Abstract
Aim. This review evaluated the effects of activity monitor-based counseling on physical activity (PA) and generic and disease-specific health-related outcomes in adults with diabetes mellitus type II (DMII), chronic obstructive pulmonary disease (COPD), or chronic heart failure (CHF).
Methods. Four electronic databases were searched for randomized controlled trials using activity monitor-based counseling versus control intervention or usual care in adults with DMII, COPD, or CHF. Pooled effect sizes were calculated using a random effects model.
Results. Twenty-four articles were included: 21 DMII studies and 3 COPD studies. No CHF studies were identified. Pooled analysis showed that activity monitor-based counseling resulted in a significantly greater improvement in PA compared to control intervention or usual care in DMII. Furthermore, these interventions had a beneficial effect on hemoglobin A1c (HbA1c), systolic blood pressure, and body mass index (BMI) (P < 0.05), whereas no differences were found on diastolic blood pressure, and health-related quality of life. Meta-analysis of COPD studies was not possible due to lack of available data.
Conclusion. Activity monitor-based counseling had a beneficial effect on PA, HbA1c, systolic blood pressure, and BMI in patients with DMII. Data in patients with COPD and CHF are limited or non-existing, respectively.
Key messages
Physical activity is associated with numerous health benefits, which indicates the importance of promoting physical activity, especially in sedentary patients with diabetes mellitus type II (DMII), chronic obstructive pulmonary disease (COPD), or chronic heart failure (CHF), who have a higher risk of hospitalization and mortality.
Activity monitor-based interventions have beneficial effects on physical activity, HbA1c, systolic blood pressure, and BMI in patients with DMII.
Data in patients with COPD and CHF are limited or non-existing, respectively.
Introduction
Regular physical activity has a positive impact on the onset and progression of prevalent chronic diseases, such as cardiovascular disease, obesity, diabetes mellitus type II (DMII), stroke, chronic obstructive pulmonary disease (COPD), chronic heart failure (CHF), and some forms of cancer (Citation1–3). This indicates the importance of promoting physical activity in the general population by organizations, such as the American College of Sports Medicine (ACSM) and the American heart Association (AHA) (Citation4). Despite substantial health benefits from regular physical activity, many older adults do not meet the ACSM/AHA physical activity recommendations (Citation5), and the level of physical activity seriously declines with increasing age (Citation5,Citation6). Having a chronic disease most probably accelerates this decline in physical activity, as patients with DMII, COPD, or CHF are markedly physically inactive compared to healthy peers (Citation7–10).
Physical inactivity in older adults, particularly in elderly with a chronic disease, results in a significant economic burden, accounting for up to 3% of the direct health care costs in developed countries (Citation11,Citation12). Moreover, sedentary patients with DMII, COPD, or CHF have a higher risk of disease-related hospitalization and mortality (Citation1,Citation13–15). Therefore, there is a need for low-cost, easily accessible, and side-effect-free programs to promote physical activity in older adults with a chronic disease.
Nowadays, activity monitors are increasingly popular to promote physical activity (Citation16,Citation17). They can be categorized as simple and inexpensive pedometers, which basically quantify steps, to technologically advanced accelerometers, which assess the amount and intensity of physical activity in daily life (Citation18). Activity monitors can be worn without major inconvenience, are compatible with most daily activities, and are easy to use (Citation18). Moreover, feedback from the activity monitors can be used to increase physical activity (Citation17). Therefore, physical activity monitors can be a very useful tool to promote physical activity.
A previous review has already shown that physical activity counseling is associated with a significant increase in self-reported daily physical activity levels (Citation16). However, self-reported physical activity measures may not provide valuable data, since they lack precision and may be influenced by social desirability bias (Citation18). Other reviews showed that interventions of physical activity counseling combined with an activity monitor have a beneficial effect on daily physical activity levels in adults and subjects living with disability and/or chronic disease (Citation17,Citation19). To date, little is known about the effects of activity monitor-based counseling on objectively measured physical activity and generic health-related outcomes in chronic diseases, such as hospitalization and survival rates; and on disease-specific health-related outcomes, such as hemoglobin A1c (HbA1c), body mass index (BMI), systemic blood pressure, and quality of life (Citation1,Citation13,Citation14, Citation20–24).
Therefore, the aim of this study was to evaluate systematically the effect of activity monitor-based counseling on physical activity levels in adults with a highly prevalent chronic disease, such as DMII, COPD, or CHF. Additionally, we sought to determine the effect of these interventions on generic and disease-specific health-related outcomes.
Methods
This systematic review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Citation25).
Eligibility criteria
Included studies met the following criteria: 1) Participants: patients with DMII, COPD, or CHF; 2) Intervention: (randomized) controlled trials using an activity monitor-based counseling intervention versus a control intervention or usual care; 3) Primary outcome: objectified physical activity; and 4) Secondary outcomes: generic and/or disease-specific health-related outcomes.
Data sources and searches
A computerized literature search was performed in Medline/PubMed, Web of Knowledge (WOK), Embase, and BIOSIS in August 2012. The following key words were used to identify relevant trials: (activit? OR “energy expenditure” OR step* OR walk?) AND (“chronic obstructive pulmonary disease” OR copd OR “chronic lung disease” OR emphysema OR “chronic bronchitis” OR “chronic heart failure” OR chf OR “congestive heart failure” OR “chronic heart disease” OR “chronic disease*” OR diabet?) AND (acceleromet? OR pedomet? OR “activity monitor?” OR “step count?” OR actigraph?).
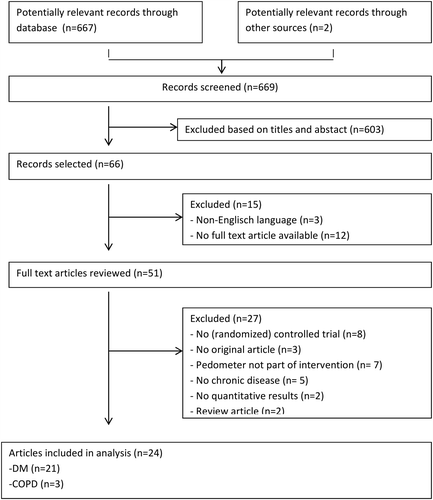
In total, 667 articles were retrieved. In addition two potentially relevant articles were found by checking reference lists of included trials ().
Study selection
Titles and abstracts were screened against inclusion criteria by two authors (A.W.V. and A.C.), and potentially eligible articles were retrieved. All articles were independently selected for inclusion by two reviewers (A.W.V. and A.C.). Disagreements could be resolved by consulting a third reviewer (M.A.). Non-English language articles, review articles, editorials, qualitative studies, and congress abstracts were excluded.
Data extraction and assessment of methodological quality
A predesigned data abstraction form was used to obtain data on study design and relevant results. For each study, authors, journal, years of publication, studied disease, patient characteristics (gender, age, and disease severity), intervention, outcome parameters, and results were recorded. Authors of included studies were contacted via (multiple) e-mail(s) and telephone call(s) to request additional data when applicable. The methodological quality of the included trials was scored independently using the Physiotherapy Evidence-based Database (PEDro) scale (Citation26). This scale is based largely on the Delphi List and expert consensus. It consists of 11 items to score the internal validity and statistics. Items scored a ‘yes’ if the criterion was clearly satisfied, and all ‘yes’ were summed. A κ coefficient was used to measure the level of inter-observer agreement. Previously, trials with a PEDro score of ≥ 6 points were classified as ‘high-quality trials’; trials with a PEDro score ≤ 5 points were classified as ‘low-quality trials’ (Citation26).
Statistical analysis
Meta-analytic techniques were conducted using a random effects model in RevMan 5. Pooled effect estimates were calculated using a random effects model for physical activity and health-related outcomes. All trials reporting these outcomes were included in the model. If a study included more than one intervention group, each comparison with the control group was entered in the meta-analysis.
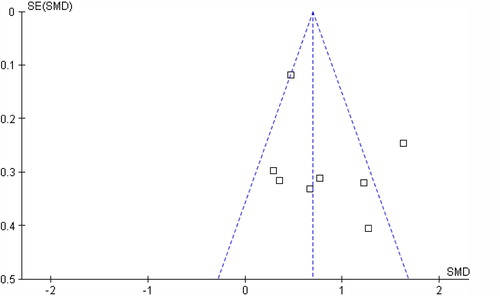
For each analysis, the I2 statistic was calculated to estimate the proportion of observed variance in effects across studies. Values of 25%, 50%, and 75% were used as boundary limits for low, moderate, or high heterogeneity, respectively (Citation27). The presence of publication bias was checked by a funnel plot for change in physical activity level.
Results
Study and participant characteristics
Twenty-four articles fulfilled the review criteria (). Twenty-one studies included subjects with DMII, and three studies included subjects with COPD. The selection included no trials that studied patients with CHF. Funnel plot did not display asymmetry, which indicates that there is no indication for publication bias (). PEDro scores ranged from 4 to 9 points (median: 7 points; ). Three studies were classified as ‘low-quality trials’ (Citation28–30). Failure to conceal allocation and blinding of the patients, therapists, and/or outcome assessors were the most prevalent methodological shortcomings.
Table I. Methodological quality studies.
A total of 2908 participants were evaluated: 2763 patients with DMII and 145 patients with COPD ( and ). About half of the patients (48.1%) were male. Reported mean age ranged from 47.0 to 70.9 years in patients with DMII, and 61.2 to 65.7 years in patients with COPD. However, in some studies data on patient characteristics were missing or incomplete ( and ) (Citation28,Citation29,Citation31–35). Drop out ranged from 0 to 38% ( and ).
Table II. Characteristics studies including patients with DMII.
Table III. Characteristics studies including patients with COPD.
Intervention characteristics
Different types of interventions were used to evaluate the effect of activity monitor-based counseling on physical activity levels ( and ). Mean duration of the interventions was 30.3 weeks (range 5 to 260 weeks). In most interventions, physical activity counseling was delivered in one or more face-to-face sessions (Citation29,Citation30,Citation32–50). These sessions were given by a nurse, physical therapist, (clinical) psychologist, and/or general practitioner. Supplementary advice or counseling was delivered by telephone (Citation32–34,Citation36,Citation48), video-conference (Citation50), video (Citation41), or in written form (Citation28,Citation46). All studies used an activity monitor device to provide feedback to patients.
In 16 studies, intervention was compared with usual care combined with one or more sessions of advice or information on physical activity (Citation28–30,Citation33–37,Citation39,Citation41–43,Citation45–48). In eight studies, control subjects received only usual care (no intervention) (Citation31,Citation32,Citation38,Citation40,Citation44,Citation49–51).
Diabetes mellitus type II
Effects on physical activity
Physical activity level at baseline and post-intervention was measured using different activity monitors (). In all studies, physical activity counseling sessions were based on data acquired from the activity monitors. The most reported outcome parameter on physical activity was number of steps per day (13 studies), but several other parameters were used, such as time spent walking (Citation42,Citation43), activity counts (Citation34,Citation35,Citation46,Citation47), activity intensity (Citation36), frequency (Citation36), and/or energy expenditure (Citation33,Citation45).
A pooled analysis to study the effect of activity monitor-based counseling on changes in steps per day included seven studies, all using a pedometer to determine step counts. Four studies were excluded because steps per day were only available in the intervention group (Citation28,Citation30,Citation37,Citation51), and in two studies absolute changes in steps per day were not available, even after contacting the corresponding authors (Citation46,Citation50).
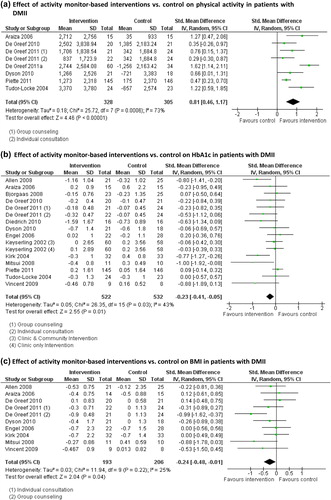
Analysis showed that activity monitor-based interventions in combination with counseling resulted in a significantly greater number of steps per day in patients with DMII compared to a usual care control group (steps per day: + 2042; 95% confidence interval 1067 to 3017; P < 0.001; ). Heterogeneity was moderate (I2 = 73%), which can be caused by differences in included patients (age, gender distribution) and interventions (methods, duration).
Figure 3. Effects of activity monitor-based counseling on physical activity and generic and disease-specific health-related outcomes in adults with DMII. A: Effect of activity monitor-based interventions versus control on physical activity (steps/day) in patients with DMII; B: Effect of activity monitor-based interventions versus control on HbA1c in patients with DMII; C: Effect of activity monitor-based interventions versus control on BMI in patients with DMII; D: Effect of activity monitor-based interventions versus control on systolic blood pressure in patients with DMII; E: Effect of activity monitor-based interventions versus control on diastolic blood pressure in patients with DMII.
Studies excluded from the meta-analysis showed contrary results. In several studies, in which steps per day was only measured in the intervention group, a significant improvement in steps per day was found after an activity monitor-based intervention in patients with DMII (Citation28,Citation30,Citation51); only one study showed no improvement (Citation37). Another study, in which absolute changes in steps per day were not available from the corresponding author, found no significant differences in change in steps per day between an activity monitor-based intervention and usual care (Citation46). In one study, data on steps per day were not presented (Citation50).
Studies using activity counts (Citation34,Citation36), time spent walking (Citation43), total physical activity (Citation43,Citation50), and energy expenditure (Citation33) as parameters to measure intervention effects on physical activity all reported significant positive benefits. Only the study of Engel and colleagues found no differences between subjects following an activity monitor-based intervention and a control group (Citation42).
Effects on generic health-related outcomes
None of the studies used hospitalization or mortality rate as primary outcomes.
Effects on disease-specific health-related outcomes
Hemoglobin A1c. Sixteen studies reported intervention effects on HbA1c. Because in two studies data were not available in absolute changes and standard deviation, even after contacting authors (Citation46,Citation50), 14 studies could be included in the pooled analysis.
Results show a significantly greater decrease in HbA1c in the intervention group compared to controls (standardized mean difference –0.23; 95% confidence interval –0.41 to –0.05; P = 0.01; ). The heterogeneity of the studies was moderate (I2 = 43%; ). Results remained significant after exclusion of the study of Allen et al., in which physical activity counseling was combined with a continuous glucose monitoring system (standardized mean difference –0.19; 95% confidence interval –0.36 to –0.02; P = 0.03) (Citation36). One study excluded from the meta-analysis did not observe between-group differences in HbA1c (Citation46).
Body mass index (BMI). Eleven articles described the effect of the intervention on BMI. Nine studies were included in the pooled analyses. The other two studies were excluded from the analysis because data were not available in absolute changes and standard deviation, even after contacting authors (Citation45,Citation46).
Results show a beneficial effect on decreasing BMI following an activity monitor-based intervention compared to control interventions (standardized mean difference –0.24; 95% confidence interval –0.48 to –0.01; P = 0.04; ). Heterogeneity of the included studies was low (I2 = 25%). Studies excluded from the meta-analysis found no significant differences between the intervention and control groups (Citation45,Citation46).
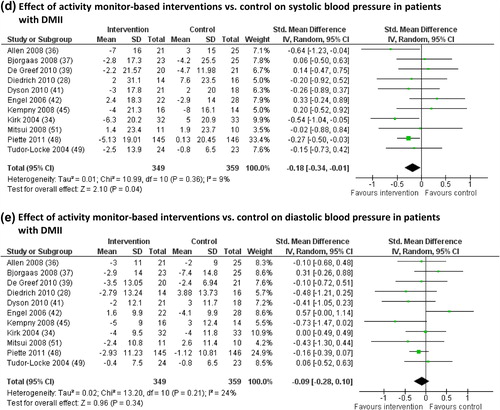
Systolic and diastolic blood pressure. Effects on systolic and diastolic blood pressure were reported in 14 studies. In three studies absolute changes in systolic and diastolic blood pressure were not available, even after contacting authors (Citation31,Citation46,Citation50). Therefore, pooled analysis was performed using 11 studies ( and 3E).
Pooled analysis showed a significant difference in changes in systolic blood pressure (P = 0.04; ), but not in diastolic blood pressure (P = 0.34; ), between the intervention and control groups. Heterogeneity of the studies was low (I2 = 9% and 24%). Two studies excluded from the meta-analysis found no significant changes in systolic or diastolic blood pressure after activity monitor-based interventions (Citation31,Citation46).
Health-related quality of life. Health-related quality of life was examined in four studies (Citation33,Citation35,Citation41,Citation50). No meta-analysis was conducted because of the use of different questionnaires (SF-36, SF-12, Mental and Social Well-Being Scale, and WHO-5 Well-Being Index), or because absolute changes in health-related quality of life were not presented (Citation41,Citation50). Studies found no significant improvement in health-related quality of life in patients with DMII, or no significant between-group differences (Citation33,Citation35,Citation41).
COPD
Effects on physical activity
Physical activity was measured using different activity monitors (). Studies used steps per day (Citation38,Citation44) or activity counts (Citation29) as outcome parameters. A pooled analysis to study the effect of an activity monitor-based intervention on changes in steps per day could not be performed, because absolute changes in steps per day were not available, even after contacting authors (Citation38,Citation44). In studies presenting only absolute changes in steps per day, one study showed a significant difference in steps per day between an activity monitor-based intervention and usual care (Citation44), whereas no significant difference was found in another study (Citation38). A study using activity counts as parameter to measure intervention effects on physical activity only reported significant short-term benefits (Citation29).
Effects on generic health-related outcomes
None of the studies used hospitalization or mortality rate as primary outcomes.
Effects on disease-specific health-related outcomes
Health-related quality of life. Health-related quality of life was examined in all studies (Citation29,Citation44). No meta-analysis was conducted, because of the use of different questionnaires (St Georges Respiratory Questionnaire (SGRQ), Clinical COPD Questionnaire (CCQ), Seattle Obstructive Lung Disease Questionnaire (SOLDQ), 36-Item Short-Form Health Survey (SF-36)). One study found a beneficial effect of an activity monitor-based intervention on health-related quality of life in patients with COPD compared to a control intervention (Citation44), whereas other studies found no significant between-group differences in health-related quality of life (Citation29).
Discussion
In this review we systematically evaluated the effect of activity monitor-based interventions on physical activity levels and health-related outcomes in patients with a DMII, COPD, or CHF. In general, interventions using an activity monitor in combination with counseling are associated with a significantly greater improvement in physical activity compared to a control intervention or usual care in patients with DMII. Moreover, these interventions had a beneficial effect on HbA1c, systolic blood pressure, and BMI in patients with DMII. Contrary results were found on physical activity and health-related quality of life in patients with COPD, although data were limited. None of the studies used hospitalization or mortality rate as primary outcomes. Moreover, CHF trials were not available.
Daily physical activity level
Pooled analysis showed that interventions using an activity monitor in combination with counseling had a beneficial effect on physical activity, measured in steps per day, compared to a control intervention (). This is consistent with findings of an earlier meta-analysis, showing that the use of a pedometer is associated with a significant increase of more than 2000 steps per day in out-patient (healthy) adults (Citation17). Avery et al. also showed an increase in physical activity levels in patients with DMII, following behavioral interventions including exercise (Citation24). Nevertheless, relevant articles seem to be missing in this review (Citation33,Citation36,Citation37,Citation42,Citation48).
The included studies used different types of activity monitors ( and ). Pedometers can be used to provide direct feedback to the participants on the level of physical activity (number of steps per day). Therefore, participants can monitor themselves and set goals for increasing their level of physical activity. In contrast, most accelerometers do not have a display and can therefore not provide direct feedback to participants. However, accelerometer data, processed by a computer, can be used for promoting physical activity by counseling sessions or by a self-monitoring approach.
It is known that simple pedometers are less sensitive to lower force accelerations, such as slow walking, than accelerometers (Citation18). Moreover, it has earlier been shown that patients with a chronic disease walk less, but also with a lower speed compared to healthy adults (Citation52), which can lead to an underestimation of steps while using a pedometer (Citation53). Furthermore, pedometers can only give an indication about the number of steps per day, whereas an accelerometer is able to give information about the quantity and intensity of performed activities (Citation18).
Health-related outcomes
To date, the effects of activity monitor-based counseling on hospitalization or survival have not been studied. Then again, this systematic review showed a significantly larger decrease in HbA1c in the intervention group compared to a control intervention or usual care in patients with DMII (). This is consistent with results of earlier reviews showing a positive effect of physical activity on the reduction of HbA1c, which was not mediated by weight loss (Citation23,Citation24,Citation54). A decrease in HbA1c level is an indication of better glucose control, which is associated with a reduced burden of cardiovascular disease and microvascular complications in people with DMII (Citation55). Indeed, current guidelines recommend intensive glycemic control as an effective treatment, with a target HbA1c of 7% or less (Citation56). However, results regarding the association between HbA1c and cardiovascular events in DMII are contrary (Citation57,Citation58). Therefore, it remains unknown whether and to what extent a reduction in HbA1c will result in a long-term health benefit in patients with DMII.
Pooled analysis showed a beneficial effect on decreasing BMI following an activity monitor-based intervention compared to control interventions, although results are limited to patients with DMII (). These results confirm findings of earlier reviews, in which it was shown that interventions promoting physical activity resulted in a significant reduction in BMI both in healthy sedentary adults and in adults with COPD, DMII, neuromuscular disease, or obesity (Citation17,Citation24). Unfortunately, data on changes in fat free mass and fat mass are limited, whereas these data provide more valuable information than changes in BMI.
The risk of additional weight gain is increased in patients with DMII as a result of a reduced level of physical activity compared to healthy peers. Earlier studies already showed that weight loss is associated with positive long-term outcomes and substantial reduction in mortality in obese patients with DMII (Citation59).
Obesity is also associated with decreased levels of physical activity in patients with COPD (Citation21). The currently available data do not suggest a reduction in BMI following activity monitor-based counseling. Then again, being overweight or obese has been associated with decreased mortality among patients with severe COPD, a phenomenon commonly referred to as the ‘obesity paradox’ (Citation60).
Elevated resting blood pressure is associated with a significantly increased risk for cardiovascular diseases (Citation61). An earlier published review already showed the positive effects of physical activity on decreasing blood pressure (Citation17), which in turn will result in a better prognosis and health benefits. Pooled analyses in the current review showed a beneficial effect on systolic blood pressure following an activity monitor-based intervention compared to a control intervention in patients with DMII, whereas no significant changes were found in diastolic blood pressure ( and 3E). Earlier it was shown that even relatively small reductions in resting diastolic blood pressure of 2 mmHg can reduce the cardiovascular risk by 6% (Citation62). Indeed, most studies showed a decrease in diastolic blood pressure of ≥ 2 mmHg following an activity monitor-based intervention (Citation34,Citation36,Citation37,Citation39,Citation41,Citation46,Citation48), which was found non-significant in some studies (Citation39,Citation41,Citation46) or non-different between intervention and control group (Citation34,Citation37,Citation48).
This systematic review found contrary results on the health-related quality of life after activity monitor-based interventions. Indeed, different questionnaires were used (SGRQ (Citation44), CCQ (Citation44), SOLDQ (Citation29), SF-36 (Citation29,Citation35,Citation44), SF-12 (Citation50), Mental and Social Well-Being Scale (Citation33) and WHO-5 Well-Being Index (Citation41)), making it difficult to compare outcomes. However, quality of life is an important measure of health, particular for older people and those suffering from a chronic disease. Indeed, it has earlier been shown that patients with DMII, COPD, or CHF have a reduced health-related quality of life compared to healthy peers, and it decreases with disease progression and complications (Citation63–65). Moreover, worsening of health-related quality of life is associated with increased overall mortality (Citation65–67), and maintaining moderate or high levels of physical activity and improvement in physical activity level are associated with a better health-related quality of life in patients with DMII, COPD, or CHF (Citation20,Citation22,Citation68).
Strengths
To the best of our knowledge, this review is the first to examine the effects of activity monitor-based counseling on objectified physical activity levels and health-related outcomes in adults with a chronic disease. Indeed, earlier reviews already studied the effectiveness of physical activity promotion in adults and subjects living with disability and/or chronic disease (Citation16,Citation17,Citation19), but these reviews only focused on physical activity (Citation19), included studies with healthy subjects (Citation17), or included studies using self-reported measures of physical activity (Citation16,Citation24). The strength of this meta-analysis is that it included only trials that objectively measured physical activity before and after the intervention, because self-reported measures are associated with a lack of precision and with sensitivity to social desirability (Citation18,Citation69). Surprisingly, studies on the effects of activity monitor-based counseling in patients with CHF are currently lacking. Therefore, we believe we have identified a major research topic within CHF. Indeed, accelerometers are already incorporated in implanted devices for continuous activity telemonitoring in patients with CHF (Citation70). Moreover, physical activity monitoring using an accelerometer during the first phase of cardiac rehabilitation (in hospital) and directly after discharge from the hospital may increase physical activity levels in patients with cardiovascular disease, such as coronary artery bypass graft, myocardial infarction, and valve replacement (Citation71). Therefore, it seems reasonable to assume that patients with CHF also may benefit from interventions using an accelerometer.
Limitations
Our review has several limitations. Firstly, the included results may have been biased, because outcome assessors, therapists, and/or patients were not blinded for the intervention arm. However, blinding of patients and therapists is probably not possible in these study designs, and most probably will not affect objective measures such as HbAc1 and blood pressure.
Secondly, this meta-analysis included trials with different intervention durations (5 weeks to 260 weeks), methods of counseling (number of sessions, individual or group, face- to-face or written, phone or video), activity monitors, and patient characteristics (age, gender, distribution). Moreover, the use of the activity monitors to measure physical activity before and after the intervention and also the use during the intervention was different in most studies, though reportings on this were often limited. Although our review demonstrated the effectiveness of activity monitor-based programs, these differences could have influenced the intervention effects. Because of the large variety in activity monitor types, intervention duration, and intervention design, a sensitivity analysis was not feasible. Therefore, we were not able to demonstrate differences between different activity monitors, intervention duration, and intervention designs. Furthermore, these differences in study designs resulted in low to moderate scores of heterogeneity (0% to 73%). However, it was earlier shown that about a quarter of meta-analyses have I2 values over 50% (Citation27).
Thirdly, data on physical activity and health-related outcomes in patients with COPD and CHF were limited or non-existing. It can be assumed that activity monitor-based interventions in patients with COPD and CHF will have similar beneficial effects as in patients with DMII. However, the number and quality of the data studies are not sufficient to support this assumption, and therefore, conclusions on health-related outcomes are mainly based on studies including patients with DMII.
Fourthly, interventions using an activity monitor in combination with counseling appear to be cost-effective in the general population (Citation72). It seems reasonable to assume that the cost-effectiveness is even higher in patients with DMII, COPD, or CHF. Nevertheless, this remains currently unknown.
Finally, 24 studies were selected for this review, but not all studies could be included in the meta-analysis, because studies used different physical activity parameters or different questionnaires to measure health-related quality of life. Furthermore, some of the authors did not reply to the request for additional data (Citation46,Citation50), or were not able to provide the requested data (Citation38,Citation44). Therefore, the observed effect of this meta-analysis may be biased by this exclusion.
To conclude, our review shows the effectiveness of activity monitor-based interventions to promote physical activity in patients with DMII. Moreover, these interventions resulted in significant reductions in HbA1c, systolic blood pressure, and BMI in patients with DMII compared to usual care. Data in patients with COPD and CHF are limited or non-existing, respectively. Future studies are needed to study the long-term effects of activity monitor-based counseling interventions on physical activity and generic and disease-specific outcomes, including a cost-effectiveness analysis.
Acknowledgements
The authors thank the corresponding authors of references (Citation28,Citation30,Citation31,Citation33,Citation34,Citation39,Citation41,Citation45,Citation48) for sending us additional data which made the meta-analyses possible.
Declaration of interest: This work was supported by ‘Stichting de Weijerhorst’ and Point-One funding from AgentschapNL, Dutch Ministry of Economic affairs, the Netherlands. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The authors report no conflicts of interest.
References
- Garcia-Aymerich J, Lange P, Benet M, Schnohr P, Anto JM. Regular physical activity reduces hospital admission and mortality in chronic obstructive pulmonary disease: a population based cohort study. Thorax. 2006;61:772–8.
- Sigal RJ, Kenny GP, Wasserman DH, Castaneda-Sceppa C, White RD. Physical activity/exercise and type 2 diabetes: a consensus statement from the American Diabetes Association. Diabetes Care. 2006; 29:1433–8.
- Lee IM, Shiroma EJ, Lobelo F, Puska P, Blair SN, Katzmarzyk PT. Effect of physical inactivity on major non-communicable diseases worldwide: an analysis of burden of disease and life expectancy. Lancet. 2012; 380:219–29.
- Nelson ME, Rejeski WJ, Blair SN, Duncan PW, Judge JO, King AC, et al. Physical activity and public health in older adults: recommendation from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc. 2007;39:1435–45.
- Hallal PC, Andersen LB, Bull FC, Guthold R, Haskell W, Ekelund U. Global physical activity levels: surveillance progress, pitfalls, and prospects. Lancet. 2012;380:247–57.
- Caspersen CJ, Pereira MA, Curran KM. Changes in physical activity patterns in the United States, by sex and cross-sectional age. Med Sci Sports Exerc. 2000;32:1601–9.
- Resnick HE, Foster GL, Bardsley J, Ratner RE. Achievement of American Diabetes Association clinical practice recommendations among U.S. adults with diabetes, 1999–2002: the National Health and Nutrition Examination Survey. Diabetes Care. 2006;29:531–7.
- Morrato EH, Hill JO, Wyatt HR, Ghushchyan V, Sullivan PW. Physical activity in U.S. adults with diabetes and at risk for developing diabetes,2003. Diabetes Care.2007;30:203–9.
- Gosker HR, Lencer NH, Franssen FM, van der Vusse GJ, Wouters EF, Schols AM. Striking similarities in systemic factors contributing to decreased exercise capacity in patients with severe chronic heart failure or COPD. Chest. 2003;123:1416–24.
- Waschki B, Spruit MA, Watz H, Albert PS, Shrikrishna D, Groenen M, et al. Physical activity monitoring in COPD: compliance and associations with clinical characteristics in a multicenter study. Respir Med. 2012; 106:522–30.
- Oldridge NB. Economic burden of physical inactivity: healthcare costs associated with cardiovascular disease. Eur J Cardiovasc Prev Rehabil. 2008;15:130–9.
- World Health Organization. The World Health Report 2002: Reducing risks to health, promoting healthy life. Geneva: WHO; 2002.
- Oerkild B, Frederiksen M, Hansen JF, Prescott E. Self-reported physical inactivity predicts survival after hospitalization for heart disease. Eur J Cardiovasc Prev Rehabil. 2011;18:475–80.
- Wei M, Gibbons LW, Kampert JB, Nichaman MZ, Blair SN. Low cardiorespiratory fitness and physical inactivity as predictors of mortality in men with type 2 diabetes. Ann Intern Med. 2000; 132:605–11.
- Waschki B, Kirsten A, Holz O, Muller KC, Meyer T, Watz H, et al. Physical activity is the strongest predictor of all-cause mortality in patients with COPD: a prospective cohort study. Chest. 2011;140: 331–42.
- Orrow G, Kinmonth AL, Sanderson S, Sutton S. Effectiveness of physical activity promotion based in primary care: systematic review and meta-analysis of randomised controlled trials. BMJ. 2012; 344:e1389.
- Bravata DM, Smith-Spangler C, Sundaram V, Gienger AL, Lin N, Lewis R, et al. Using pedometers to increase physical activity and improve health: a systematic review. JAMA. 2007;298:2296–304.
- Pitta F, Troosters T, Probst VS, Spruit MA, Decramer M, Gosselink R. Quantifying physical activity in daily life with questionnaires and motion sensors in COPD. Eur Respir J. 2006;27:1040–55.
- Tudor-Locke C, Craig CL, Aoyagi Y, Bell RC, Croteau KA, De Bourdeaudhuij I, et al. How many steps/day are enough? For older adults and special populations. Int J Behav Nutr Phys Act. 2011;8:80.
- Esteban C, Quintana JM, Aburto M, Moraza J, Egurrola M, Perez-Izquierdo J, et al. Impact of changes in physical activity on health-related quality of life among patients with COPD. Eur Respir J. 2010;36:292–300.
- Watz H, Waschki B, Kirsten A, Muller KC, Kretschmar G, Meyer T, et al. The metabolic syndrome in patients with chronic bronchitis and COPD: frequency and associated consequences for systemic inflammation and physical inactivity. Chest. 2009;136:1039–46.
- Eckert K. Impact of physical activity and bodyweight on health-related quality of life in people with type 2 diabetes. Diabetes Metab Syndr Obes. 2012;5:303–11.
- Umpierre D, Ribeiro PA, Kramer CK, Leitao CB, Zucatti AT, Azevedo MJ, et al. Physical activity advice only or structured exercise training and association with HbA1c levels in type 2 diabetes: a systematic review and meta-analysis. JAMA. 2011;305:1790–9.
- Avery L, Flynn D, van Wersch A, Sniehotta FF, Trenell MI. Changing physical activity behavior in type 2 diabetes: a systematic review and meta-analysis of behavioral interventions. Diabetes Care. 2012;35:2681–9.
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097.
- Foley NC, Bhogal SK, Teasell RW, Bureau Y, Speechley MR. Estimates of quality and reliability with the physiotherapy evidence-based database scale to assess the methodology of randomized controlled trials of pharmacological and nonpharmacological interventions. Phys Ther. 2006;86:817–24.
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60.
- Diedrich A, Munroe DJ, Romano M. Promoting physical activity for persons with diabetes. Diabetes Educ. 2010;36:132–40.
- Steele BG, Belza B, Cain KC, Coppersmith J, Lakshminarayan S, Howard J, et al. A randomized clinical trial of an activity and exercise adherence intervention in chronic pulmonary disease. Arch Phys Med Rehabil. 2008;89:404–12.
- Vincent D. Culturally tailored education to promote lifestyle change in Mexican Americans with type 2 diabetes. J Am Acad Nurse Pract. 2009;21:520–7.
- Araiza P, Hewes H, Gashetewa C, Vella CA, Burge MR. Efficacy of a pedometer-based physical activity program on parameters of diabetes control in type 2 diabetes mellitus. Metabolism. 2006; 55:1382–7.
- De Greef KP, Deforche BI, Ruige JB, Bouckaert JJ, Tudor-Locke CE, Kaufman JM, et al. The effects of a pedometer-based behavioral modification program with telephone support on physical activity and sedentary behavior in type 2 diabetes patients. Patient Educ Couns. 2011;84:275–9.
- Keyserling TC, Samuel-Hodge CD, Ammerman AS, Ainsworth BE, Henriquez-Roldan CF, Elasy TA, et al. A randomized trial of an intervention to improve self-care behaviors of African-American women with type 2 diabetes: impact on physical activity. Diabetes Care. 2002;25:1576–83.
- Kirk A, Mutrie N, MacIntyre P, Fisher M. Effects of a 12-month physical activity counselling intervention on glycaemic control and on the status of cardiovascular risk factors in people with type 2 diabetes. Diabetologia. 2004;47:821–32.
- Kirk AF, Higgins LA, Hughes AR, Fisher BM, Mutrie N, Hillis S, et al. A randomized, controlled trial to study the effect of exercise consultation on the promotion of physical activity in people with type 2 diabetes: a pilot study. Diabet Med. 2001;18:877–82.
- Allen NA, Fain JA, Braun B, Chipkin SR. Continuous glucose monitoring counseling improves physical activity behaviors of individuals with type 2 diabetes: a randomized clinical trial. Diabetes Res Clin Pract. 2008;80:371–9.
- Bjorgaas MR, Vik JT, Stolen T, Lydersen S, Grill V. Regular use of pedometer does not enhance beneficial outcomes in a physical activity intervention study in type 2 diabetes mellitus. Metabolism. 2008;57:605–11.
- de Blok BM, de Greef MH, ten Hacken NH, Sprenger SR, Postema K, Wempe JB. The effects of a lifestyle physical activity counseling program with feedback of a pedometer during pulmonary rehabilitation in patients with COPD: a pilot study. Patient Educ Couns. 2006;61: 48–55.
- De Greef K, Deforche B, Tudor-Locke C, De Bourdeaudhuij I. A cognitive-behavioural pedometer-based group intervention on physical activity and sedentary behaviour in individuals with type 2 diabetes. Health Educ Res. 2010;25:724–36.
- De Greef K, Deforche B, Tudor-Locke C, De Bourdeaudhuij I. Increasing physical activity in Belgian type 2 diabetes patients: a three-arm randomized controlled trial. Int J Behav Med. 2011;18:188–98.
- Dyson PA, Beatty S, Matthews DR. An assessment of lifestyle video education for people newly diagnosed with type 2 diabetes. J Hum Nutr Diet. 2010;23:353–9.
- Engel L, Lindner H. Impact of using a pedometer on time spent walking in older adults with type 2 diabetes. Diabetes Educ. 2006;32: 98–107.
- Furber S, Monger C, Franco L, Mayne D, Jones LA, Laws R, et al. The effectiveness of a brief intervention using a pedometer and step-recording diary in promoting physical activity in people diagnosed with type 2 diabetes or impaired glucose tolerance. Health Promot J Austr. 2008;19:189–95.
- Hospes G, Bossenbroek L, Ten Hacken NH, van Hengel P, de Greef MH. Enhancement of daily physical activity increases physical fitness of outclinic COPD patients: results of an exercise counseling program. Patient Educ Couns. 2009;75:274–8.
- Kempny A, Moczulski D, Kempny A. [Pedometer improves doctor's advice to enhance physical activity in type 2 diabetes]. Diabetologia Doswiadczalna i Klinicza. 2008;8:33–7.
- Kirk A, Barnett J, Leese G, Mutrie N. A randomized trial investigating the 12-month changes in physical activity and health outcomes following a physical activity consultation delivered by a person or in written form in Type 2 diabetes: Time2Act. Diabet Med. 2009;26:293–301.
- Paschali AA, Goodrick GK, Kalantzi-Azizi A, Papadatou D, Balasubramanyam A. Accelerometer feedback to promote physical activity in adults with type 2 diabetes: a pilot study. Percept Mot Skills. 2005;100:61–8.
- Piette JD, Richardson C, Himle J, Duffy S, Torres T, Vogel M, et al. A randomized trial of telephonic counseling plus walking for depressed diabetes patients. Med Care. 2011;49:641–8.
- Tudor-Locke C, Bell RC, Myers AM, Harris SB, Ecclestone NA, Lauzon N, et al. Controlled outcome evaluation of the First Step Program: a daily physical activity intervention for individuals with type II diabetes. Int J Obes Relat Metab Disord. 2004;28:113–9.
- Weinstock RS, Brooks G, Palmas W, Morin PC, Teresi JA, Eimicke JP, et al. Lessened decline in physical activity and impairment of older adults with diabetes with telemedicine and pedometer use: results from the IDEATel study. Age Ageing. 2011;40:98–105.
- Mitsui T, Shimaoka K, Kobayashi T.. [Examining a 30-minute walking program using a pedometer in the management of type 2 diabetes]. Gazzetta Medica Italiana Archivio per le Scienze Mediche. 2008; 167:293–8.
- Pitta F, Troosters T, Spruit MA, Probst VS, Decramer M, Gosselink R. Characteristics of physical activities in daily life in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;171:972–7.
- Cyarto EV, Myers AM, Tudor-Locke C. Pedometer accuracy in nursing home and community-dwelling older adults. Med Sci Sports Exerc. 2004;36:205–9.
- Boule NG, Haddad E, Kenny GP, Wells GA, Sigal RJ. Effects of exercise on glycemic control and body mass in type 2 diabetes mellitus: a meta-analysis of controlled clinical trials. JAMA. 2001;286:1218–27.
- Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560–72.
- American Diabetes Association. Standards of medical care in diabetes-2010. Diabetes Care. 2010;33(Suppl 1):S11–61.
- Gerstein HC, Miller ME, Genuth S, Ismail-Beigi F, Buse JB, Goff DC Jr, et al. Long-term effects of intensive glucose lowering on cardiovascular outcomes. N Engl J Med. 2011;364:818–28.
- Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360:129–39.
- Williamson DF, Thompson TJ, Thun M, Flanders D, Pamuk E, Byers T. Intentional weight loss and mortality among overweight individuals with diabetes. Diabetes Care. 2000;23:1499–504.
- Franssen FM, O’Donnell DE, Goossens GH, Blaak EE, Schols AM. Obesity and the lung: 5. Obesity and COPD. Thorax. 2008;63: 1110–7.
- Vasan RS, Larson MG, Leip EP, Evans JC, O’Donnell CJ, Kannel WB, et al. Impact of high-normal blood pressure on the risk of cardiovascular disease. N Engl J Med. 2001;345:1291–7.
- Cook NR, Cohen J, Hebert PR, Taylor JO, Hennekens CH. Implications of small reductions in diastolic blood pressure for primary prevention. Arch Intern Med. 1995;155:701–9.
- Wexler DJ, Grant RW, Wittenberg E, Bosch JL, Cagliero E, Delahanty L, et al. Correlates of health-related quality of life in type 2 diabetes. Diabetologia. 2006;49:1489–97.
- Janssen DJ, Spruit MA, Leue C, Gijsen C, Hameleers H, Schols JM, et al. Symptoms of anxiety and depression in COPD patients entering pulmonary rehabilitation. Chron Respir Dis. 2010;7:147–57.
- Iqbal J, Francis L, Reid J, Murray S, Denvir M. Quality of life in patients with chronic heart failure and their carers: a 3-year follow-up study assessing hospitalization and mortality. Eur J Heart Fail. 2010; 12:1002–8.
- Kleefstra N, Landman GW, Houweling ST, Ubink-Veltmaat LJ, Logtenberg SJ, Meyboom-de Jong B, et al. Prediction of mortality in type 2 diabetes from health-related quality of life (ZODIAC-4). Diabetes Care. 2008;31:932–3.
- Domingo-Salvany A, Lamarca R, Ferrer M, Garcia-Aymerich J, Alonso J, Felez M, et al. Health-related quality of life and mortality in male patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2002;166:680–5.
- van Tol BA, Huijsmans RJ, Kroon DW, Schothorst M, Kwakkel G. Effects of exercise training on cardiac performance, exercise capacity and quality of life in patients with heart failure: a meta-analysis. Eur J Heart Fail. 2006;8:841–50.
- Annegarn J, Spruit MA, Uszko-Lencer NH, Vanbelle S, Savelberg HH, Schols AM, et al. Objective physical activity assessment in patients with chronic organ failure: a validation study of a new single-unit activity monitor. Arch Phys Med Rehabil. 2011;92:1852–7 e1.
- Shoemaker MJ, Curtis AB, Vangsnes E, Dickinson MG, Paul R. Analysis of daily activity data from implanted cardiac defibrillators: the minimum clinically important difference and relationship to mortality/life expectancy. World Journal Of Cardiovascular Diseases. 2012;2:129–35.
- Izawa KP, Watanabe S, Hiraki K, Morio Y, Kasahara Y, Takeichi N, et al. Determination of the effectiveness of accelerometer use in the promotion of physical activity in cardiac patients: a randomized controlled trial. Arch Phys Med Rehabil. 2012;93:1896–902.
- De Smedt D, De Cocker K, Annemans L, De Bourdeaudhuij I, Cardon G. A cost-effectiveness study of the community-based intervention ‘10 000 Steps Ghent’. Public Health Nutr. 2012;15:442–51.