Other neuropathies are sometimes indistinguishable from chronic inflammatory demyelinating polyneuropathies and may be treated by steroids, intravenous immunoglobulins, or plasma exchanges. Once stopped, the neuropathy can reappear several weeks or months later, requiring a less intensive maintenance treatment.
Other complications
According to a large Swedish study, MGUS patients may have a 2-fold increased risk of developing any infection at 5 and 10 years after diagnosis compared to the general population (Citation54). Increased risk of bacterial and viral infections mostly concerns patients with M-protein concentrations above 25 g/L. Hypogammaglobulinemia occurs in 25%–28% of MGUS and leads to a defective antibody response partially responsible for these complications (Citation15).
A variety of renal lesions have been reported in association with monoclonal gammopathy. Mild renal impairment can be seen in 1% to 2% of MGUS patients and could be attributed to underlying amyloidosis, cryoglobulinemia, or monoclonal immunoglobulin deposition disease. When renal insufficiency is present at diagnosis or occurs in the follow-up of a MGUS, progression to MM should be excluded by serum and urine analysis for paraprotein measurement and BM examination. Urine analysis for proteinuria can confirm a nephrotic syndrome. In this setting, renal biopsy can be considered to characterize the type of renal disease. However, the majority of patients with serum and/or urine monoclonal gammopathy who undergo a renal biopsy present a disease unrelated to monoclonal gammopathy deposits, due to a high incidence of incidental gammopathies in this population. The increased diagnosis of monoclonal gammopathies can be explained by the frequent use of protein electrophoresis as a screening tool in adult patients with renal dysfunction and the increased incidence of monoclonal gammopathy in elderly patients (Citation55).
Several skin disorders have been recognized in association with a monoclonal gammopathy and could be divided into four categories: disorders potentially associated with monoclonal gammopathies (scleromyxedema, plane xanthoma, pyoderma gangrenosa, Schnitzler syndrome), skin disorders due to the infiltration of the skin by malignant plasma cells or secreted paraproteins, dermatoses linked to a paraproteinemia without a clear association, and finally non-specific cutaneous complications due to the monoclonal paraprotein (Citation56).
Follow-up of MGUS patients
It is widely accepted that MGUS requires no treatment, except in cases where the monoclonal immunoglobulin itself is responsible for a complication such as cryoglobulinemia or peripheral neuropathy. The clinical and laboratory monitoring should be regular and prolonged due to the permanent risk of evolution over time.
Surveillance of MGUS is based on clinical (general condition, bone pain, tumoral syndrome) and laboratory criteria (blood count, serum calcium, creatinine, protein electrophoresis, 24-h proteinuria). The monitoring of the monoclonal component is based on the evaluation of the monoclonal peak on protein electrophoresis.
Several prognostic risk scores have been described for the initial assessment of MGUS patients. However, a recent study showed that close follow-up of MGUS patients was rarely able to identify progression before serious complications occurred (Citation57). Among 80 MGUS patients with a regular follow-up (defined in this study as monitoring realized at least every 24 months), routine laboratory follow-up led to the diagnosis of MM in only 16%, whereas MM was diagnosed only after serious MM-related complications (pathological fracture, anemia, recurrent infection, hypercalcemia, or renal failure) in 45%. In patients without risk factors (M- protein < 15 g/L and/or non-IgG M-protein), the fraction of patients diagnosed on the basis of laboratory abnormalities was low (7%) (Citation57). These results suggest that education of low-risk patients for alarming signs can help in a timely recognition of a transformation and that follow-up can be organized in collaboration with a general practitioner. On the other hand, ameliorated follow-up strategies, prevention trials, and continued research on predictive biomarkers are needed for high-risk patients (Citation58).
Prognostic scores are currently based on laboratory parameters obtained from peripheral blood or bone marrow aspirate. Since BM aspiration is not performed in every patient with a MGUS, and flow cytometric analysis of the plasma cell immunophenotype and ploidy is not routinely available, the authors agreed to recommend the Mayo Clinic risk score, that is based on the isotype, the level of monoclonal component and the serum free light chain ratio at diagnosis, and the evolution of the M-protein over time. In 2010, the International Myeloma Working Group published their recommendations for the diagnosis and follow-up of MGUS patients (Citation3).
Follow up of low-risk MGUS
In the presence of a serum M-protein inferior to 15 g/L, IgG isotype, and normal free light chain ratio, the risk of eventual progression to MM or related malignancy is low. In this setting, a baseline BM examination or skeletal survey is not routinely indicated if the clinical and biological evaluation suggests a MGUS. Patients should be followed with serum protein electrophoresis after 6 months and, if stable, every 2–3 years or when symptoms suggestive of a plasma cell malignancy arise.
Intermediate and high-risk MGUS
In the presence of a serum M-protein over 15 g/L, an IgA or IgM isotype, or an abnormal free light chain ratio, a BM aspirate and biopsy should be carried out at baseline to rule out an underlying malignancy. A BM aspirate is always required if a patient with presumed MGUS has unexplained anemia, renal insufficiency, hypercalcemia, bone lesions, or a suspicion of amyloid light chain amyloidosis. Both conventional cytogenetics and fluorescence in situ hybridization should be performed on the BM examination. A computerized tomography of the abdomen should be done in the presence of an IgM M-protein because asymptomatic retroperitoneal lymph nodes may be present. Lactate dehydrogenase, β-2-microglobulin, and C-reactive protein should be determined if there is evidence of MM or WM. If the results of these tests exclude an underlying malignancy, patients should be followed with serum protein electrophoresis and a complete blood count at 6 months and then annually for life. Patients should be educated to contact their physician if there is any change in their clinical condition.
Recommendations for follow-up
Patients with a low risk profile, especially those with an IgG isotype, a low paraprotein level, and a normal free light chain ratio, can be followed every 2 years with a laboratory testing and do not require a BM examination when there are no other signs of organ damage (defined earlier as CRAB criteria). This follow-up can be performed in close collaboration with the general practitioner.
For patients with an intermediate or high risk profile, the complete staging procedure (including a skeletal survey and BM examination) should be carried out, especially in case of doubt.
Monitoring visits should include a medical history, physical examination, and further laboratory testing (quantification of the M-protein and immunoglobulin levels, full blood count, renal function, electrolytes, and calcium). When the patient is not monitored by a hematologist, he should be re-referred when the concentration of the M-protein increases by more than 25% or when symptoms compatible with a diagnosis of MM or lymphoma develop, or in case of unexplained anemia, other cytopenias, renal failure, or hypercalcemia. The patient is best positioned to pick up relevant symptoms. Patients should be informed of relevant symptoms that can occur in the context of MGUS and be encouraged to report them outside appointment visits if they occur.
Conclusions
The incidence of MGUS is estimated at 3.4% in the general population over 50 years. Its possible progression into a lymphoplasmocytic malignancy requires regular and prolonged clinical monitoring. By improving the risk stratification of each patient, we may be able to optimize the management of MGUS patients. Several laboratory criteria are identified as predictors of malignant transformation, and predicting scores combining several factors have been proposed.
Take-home messages
A monoclonal gammopathy is a laboratory finding and a sign of an underlying clonal B cell disorder that can be seen in monoclonal lymphoproliferative disorders but also in inflammatory conditions such as infections or auto- immune diseases.
MGUS is defined by the presence of a monoclonal immunoglobulin in the serum up to 30 g/L in the absence of lytic bone lesions, anemia, hypercalcemia, and renal insufficiency related to the underlying monoclonal plasma cell proliferation, and less than 10% plasma cells in the BM.
The prevalence of MGUS is 3% in persons over 70 years and is higher in persons of African/Caribbean origin compared to Caucasians.
The commonest type of M-protein (isotype) is IgG, followed by IgM and then IgA. Monoclonal IgM can later transform into lymphoproliferative conditions such as Waldenström's macroglobulinemia, B cell non-Hodgkin's lymphoma, or chronic lymphocytic leukemia, while IgA and IgG gammopathies generally transform into MM.
Once detected, a series of staging investigations are undertaken, depending on isotype, age of the patient, and results of initial blood profile. A bone marrow aspiration or biopsy can be indicated to distinguish a MGUS from other lymphoproliferative disorders.
Predictive scores, based on isotype, level of M-protein, and free light chain (kappa/lambda) ratio, are able to identify patients with a high risk of transformation who require a close follow-up. Unfortunately transformation may occur outside the follow-up visits, and patients should be educated about alarming signs for progression.
Although a pre-malignant condition, MGUS can be associated with an increased risk of fractures, thromboembolic diseases, and neurological complications. Initial clinical work-up should pay attention to these complications.
Declaration of interest: This work was funded by a grant from the Belgian Foundation for Cancer, the Fonds de la Recherche Scientifique Médicale, the Fonds National de la Recherche Scientifique (F.N.R.S., Belgium), the Fonds spéciaux de la Recherche (University of Liège). The authors report no conflicts of interest.
References
- Dammacco F, Waldenstrom J. Serum and urine light chain levels in benign monoclonal gammapathies, multiple myeloma and Waldenstrom's macroglobulinaemia. Clin Exp Immunol. 1968;3:911–21.
- Kyle RA. Monoclonal gammopathy of undetermined significance. Natural history in 241 cases. Am J Med. 1978;64:814–26.
- Kyle RA, Durie BG, Rajkumar SV, Landgren O, Blade J, Merlini G, et al. Monoclonal gammopathy of undetermined significance (MGUS) and smoldering (asymptomatic) multiple myeloma: IMWG consensus perspectives risk factors for progression and guidelines for monitoring and management. Leukemia. 2010;24:1121–7.
- Kyle RA, Therneau TM, Rajkumar SV, Offord JR, Larson DR, Plevak MF, et al. A long-term study of prognosis in monoclonal gammopathy of undetermined significance. N Engl J Med. 2002;346:564–9.
- Dispenzieri A, Katzmann JA, Kyle RA, Larson DR, Melton LJ III, Colby CL, et al. Prevalence and risk of progression of light-chain monoclonal gammopathy of undetermined significance: a retrospective population-based cohort study. Lancet. 2010;375:1721–8.
- Blade J, Lopez-Guillermo A, Rozman C, Cervantes F, Salgado C, Aguilar JL, et al. Malignant transformation and life expectancy in monoclonal gammopathy of undetermined significance. Br J Haematol. 1992;81:391–4.
- Baldini L, Guffanti A, Cesana BM, Colombi M, Chiorboli O, Damilano I, et al. Role of different hematologic variables in defining the risk of malignant transformation in monoclonal gammopathy. Blood. 1996; 87:912–18.
- Pasqualetti P, Casale R. Risk of malignant transformation in patients with monoclonal gammopathy of undetermined significance. Biomed Pharmacother. 1997;51:74–8.
- Gregersen H, Mellemkjaer L, Ibsen JS, Dahlerup JF, Thomassen L, Sorensen HT. The impact of M-component type and immunoglobulin concentration on the risk of malignant transformation in patients with monoclonal gammopathy of undetermined significance. Haematologica. 2001;86:1172–9.
- Cesana C, Klersy C, Barbarano L, Nosari AM, Crugnola M, Pungolino E, et al. Prognostic factors for malignant transformation in monoclonal gammopathy of undetermined significance and smoldering multiple myeloma. J Clin Oncol. 2002;20:1625–34.
- Rosinol L, Cibeira MT, Montoto S, Rozman M, Esteve J, Filella X, et al. Monoclonal gammopathy of undetermined significance: predictors of malignant transformation and recognition of an evolving type characterized by a progressive increase in M protein size. Mayo Clin Proc. 2007; 82:428–34.
- Perez-Persona E, Vidriales MB, Mateo G, Garcia-Sanz R, Mateos MV, de Coca AG, et al. New criteria to identify risk of progression in monoclonal gammopathy of uncertain significance and smoldering multiple myeloma based on multiparameter flow cytometry analysis of bone marrow plasma cells. Blood. 2007;110:2586–92.
- Ucci G, Riccardi A, Luoni R, Ascari E. Presenting features of monoclonal gammopathies: an analysis of 684 newly diagnosed cases. Cooperative Group for the Study and Treatment of Multiple Myeloma. J Intern Med. 1993;234:165–73.
- Berenson JR, Anderson KC, Audell RA, Boccia RV, Coleman M, Dimopoulos MA, et al. Monoclonal gammopathy of undetermined significance: a consensus statement. Br J Haematol. 2010;150:28–38.
- Kyle RA, Therneau TM, Rajkumar SV, Larson DR, Plevak MF, Offord JR, et al. Prevalence of monoclonal gammopathy of undetermined significance. N Engl J Med. 2006;354:1362–9.
- Wadhera RK, Rajkumar SV. Prevalence of monoclonal gammopathy of undetermined significance: a systematic review. Mayo Clin Proc. 2010;85:933–42.
- Greenberg AJ, Vachon CM, Rajkumar SV. Disparities in the prevalence, pathogenesis and progression of monoclonal gammopathy of undetermined significance and multiple myeloma between blacks and whites. Leukemia. 2012;26:609–14.
- Landgren O, Kristinsson SY, Goldin LR, Caporaso NE, Blimark C, Mellqvist UH, et al. Risk of plasma cell and lymphoproliferative disorders among 14621 first-degree relatives of 4458 patients with monoclonal gammopathy of undetermined significance in Sweden. Blood. 2009;114:791–5.
- Landgren O, Kyle RA, Hoppin JA, Beane Freeman LE, Cerhan JR, Katzmann JA, et al. Pesticide exposure and risk of monoclonal gammopathy of undetermined significance in the Agricultural Health Study. Blood. 2009;113:6386–91.
- Landgren O, Rajkumar SV, Pfeiffer RM, Kyle RA, Katzmann JA, Dispenzieri A, et al. Obesity is associated with an increased risk of monoclonal gammopathy of undetermined significance among black and white women. Blood. 2010;116:1056–9.
- Kyle RA, Therneau TM, Rajkumar SV, Larson DR, Plevak MF, Melton LJ III. Long-term follow-up of 241 patients with monoclonal gammopathy of undetermined significance: the original Mayo Clinic series 25 years later. Mayo Clin Proc. 2004;79:859–66.
- Kristinsson SY, Bjorkholm M, Andersson TML, Eloranta S, Dickman PW, Goldin LR, et al. Patterns of survival and causes of death following a diagnosis of monoclonal gammopathy of undetermined significance: a population-based study. Haematologica. 2009;94: 1714–20.
- Kyle RA, Kumar S. The significance of monoclonal gammopathy of undetermined significance. Haematologica. 2009;94:1641–4.
- van de Poel MH, Coebergh JW, Hillen HF. Malignant transformation of monoclonal gammopathy of undetermined significance among out-patients of a community hospital in southeastern Netherlands. Br J Haematol. 1995;91:121–5.
- Landgren O, Kyle RA, Pfeiffer RM, Katzmann JA, Caporaso NE, Hayes RB, et al. Monoclonal gammopathy of undetermined significance (MGUS) consistently precedes multiple myeloma: a prospective study. Blood. 2009;113:5412–17.
- Weiss BM, Abadie J, Verma P, Howard RS, Kuehl WM. A monoclonal gammopathy precedes multiple myeloma in most patients. Blood. 2009; 113:5418–22.
- Ogmundsdottir HM, Haraldsdottir V, Johannesson M, Olafsdottir G, Bjarnadottir K, Sigvaldason H, et al. Monoclonal gammopathy in Iceland: a population-based registry and follow-up. Br J Haematol. 2002; 118:166–73.
- Decaux O, Cuggia M, Ruelland A, Cazalets C, Cador B, Jego P, et al. [Monoclonal gammopathies of undetermined significance and their progression over time. Retrospective study of 190 patients]. Presse Med. 2006;35:1143–50.
- Rajkumar SV, Kyle RA, Therneau TM, Melton LJ III, Bradwell AR, Clark RJ, et al. Serum free light chain ratio is an independent risk factor for progression in monoclonal gammopathy of undetermined significance. Blood. 2005;106:812–17.
- Ocqueteau MF, Orfao AF, Almeida JF, Blade JF, Gonzalez MF, Garcia-Sanz RF, et al. Immunophenotypic characterization of plasma cells from monoclonal gammopathy of undetermined significance patients. Implications for the differential diagnosis between MGUS and multiple myeloma.Am J Pathol. 1998;152:1655–65.
- Pepe J, Petrucci MT, Nofroni I, Fassino V, Diacinti D, Romagnoli E, et al. Lumbar bone mineral density as the major factor determining increased prevalence of vertebral fractures in monoclonal gammopathy of undetermined significance. Br J Haematol. 2006;134 :485–90.
- Melton LJ III, Rajkumar SV, Khosla S, Achenbach SJ, Oberg AL, Kyle RA. Fracture risk in monoclonal gammopathy of undetermined significance. J Bone Miner Res. 2004;19:25–30.
- Kristinsson SY, Tang M, Pfeiffer RM, Bjorkholm M, Blimark C, Mellqvist UH, et al. Monoclonal gammopathy of undetermined significance and risk of skeletal fractures: a population-based study. Blood. 2010;116:2651–5.
- Bida JP, Kyle RA, Therneau TM, Melton LJ III, Plevak MF, Larson DR, et al. Disease associations with monoclonal gammopathy of undetermined significance: a population-based study of 17,398 patients. Mayo Clin Proc. 2009;84:685–93.
- Pepe J, Petrucci MT, Mascia ML, Piemonte S, Fassino V, Romagnoli E, et al. The effects of alendronate treatment in osteoporotic patients affected by monoclonal gammopathy of undetermined significance. Calcif Tissue Int. 2008;82:418–26.
- Berenson JR, Yellin O, Boccia RV, Flam M, Wong SF, Batuman O, et al. Zoledronic acid markedly improves bone mineral density for patients with monoclonal gammopathy of undetermined significance and bone loss. Clin Cancer Res. 2008;14:6289–95.
- Kristinsson SY, Pfeiffer RM, Bjorkholm M, Goldin LR, Schulman S, Blimark C, et al. Arterial and venous thrombosis in monoclonal gammopathy of undetermined significance and multiple myeloma: a population-based study. Blood. 2010;115:4991–8.
- Kristinsson SY, Fears TR, Gridley G, Turesson I, Mellqvist UH, Bjorkholm M, et al. Deep vein thrombosis after monoclonal gammopathy of undetermined significance and multiple myeloma. Blood. 2008;112:3582–6.
- Gregersen H, Norgaard M, Severinsen MT, Engebjerg MC, Jensen P, Sorensen HT. Monoclonal gammopathy of undetermined significance and risk of venous thromboembolism. Eur J Haematol. 2011;86: 129–34.
- Sallah S, Husain A, Wan J, Vos P, Nguyen NP. The risk of venous thromboembolic disease in patients with monoclonal gammopathy of undetermined significance. Ann Oncol. 2004;15:1490–4.
- Srkalovic G, Cameron MG, Rybicki L, Deitcher SR, Kattke-Marchant K, Hussein MA. Monoclonal gammopathy of undetermined significance and multiple myeloma are associated with an increased incidence of venothromboembolic disease. Cancer. 2004;101:558–66.
- Muslimani AA, Spiro TP, Chaudhry AA, Taylor HC, Jaiyesimi I, Daw HA. Venous thromboembolism in patients with monoclonal gammopathy of undetermined significance. Clin Adv Hematol Oncol. 2009;7:827–32.
- Cohen AL, Sarid R. The relationship between monoclonal gammopathy of undetermined significance and venous thromboembolic disease. Thromb Res. 2010;125:216–19.
- Lamboley V, Zabraniecki L, Sie P, Pourrat J, Fournie B. Myeloma and monoclonal gammopathy of uncertain significance associated with acquired von Willebrand's syndrome. Seven new cases with a literature review. Joint Bone Spine. 2002;69:62–7.
- Federici AB, Stabile F, Castaman G, Canciani MT, Mannucci PM. Treatment of acquired von Willebrand syndrome in patients with monoclonal gammopathy of uncertain significance: comparison of three different therapeutic approaches. Blood. 1998;92:2707–11.
- Sanchorawala V. Light-chain (AL) amyloidosis: diagnosis and treatment. Clin J Am Soc Nephrol. 2006;1:1331–41.
- Nobile-Orazio E. IgM paraproteinaemic neuropathies. Curr Opin Neurol. 2004;17:599–605.
- European Federation of Neurological Societies/Peripheral Nerve Society Guideline on management of paraproteinemic demyelinating neuropathies. Report of a Joint Task Force of the European Federation of Neurological Societies and the Peripheral Nerve Society–first revision. J Peripher Nerv Syst. 2010;15:185–95.
- Gorson KC, Ropper AH. Axonal neuropathy associated with monoclonal gammopathy of undetermined significance. J Neurol Neurosurg Psychiatry. 1997;63:163–8.
- Nobile-Orazio E, Meucci N, Baldini L, Di Troia A, Scarlato G. Long-term prognosis of neuropathy associated with anti-MAG IgM M-proteins and its relationship to immune therapies. Brain. 2000; 123:710–17.
- Oksenhendler E, Chevret S, Leger JM, Louboutin JP, Bussel A, Brouet JC. Plasma exchange and chlorambucil in polyneuropathy associated with monoclonal IgM gammopathy. IgM-associated Polyneuropathy Study Group. J Neurol Neurosurg Psychiatry. 1995; 59:243–7.
- Léger JM, Viala K, Nicolas G, Créange A, Vallat JM, Pouget J, Clavelou P, Vial C, Steck A, Musset L, Marin B ; For the RIMAG Study Group (France and Switzerland). Placebo-controlled trial of rituximab in IgM anti-myelin-associated glycoprotein neuropathy. Neurology. 2013 May 10.
- Dalakas MC, Rakocevic G, Salajegheh M, Dambrosia JM, Hahn AF, Raju R, et al. Placebo-controlled trial of rituximab in IgM anti-myelin-associated glycoprotein antibody demyelinating neuropathy. Ann Neurol. 2009;65:286–93.
- Kristinsson SY, Tang M, Pfeiffer RM, Bjorkholm M, Goldin LR, Blimark C, et al. Monoclonal gammopathy of undetermined significance and risk of infections: a population-based study. Haematologica. 2012;97:854–8.
- Paueksakon P, Revelo MP, Horn RG, Shappell S, Fogo AB. Monoclonal gammopathy: significance and possible causality in renal disease. Am J Kidney Dis. 2003;42:87–95.
- Daoud MS, Lust JA, Kyle RA, Pittelkow MR. Monoclonal gammopathies and associated skin disorders. J Am Acad Dermatol. 1999;40 :507–35.
- Bianchi G, Kyle RA, Colby CL, Larson DR, Kumar S, Katzmann JA, et al. Impact of optimal follow-up of monoclonal gammopathy of undetermined significance on early diagnosis and prevention of myeloma- related complications. Blood. 2010;116:2019–25.
- Rajkumar SV, Kyle RA, Buadi FK. Advances in the diagnosis, classification, risk stratification, and management of monoclonal gammopathy of undetermined significance: implications for recategorizing disease entities in the presence of evolving scientific evidence. Mayo Clin Proc. 2010;85:945–8.