Abstract
Inferior vena cava filter (IVCF) use continues to increase in the United States (US) despite questionable clinical benefit and increasing concerns over long-term complications. For this review we comprehensively examine the randomized, prospective data on IVC filter efficacy, compare relative rates of IVCF placement in the US and Europe, compare commonly considered guidelines for IVCF indications, and the current data on IVCF complications. Searches of MEDLINE and Cochrane databases were conducted for randomized prospective IVCF studies. Only three randomized prospective studies for IVCFs were identified. Commonly cited IVCF guidelines were reviewed with attention to their evolution over time. No evidence has shown a survival benefit with IVCF use. Despite this, continued rising utilization, especially for primary prophylactic indications, is concerning, given increasing evidence of long-term filter-related complications. This is particularly noted in the US where IVCF placements for 2012 are projected to be 25 times that of an equivalent population in Europe (224,700 versus 9,070). Pending much-needed randomized controlled trials that also evaluate long-term safety, we support the more stringent American College of Chest Physicians (ACCP) guidelines for IVCF placement indications and advocate a close, structured follow-up of retrievable IVCFs to improve filter retrieval rates.
Key messages
We recommend using the more stringent American College of Chest Physicians (ACCP) guidelines for inferior vena cava (IVC) filter placement indications.
Recent concerns over retrievable filter complications including filter fracture have led to an FDA advisory recommending removal of retrievable filters as soon as protection from pulmonary embolism (PE) is no longer needed.
Close, structured follow-up of retrievable IVC filter patients is recommended with the goal of removal as soon as protection from PE is no longer needed.
Prospective randomized studies on IVC filter efficacy and long-term safety are critically needed.
IVC filter utilization per capita in the US is 25 times that of Europe's ‘Big 5’, and some studies suggest that medical insurance coverage and highly litigious regional medico-legal environments (defensive medicine) are correlated with increased IVC filter use in the US.
Introduction and history
Pulmonary embolism (PE) and related mortality are major concerns in patients with deep vein thrombosis (DVT). PE is responsible for 100,000–200,000 deaths in the United States (US) annually (Citation1). Early attempts at surgical interruption of the inferior vena cava (IVC) date back to the late 1800s by Trousseau (Citation2). The first endoluminal device, the Mobin-Uddin filter, was placed in 1967 (Citation3). Inferior vena cava filter (IVCF) designs evolved over the next decade, and placement via percutaneous approaches became preferred to open surgery with the advent of the Greenfield filter in the early 1970s (Citation4).
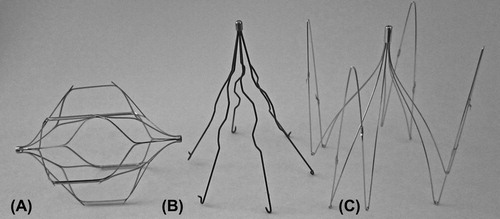
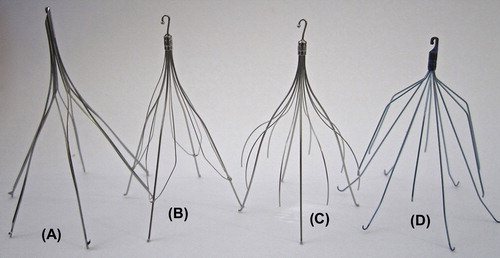
While the concept of IVC interruption appears simple, designing a device that is safe, traps thrombus while preserving natural flow in the IVC, and avoids the inherent thrombogenicity of a foreign body is challenging. Since the introduction of early endoluminal IVCFs in the 1960s, numerous IVCFs, both permanent () and ‘retrievable’ (), have been introduced into the market-place. This article reviews the published data on IVCF usage, clinical applications, current guidelines, filter complications, and obstacles related to filter success.
Materials and methods
The authors searched the Medline Database (PubMed) with the search terms: ‘randomized clinical trial’ (publication type) and the MeSH terms: ‘prospective studies’, ‘vena cava filter’, and ‘adult’. Also included in the search were the major search terms (MAJR) ‘filtration’ and ‘thrombophlebitis/prevention and control’. Search results were limited to English language and human subjects, and review articles were excluded. The search yielded 99 references. Additionally, a search of the Cochrane collaborative was included. Each article was examined and included in this review if it presented data that evaluated clinical outcomes from IVCF use including PE, DVT, filter-related complications (FRC), and cost-effectiveness of IVCFs. Using these criteria, only three prospective, randomized clinical trials on the efficacy of IVCFs were identified () (Citation5–8).
Table I. Prospective randomized trials on inferior vena cava filters (Citation5–8).
Patterns of IVCF placement
IVCF placement in the US has increased steadily over the last 30 years. From 1979 to 1999, US filter placement increased 25-fold (Citation9). More recently, Stein et al. demonstrated a sustained increase in US IVCF placements from 2001 to 2006 (Citation10). Diagnosis of DVT and PE also increased from 2001 to 2006, primarily due to an increasing availability of computed tomographic pulmonary angiography for PE detection (Citation11). However, IVCF placement in patients with known PE and/or DVT increased linearly while IVCF placement in patients without a diagnosis of DVT or PE (the so-called ‘prophylactic indication’) was superlinear, increasing at a rate nearly 7-fold from 2003 to 2006 compared to 1985–2003 (P < 0.0001) (Citation10). The timing of this rapid increase in prophylactic filter placement coincided with the FDA's approval of retrievable filter devices in 2003. Approximately 224,700 IVCFs are projected to be deployed in the US in 2012 (Citation12). The Society of Interventional Radiology (SIR) 2009 consensus panel on IVCFs noted that over half of all filter placements in the US are for prophylactic indications, with a large proportion of these being retrievable-type filters (Citation13).
Within the US, marked regional variation in IVCF usage is well documented (Citation9,Citation14,Citation15). The New England region leads the US with rates of IVCF placement double that of the Western US (Citation15). Reasons for this wide geographic variation are unclear, though lack of uniform guidelines is a common theory. Furthermore, correlational studies have shown that the presence of medical insurance coverage and highly litigious regional medico-legal environments are associated with higher utilization in the US (Citation14,Citation16).
Globally, the US dominates the IVCF placement market. Although 2010 estimates of the US population (309 million) are similar to Europe's ‘Big 5’ (France, Germany, Italy, Spain, and the United Kingdom: with a combined population of 313 million), the estimated number of IVCF placements in 2012 is 224,700 for the US and only 9,070 for Europe's Big 5 (Citation12,Citation17–20). This 25:1 (US: Europe) IVCF placement ratio is staggering, and although it is difficult to quantify accurately, the number of annual venous thromboembolism (VTE)-related deaths (with respect to population) remains similar in the US and Europe (Citation21,Citation22). A complete explanation for this marked intercontinental disparity remains unclear, but given the positive correlation of US IVC filter utilization with insurance payer status and highly litigious medico-legal regions, intercontinental differences in financial reimbursement and defensive medicine are likely at least partially responsible (Citation14,Citation16).
Indications for IVCFs
Adjuvant to chemoprophylaxis
The benefit of adding IVCFs to chemoprophylaxis has been suggested and questioned in the clinical literature (Citation23). Randomized studies that evaluate adjuvant filter placement are limited to the Prévention du Risque d’Embolie Pulmonaire par Interruption Cave (PREPIC) (Citation5,Citation7). PREPIC evaluated 400 patients with acute proximal DVT with or without a PE and randomized patients to a permanent IVCF group or to a group with no IVCF; both groups received systemic anticoagulation (). The primary outcome was symptomatic or asymptomatic PE after filter placement. Secondary outcomes included PE, recurrent DVT, death, and major complications. The PREPIC investigators found that IVCFs reduced the PE rate in the first 12 days and at 8 years post filter placement, but mortality rates were not statistically different. However, there was a 1.5-fold increased risk of recurrent DVT in the IVCF group ().
Several limitations of the PREPIC study deserve mention. PREPIC had relatively few patients, and anticoagulation regimens were not standardized after the first three months. The study also focused on early-generation IVCFs which are no longer clinically used, and failed to stratify outcomes based on specific filter device type. Lack of stratification by filter type is a critical point, since recent studies have shown that IVCF devices vary in performance and complication rates (Citation24,Citation25). Additionally, the clinical impact of the recorded decrease in PE may be overestimated. PREPIC follow-up protocols were based on annual phone interviews, eliciting symptoms of PE or DVT and recommended hospitalization or imaging based on these phone interviews. Thus, many of the PEs may well have been subclinical and only evident on probing by the physician (Citation26). Decreasing such subclinical PEs may not be of clear benefit, as suggested by the lack of mortality benefit in the IVCF group (Citation5,Citation7,Citation26).
Large, population-based studies show varying results. A study of the California patient discharge data (risk-adjusted) showed that IVCF placement was associated with significantly higher relative hazard (RH) of rehospitalization for venous thrombosis among patients who initially manifested with PE (RH 2.62; 95% confidence interval 2.09–3.29) compared to the control group without IVCF (Citation27). Stein and colleagues evaluated in-hospital case fatality rates of the National Inpatient Sample cohort, consisting of 2.1 million patients with PE (Citation28). Their study showed that for stable patients who presented with PE, patients with an IVCF had marginally improved in-hospital case fatality rates compared to patients with no IVCF (7.2% versus 7.9%, P <0.0001). However, for stable patients diagnosed with DVT, there was a higher in-hospital case fatality rate in the IVCF group compared to the control group (6.7% versus 5.3%, P < 0.0001). Unstable patients (defined as having ICD-9 code for ventilator dependence or shock) with an IVCF had a lower in-hospital case fatality rate than the control group (33% versus 51%, P < 0.0001). However, the study did not evaluate post-discharge or long-term outcomes; moreover, these population studies were limited by the retrospective design and were predicated on accurate coding.
Trauma patients
The Eastern Association of Trauma Guidelines (EAST) for prophylactic filter insertion suggest that IVCFs should be considered for high-risk trauma patients (Glasgow Coma Score < 8, incomplete spinal cord injury, closed head injury, complex pelvic and long bone fractures, and paresis) who have sustained an injury that renders them immobilized for a prolonged period of time and cannot receive anticoagulation due to bleeding (Citation29). However, the only published prospective study is a small feasibility study (n = 34) () (Citation8). A recent review of prophylactic IVCF placement for trauma patients notes that the data are mixed and high-quality studies are lacking (Citation29). This lack of high-quality data is likely responsible for the wide variation in IVCF use in trauma patients (Citation30). Furthermore, a recent cost analysis noted that compliance with the EAST guidelines is not cost-effective, costing upwards of $380,000 per quality-adjusted life year saved (Citation31). A study on spinal cord injury patients notes that if given appropriate DVT prophylaxis, the cost to prevent one additional episode of PE by placing an IVCF approaches $150,000, surpassing an accepted threshold of $50,000 (Citation32,Citation33).
Bariatric surgery
Bariatric patients represent another patient population at high risk for venous thromboembolism. Nationwide, approximately 9% of patients undergoing bariatric surgery have a preoperative, prophylactic IVCF placed (Citation34). There are several retrospective studies, limited by low enrollment, which report mixed clinical outcomes (Citation35–38). Results of a recent prospective registry of IVCFs in bariatric patients (n = 6,376, with 542 IVCF) concluded that prophylactic IVCFs do not reduce the risk of PE and may lead to additional complications. Alarmingly, among patients who had severe perioperative complications, more than half were directly related to the filter, including fatal PE, filter migration, or IVC thrombosis (Citation34). Similarly, a systematic review of the literature for preoperative IVCF use in bariatric surgery concluded that, pending the results of controlled studies, prophylactic IVCF placement cannot be recommended for routine patients undergoing bariatric surgery (Citation39).
Orthopedic patients
IVCFs are often used prophylactically in orthopedic applications. A single quasi-randomized trial involving IVCF placement for orthopedic patients was published in 1973 () (Citation6). In that study, 129 patients with proximal femoral fractures were randomized either to placement of an IVCF (Mobin-Uddin filter type) or no IVCF. Neither group received systemic anticoagulation. The study found a decreased rate of PE and absolute mortality in the IVCF group; however, a complete statistical analysis was not performed, and follow-up was limited to 34 days. The study utilized a first-generation, permanent IVCF that is no longer available. More recently, a retrospective review of 9,300 orthopedic cases found that 0.96% of these patients had a filter placed, and 61% were for prophylactic indications (Citation40).
Among prophylactic filter placements, only 42% of patients had a contraindication to anticoagulation. Five percent of the prophylactic filter patients developed a DVT with filter in place. Only 40% of retrievable filters placed for prophylactic indications were retrieved, and 11% suffered complications during retrieval (including carotid artery puncture and filter fracture with cardiac embolization of filter fragments).
The 2007 American Academy of Orthopedic Surgeons guidelines acknowledge chemoprophylaxis as first-line therapy for the prevention of venous thromboembolism and suggest IVCF use for PE prevention in patients unable to tolerate chemoprophylaxis (Citation41). However, the guidelines also note the ‘very low level of evidence’ supporting IVCFs for that indication. The Agency for Healthcare Research and Quality could not determine the efficacy of prophylactic IVCFs in orthopedic patients due to the lack of acceptable randomized trials or controlled observation studies (Citation42). Similarly, a systematic review of the literature for prophylactic IVCFs in trauma recently concluded that no recommendations for or against IVCFs could be made due to a lack of data (Citation43).
Society guidelines
Accepted indications for IVCF placement vary among medical societies and remain controversial. The most common indication for IVCF placement is prevention of PE in patients who cannot tolerate systemic anticoagulation.
Two commonly referenced societal guidelines for IVCFs include those from the Society of Interventional Radiology (SIR) and the American College of Chest Physicians (ACCP) () (Citation44–48). Compared to the SIR document, the ACCP guidelines have more stringent criteria for appropriate IVCF placement. A general trend toward more stringent criteria in ACCP guidelines for IVCF use is seen from 2004 to 2012 guidelines (). Notable recommendations in the ACCP guidelines include:
recommend against the use of IVCFs in addition to anticoagulation (grade 1B)
recommend for IVCF placement in cases of acute proximal DVT with contraindication for anticoagulation (grade 1B)
recommend initiating anticoagulation in a patient with a filter once the contraindication to anticoagulation has passed (at which point the patient should complete a conventional course of anticoagulation regardless of the presence of a filter) (grade 2B)
no longer recommends IVCF placement for recurrent DVT despite adequate anticoagulation (this is omitted from the 2004 ACCP guidelines ())
recommend against the use of IVCF for thromboprophylaxis in cases of trauma and spinal cord injury (grade 2C)
Table II. Comparison of recommendations for IVCF between the 2008 and 2012 ACCP guidelines and the current ACR–SIR guidelines (Citation44–48).
In contrast, 2011 American College of Radiology (ACR)–SIR guidelines approve IVCF placement in patients already on anticoagulation with specific clinical scenarios (). SIR guidelines also approve prophylactic indications for IVCF placement in severe trauma and high-risk/immobilized patients (Citation44).
Significant variations in IVCF indication guidelines among professional societies are apparent, which likely explains the observed variability and inconsistency with regard to IVCF patient selection. A recent single-institution study on guideline compliance demonstrated poor compliance with ACCP guidelines (43.5%), while SIR guidelines were more commonly satisfied (77.5%) (Citation49).
Complications of IVCF
IVCF placement is usually performed through common femoral or jugular vein access. Minor complications include access site hematoma/thrombosis and bleeding. Major complications and their frequencies vary in the literature, but include IVC thrombosis (2%–30%), recurrent PE despite filter placement (0.5%–6%), filter fracture (2%–10%), filter embolization, filter migration (0%–18%), and IVC penetration/perforation (0%–86%) (Citation44,Citation50,Citation51). Immediate periprocedural-related mortality is exceedingly rare (0.12%) (Citation52).
Retrievable filters may be removed or left in place permanently, as all currently available retrievable filters are FDA-approved for permanent placement. IVCF retrieval is generally performed from jugular vein access, though some filters may also be retrieved from common femoral vein access. Filter retrieval is successful in approximately 85% of attempts, and retrieval is typically most successful when performed within 9–12 weeks of placement (Citation24,Citation53–55). Longer-term dwell times increase the likelihood of filter adherence to the cava wall with corresponding decreased rates of successful retrieval and increased risk of complications (Citation24,Citation54). Major complications of filter retrieval include IVC thrombosis (4.3%) and injury/laceration of the IVC (0.88%) (Citation24,Citation56).
IVCF fracture
In 2010 Nicholson et al. published their findings on filter fracture and embolization of the Bard Recovery and Bard G2 IVCFs (Bard Peripheral Vascular, Tempe, AZ, USA) (Citation57). Nearly 65,000 Bard G2 filters have been placed since 2005. Eighty patients were followed for an average of 2–4 years, demonstrating fracture or fracture and embolization rates of 25% for the Bard Recovery and 16% for the Bard G2 filters (the study projected that with equivalent 50 month dwell times, G2 filters would also be at 25% fracture rates). Of the seven patients who had fracture with embolization, five of the embolized fragments lodged in the heart, and three patients experienced life-threatening ventricular tachycardia or cardiac tamponade; one patient died. Shortly after the Nicholson et al. results were published, the FDA issued an advisory on all IVCFs noting that it is ‘reviewing the literature and conducting quantitative decision analysis modeling to evaluate the change in the risk/benefit profile after retrievable IVCF implantation, over time’ (Citation58). The advisory further states: ‘FDA recommends that implanting physicians and clinicians responsible for the ongoing care of patients with retrievable IVC filters consider removing the filter as soon as protection from PE is no longer needed’.
Since the FDA advisory, other studies have assessed IVCF fractures. A recent study retrospectively evaluated 363 Bard Recovery IVCFs and found a projected 5.5 year fracture rate of 40% (Citation59). The Cordis TrapEase permanent IVCF (Cordis, Miami Lakes, FL, USA), was recently shown to exhibit a fracture rate of 50% at 50 months (Citation60). Interestingly, all filters implicated with high fracture rates are made of nitinol, and metal fatigue is one possible mechanism of fracture (Citation57).
IVC thrombosis and DVT
IVC thrombosis and iliofemoral DVT are other concerning complications of IVCF placement. IVC thrombosis rates in patients with IVCFs vary widely in the literature, from 2% to 30% of cases (Citation44). A randomized prospective trial evaluated outcomes related to specific filter type (Citation25). Usoh et al. planned to randomize 360 patients into two groups to compare clinical outcomes: one group with Greenfield permanent IVCFs implanted (Boston Scientific, Natick, MA, USA), and another group with TrapEase permanent IVCFs. Enrollment for this study was stopped early due to statistically significant higher rates of symptomatic iliac DVT and caval thrombosis in the TrapEase filter group compared to the Greenfield filter group (6.94% versus 0%, P = 0.019).
IVC perforation/penetration
Two recent reports have demonstrated very high rates of observed IVC perforation with retrievable IVCFs (86% and 85.9%) (Citation51,Citation61). This high rate of perforation is well above previous reports (9%–24%) and could be due to improved detection by use of computed tomography and newer retrievable filter devices (Citation62). Within the US FDA Manufacturer and User Facility Device Experience (MAUDE) database, 20% of all IVCF complications were reported as perforations (Citation56). Although the vast majority of such IVC perforations are asymptomatic, increasing numbers of duodenal, pancreatic, aortic, and retroperitoneal injury have been reported (Citation63,Citation64). More disconcerting is the suggestion that the severity of perforation tends to progress over time (Citation61).
Filter retrieval
The theoretical advantage of a retrievable IVCF is predicated on its removal after the filter is no longer clinically needed. This raises three important questions: 1) Are there any data that show retrievable filters are better than permanent filters? 2) Are retrievable filters actually retrieved? 3) Is there a system-based process to maximize the potential benefits of filter retrieval?
As regards question 1, there are no prospective randomized trials that show superiority of retrievable IVCFs as compared to permanent IVCFs. Retrospective data show similar efficacy and risk between permanent and retrievable filters (Citation65,Citation66). Regarding question 2, the dwell time for retrievable IVCFs depends on the clinical scenario. However, for the most part, retrievable filters are not being retrieved. IVCFs may not be retrieved for a variety of reasons: persistent clinical need for the filter, thrombus trapped in the filter, decline in patient health, and technical difficulty retrieving the filter (approximately 15% of filter retrieval attempts fail) (Citation24,Citation53). The most common reason, however, is lack of patient follow-up (Citation67,Citation68). Reported attempted retrieval rates range widely from 2% to 40%—with higher percentages of retrieval reported by centers with particular interest in IVCFs (Citation69,Citation70). A community hospital-based study showed a 2.4% retrieval rate similar to the results of a Medicare population study that reported a retrieval rate of 1.2%–5.1% (Citation68,Citation71). A large, multicenter study in trauma patients reported a 22% retrieval rate (Citation67). Regarding question 3, barriers to IVCF patient follow-up may impact retrieval rates. Outpatient providers may feel poorly informed to address IVCF aftercare and to schedule retrievals. Due to this lack of preparedness, the 9–12 week time window for safest filter retrieval may lapse. Several studies have shown that dedicated IVCF clinics with a structured follow-up strategy result in improved removal rate of retrievable filters (Citation72,Citation73).
Conclusions and future directions
IVCFs have been ingrained in our clinical subculture for over 30 years. With a projected 224,700 filters to be placed in the US in 2012, it is remarkable that only three randomized prospective studies exist, and that none has demonstrated a survival benefit with IVCF use. Several medical assessment forums have arrived at similar conclusions after reviewing the evidence. The 2010 Cochrane collaborative noted a lack of data and clinical evidence to draw any conclusions or recommendations (Citation74). The California Technology Assessment Forum (CTAF), a public service forum, recently concluded that a review of IVCFs did not meet criteria for improved health outcomes and therefore did not recommend their use (Citation75).
Certain retrievable IVCF models have been implicated with IVCF fractures and adherence to the caval wall (Citation55). These processes are generally a function of dwell time (2–4 years for fracture and 9–12 weeks for adherence to cava wall). The degree of IVC perforation by some retrievable IVCFs has been noted to increase with dwell time (Citation50). A recent systematic review of retrievable IVCFs (37 studies) reported a mean dwell time of only 52 days with mean follow-up of only 7.3 months (range 12–15 months) (Citation56). Clearly, longer-term performance studies (e.g. > 2 years) that specifically stratify for device type are needed.
Promoting prophylactic IVC filter placements with the ‘retrievable filter’ concept is an uncertain, and potentially dangerous, proposition. The majority of IVCFs are lost to follow-up, often in the hand-off from inpatient to outpatient medicine, and never retrieved (Citation67,Citation68). Even under ideal dwell times, approximately 15% of IVCFs may fail retrieval due to technical reasons (Citation24). Furthermore, for patients with either permanent IVCFs or retrievable IVCFs left to become permanent, the need for long-term and potentially lifelong anticoagulation for these patients may be warranted and continues to be debated in the medical community (Citation76,Citation77). A systematic review of anticoagulation in IVCF patients showed a statistical trend toward decreased VTE rates in patients with filters and anticoagulation versus filter alone (12.3% versus 15.8%), though the differences were not statistically significant (P = 0.141) (Citation77). Full disclosure of these risks and the potential for a retrievable filter to become permanent should be discussed with each patient.
Further research is needed to address several important facets of IVCF therapy and aftercare, and research should focus on answering the following four questions:
1) Do IVCFs confer a survival benefit in patients who cannot be anticoagulated?
2) Is there a subset of patients who would benefit from IVCFs—perhaps the critically ill, or patients with right heart strain or massive PE?
3) Are retrievable IVCFs safer than permanent IVCFs?
4) Which specific IVCF devices are the safest and most effective?
Prospective, randomized studies are essential to answer these questions.
Due to limited available clinical data and the need for on-going research, we strongly advocate the use of the more stringent/evidence-based ACCP guidelines for IVCF patient selection. Since the majority of retrievable filters are never retrieved, indications should be carefully reviewed prior to placement. Eliminating non-indicated filters is both cost- effective and obviates the need for dealing with difficulties of follow-up and possible retrieval. We also advocate organized, timely follow-up of retrievable IVCFs in a dedicated IVCF clinic or similar environment, the use of which has shown improved filter retrieval rates (Citation72,Citation73).
In summary, despite rising IVCF placement for many years, prospective randomized data on IVCFs are sparse, and most evidence for IVCF use does not indicate improved survival. More data are needed, particularly larger, long-term studies that specifically evaluate patients who cannot be anticoagulated, stratify device performance/safety by specific filter, and evaluate overall efficacy and safety of retrievable and permanent filters. Carefully reviewing the indication of each IVCF, avoiding specific filter devices with higher complication rates, and ensuring timely follow-up of retrievable filters within the ideal retrieval window is ultimately in the best interest of all patients.
Notice of correction
Since the original online publication of this article the abstract has been corrected. The publisher wishes to apologize for this error.
Declaration of interest: Dr Wang has no conflicts of interest or financial disclosures to make. Dr Lloyd has no conflicts of interest or financial disclosures to make.
References
- Park B, Messina L, Dargon P, Huang W, Ciocca R, Anderson FA. Recent trends in clinical outcomes and resource utilization for pulmonary embolism in the United States: findings from the nationwide inpatient sample. Chest. 2009;136:983–90.
- Hann CL, Streiff MB. The role of vena caval filters in the management of venous thromboembolism. Blood Rev. 2005;19: 179–202.
- Mobin-Uddin K, Callard GM, Bolooki H, Rubinson R, Michie D, Jude JR. Transvenous caval interruption with umbrella filter. N Engl J Med. 1972;286:55–8.
- Greenfield LJ, McCurdy JR, Brown PP, Elkins RC. A new intracaval filter permitting continued flow and resolution of emboli. Surgery. 1973; 73:599–606.
- Decousus H, Leizorovicz A, Parent F, Page Y, Tardy B, Girard P, et al. A clinical trial of vena caval filters in the prevention of pulmonary embolism in patients with proximal deep-vein thrombosis. Prevention du Risque d’Embolie Pulmonaire par Interruption Cave Study Group. N Engl J Med. 1998;338:409–15.
- Fullen WD, Miller EH, Steele WF, McDonough JJ. Prophylactic vena caval interruption in hip fractures. J Trauma Acute Care Surg. 1973;13:403–10.
- PREPIC Study Group. Eight-year follow-up of patients with permanent vena cava filters in the prevention of pulmonary embolism: the PREPIC (Prevention du Risque d’Embolie Pulmonaire par Interruption Cave) randomized study. Circulation. 2005;112:416–22.
- Rajasekhar A, Lottenberg L, Lottenberg R, Feezor RJ, Armen SB, Liu H, et al. A pilot study on the randomization of inferior vena cava filter placement for venous thromboembolism prophylaxis in high-risk trauma patients. J Trauma Acute Care Surg. 2011;71:323–8; discussion 328–9.
- Stein PD, Kayali F, Olson RE. Twenty-one-year trends in the use of inferior vena cava filters. Arch Intern Med. 2004;164:1541–5.
- Stein PD, Matta F, Hull RD. Increasing use of vena cava filters for prevention of pulmonary embolism. Am J Med. 2011;124:655–61.
- Wiener RS, Schwartz LM, Woloshin S. Time trends in pulmonary embolism in the United States: evidence of overdiagnosis. Arch Intern Med. 2011;171:831–7.
- LaBelle S. Markets for Clot Management Devices 2011. Toronto, Canada: © 2011 Millennium Research Group, A Decision Resources Group Company; 2011.
- Kaufman JA, Rundback JH, Kee ST, Geerts W, Gillespie D, Kahn SR, et al. Development of a research agenda for inferior vena cava filters: proceedings from a multidisciplinary research consensus panel. J Vasc Interv Radiol. 2009;20:697–707.
- Meltzer AJ, Graham A, Kim JH, Connolly PH, Karwowski JK, Bush HL, et al. Clinical, demographic, and medicolegal factors associated with geographic variation in inferior vena cava filter utilization: an interstate analysis. Surgery. 2013;153:683–8.
- Walsh DB, Birkmeyer JD, Barrett JA, Kniffin WD, Cronenwett JL, Baron JA. Use of inferior vena cava filters in the Medicare population. Ann Vasc Surg. 1995;9:483–7.
- Pickham DM, Callcut RA, Maggio PM, Mell MW, Spain DA, Bech F, et al. Payer status is associated with the use of prophylactic inferior vena cava filter in high-risk trauma patients. Surgery. 2012; 152:232–7.
- European Union: European Commission; 2010 [updated 27 July 2010]. Available from: http://epp.eurostat.ec.europa.eu/cache/ITY_PUBLIC/3-27072010-AP/EN/3-27072010-AP-EN.PDF (accessed 4 November 2012).
- Census Data: US Census Bureau; 2011. Available from: http://2010.census.gov/2010census/data/ (accessed31 October 2012).
- Hameed F, Chan A, Gierszewski K, Heilala S, Huynh N, Thevaratnam T. European Markets for Clot Management Devices 2012. Toronto, Canada: © 2011 Millennium Research Group, A Decision Resources Group Company; 2011.
- Hameed F. European Markets for Peripheral Vascular Devices 2011. Toronto, Canada: © 2010 Millennium Research Group, A Decision Resources Group Company; 2010.
- Heit JA, Cohen AT, Anderson FA Jr; on Behalf of the VTE Impact Assessment Group. Estimated annual number of incident and recurrent, non-fatal and fatal venous thromboembolism (VTE) events in the US. ASH Annual Meeting Abstracts. 2005;106(11):Abstract 910.
- Cohen AT, Agnelli G, Anderson FA, Arcelus JI, Bergqvist D, Brecht JG, et al. Venous thromboembolism (VTE) in Europe. The number of VTE events and associated morbidity and mortality. Thromb Haemost. 2007;98:756–64.
- Goldhaber SZ. A free-floating approach to filters. Arch Intern Med. 1997;157:264–5.
- Uberoi R, Chalmers N, Kinsman R, Walton P. The first BSIR inferior vena cava filter registry report. Oxfordshire, UK: Dendrite Clinical Systems Ltd.; 2011.
- Usoh F, Hingorani A, Ascher E, Shiferson A, Patel N, Gopal K, et al. Prospective randomized study comparing the clinical outcomes between inferior vena cava Greenfield and TrapEase filters. J Vasc Surg. 2010; 52:394–9.
- Prasad V, Rho J, Cifu A. The inferior vena cava filter: how could a medical device be so well accepted without any evidence of efficacy? JAMA Intern Med. 2013;173:493–5; discussion 495.
- White RH, Zhou H, Kim J, Romano PS. A population-based study of the effectiveness of inferior vena cava filter use among patients with venous thromboembolism. Arch Intern Med. 2000;160:2033–41.
- Stein PD, Matta F, Keyes DC, Willyerd GL. Impact of vena cava filters on in-hospital case fatality rate from pulmonary embolism. Am J Med. 2012;125:478–84.
- Aryafar H, Kinney TB. Optional inferior vena cava filters in the trauma patient. Semin Intervent Radiol. 2010;27:68–80.
- Dossett LA, Adams RC, Cotton BA. Unwarranted national variation in the use of prophylactic inferior vena cava filters after trauma: an analysis of the National Trauma Databank. J Trauma Acute Care Surg. 2011;70:1066–70; discussion 1070–1.
- Spangler EL, Dillavou ED, Smith KJ. Cost-effectiveness of guidelines for insertion of inferior vena cava filters in high-risk trauma patients. J Vasc Surg. 2010;52:1537–45 e1-2.
- Maxwell RA, Chavarria-Aguilar M, Cockerham WT, Lewis PL, Barker DE, Durham RM, et al. Routine prophylactic vena cava filtration is not indicated after acute spinal cord injury. J Trauma Acute Care Surg. 2002;52:902–6.
- Velmahos GC, Oh Y, McCombs J, Oder D. An evidence-based cost-effectiveness model on methods of prevention of posttraumatic venous thromboembolism. J Trauma Acute Care Surg. 2000;49:1059–64.
- Birkmeyer NJ, Share D, Baser O, Carlin AM, Finks JF, Pesta CM, et al. Preoperative placement of inferior vena cava filters and outcomes after gastric bypass surgery. Ann Surg. 2010;252:313–18.
- Gargiulo NJ 3rd, O’Connor DJ, Veith FJ, Lipsitz EC, Vemulapalli P, Gibbs K, et al. Long-term outcome of inferior vena cava filter placement in patients undergoing gastric bypass. Ann Vasc Surg. 2010; 24:946–9.
- Obeid FN, Bowling WM, Fike JS, Durant JA. Efficacy of prophylactic inferior vena cava filter placement in bariatric surgery. Surg Obes Relat Dis. 2007;3:606–8; discussion 609–10.
- Overby DW, Kohn GP, Cahan MA, Dixon RG, Stavas JM, Moll S, et al. Risk-group targeted inferior vena cava filter placement in gastric bypass patients. Obes Surg. 2009;19:451–5.
- Trigilio-Black CM, Ringley CD, McBride CL, Sorensen VJ, Thompson JS, Longo GM, et al. Inferior vena cava filter placement for pulmonary embolism risk reduction in super morbidly obese undergoing bariatric surgery. Surg Obes Relat Dis. 2007;3:461–4.
- Rajasekhar A, Crowther M. Inferior vena caval filter insertion prior to bariatric surgery: a systematic review of the literature. J Thromb Haemost. 2010;8:1266–70.
- Bass AR, Mattern CJ, Voos JE, Peterson MG, Trost DW. Inferior vena cava filter placement in orthopedic surgery. Am J Orthop (Belle Mead NJ). 2010;39:435–9.
- Johanson NA, Lachiewicz PF, Lieberman JR, Lotke PA, Parvizi J, Pellegrini V, et al. Prevention of symptomatic pulmonary embolism in patients undergoing total hip or knee arthroplasty. J Am Acad Orthop Surg. 2009;17:183–96.
- Sobieraj DM, Coleman CI, Tongbram V, Lee S, Colby J, Chen WT, et al. Venous thromboembolism prophylaxis in orthopedic surgery. AHRQ Comparative Effectiveness Reviews. Rockville, MD: University of Connecticut/Hartford Hospital Evidence-Based Practive Center Hartford Connecticut; 2012.
- Rajasekhar A, Lottenberg R, Lottenberg L, Liu H, Ang D. Pulmonary embolism prophylaxis with inferior vena cava filters in trauma patients: a systematic review using the meta-analysis of observational studies in epidemiology (MOOSE) guidelines. J Thromb Thrombolysis. 2011;32: 40–6.
- Caplin DM, Nikolic B, Kalva SP, Ganguli S, Saad WE, Zuckerman DA. Quality improvement guidelines for the performance of inferior vena cava filter placement for the prevention of pulmonary embolism. J Vasc Interv Radiol. 2011;22:1499–506.
- Guyatt GH, Akl EA, Crowther M, Gutterman DD, Schuunemann HJ. Executive summary: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):7S–47S.
- Geerts WH, Pineo GF, Heit JA, Bergqvist D, Lassen MR, Colwell CW, et al. Prevention of venous thromboembolism: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004; 126(3 Suppl):338S–400S.
- Buller HR, Agnelli G, Hull RD, Hyers TM, Prins MH, Raskob GE. Antithrombotic therapy for venous thromboembolic disease: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126(3 Suppl):401S–28S.
- Hirsh J, Guyatt G, Albers GW, Harrington R, Schunemann HJ. Executive summary: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133(6 Suppl): 71S–109S.
- Baadh AS, Zikria JF, Rivoli S, Graham RE, Javit D, Ansell JE. Indications for inferior vena cava filter placement: do physicians comply with guidelines?J Vasc Interv Radiol. 2012;23:989–95.
- Durack JC, Wang JH, Schneider DB, Kerlan RK. Vena cava filter scaffold to prevent migration of embolic materials in the treatment of a massive renal arteriovenous malformation. J Vasc Interv Radiol. 2012;23: 413–16.
- Oh JC, Trerotola SO, Dagli M, Shlansky-Goldberg RD, Soulen MC, Itkin M, et al. Removal of retrievable inferior vena cava filters with computed tomography findings indicating tenting or penetration of the inferior vena cava wall. J Vasc Interv Radiol. 2011;22:70–4.
- Becker DM, Philbrick JT, Selby JB. Inferior vena cava filters. Indications, safety, effectiveness. Arch Intern Med. 1992;152:1985–94.
- Ray CE Jr, Mitchell E, Zipser S, Kao EY, Brown CF, Moneta GL. Outcomes with retrievable inferior vena cava filters: a multicenter study. J Vasc Interv Radiol. 2006;17:1595–604.
- Marquess JS, Burke CT, Beecham AH, Dixon RG, Stavas JM, Sag AA, et al. Factors associated with failed retrieval of the Gunther Tulip inferior vena cava filter. J Vasc Interv Radiol. 2008;19:1321–7.
- Smouse HB, Rosenthal D, Thuong VH, Knox MF, Dixon RG, Voorhees WD 3rd, et al. Long-term retrieval success rate profile for the Gunther Tulip vena cava filter. J Vasc Interv Radiol. 2009;20:871–7; quiz 878.
- Angel LF, Tapson V, Galgon RE, Restrepo MI, Kaufman J. Systematic review of the use of retrievable inferior vena cava filters. J Vasc Interv Radiol. 2011;22:1522–30 e3.
- Nicholson W, Nicholson WJ, Tolerico P, Taylor B, Solomon S, Schryver T, et al. Prevalence of fracture and fragment embolization of Bard retrievable vena cava filters and clinical implications including cardiac perforation and tamponade. Arch Intern Med. 2010;170:1827–31.
- Inferior vena cava (IVC) filters: initial communication: risk of adverse events with long term use [updated 08/09/2010]. Available from: http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm221707.htm (accessed 30 May 2011).
- Tam MD, Spain J, Lieber M, Geisinger M, Sands MJ, Wang W. Fracture and distant migration of the bard recovery filter: a retrospective review of 363 implantations for potentially life-threatening complications. J Vasc Interv Radiol. 2012;23:199–205 e1.
- Sano M, Unno N, Yamamoto N, Tanaka H, Konno H. Frequent fracture of TrapEase inferior vena cava filters: a long-term follow-up assessment. Arch Intern Med. 2012;172:189–91.
- Durack JC, Westphalen AC, Kekulawela S, Bhanu SB, Avrin DE, Gordon RL, et al. Perforation of the IVC: rule rather than exception after longer indwelling times for the Gunther Tulip and Celect retrievable filters. Cardiovasc Intervent Radiol. 2012;35:299–308.
- Kinney TB. Update on inferior vena cava filters. J Vasc Interv Radiol. 2003;14:425–40.
- Arabi M, Willatt JM, Shields JJ, Cho KJ, Cwikiel WB. Retrievability of optional inferior vena cava filters with caudal migration and caval penetration: report of three cases. J Vasc Interv Radiol. 2010;21: 923–6.
- Ford ME, Lippert JA, McGraw JK. Symptomatic filter penetration presenting as pancreatitis. J Vasc Interv Radiol. 2010;21:574–6.
- Van Ha TG, Chien AS, Funaki BS, Lorenz J, Piano G, Shen M, et al. Use of retrievable compared to permanent inferior vena cava filters: a single-institution experience. Cardiovasc Intervent Radiol. 2008;31: 308–15.
- Kim HS, Young MJ, Narayan AK, Hong K, Liddell RP, Streiff MB. A comparison of clinical outcomes with retrievable and permanent inferior vena cava filters. J Vasc Interv Radiol. 2008;19:393–9.
- Karmy-Jones R, Jurkovich GJ, Velmahos GC, Burdick T, Spaniolas K, Todd SR, et al. Practice patterns and outcomes of retrievable vena cava filters in trauma patients: an AAST multicenter study. J Trauma Acute Care Surg. 2007;62:17–24; discussion 25.
- Yunus TE, Tariq N, Callahan RE, Niemeyer DJ, Brown OW, Zelenock GB, et al. Changes in inferior vena cava filter placement over the past decade at a large community-based academic health center. J Vasc Surg. 2008;47:157–65.e4.
- Janjua M, Younas F, Moinuddin I, Badshah A, Basoor A, Yaekoub AY, et al. Outcomes with retrievable inferior vena cava filters. J Invasive Cardiol. 2010;22:235–9.
- Mission JF, Kerlan RK Jr, Tan JH, Fang MC. Rates and predictors of plans for inferior vena cava filter retrieval in hospitalized patients. J Gen Intern Med. 2010;25:321–5.
- Duszak R Jr, Parker L, Levin DC, Rao VM. Placement and removal of inferior vena cava filters: national trends in the medicare population. J Am Coll Radiol. 2011;8:483–9.
- Irwin E, Byrnes M, Schultz S, Chipman J, Beal A, Ahrendt M, et al. A systematic method for follow-up improves removal rates for retrievable inferior vena cava filters in a trauma patient population. J Trauma. 2010;69:866–9.
- Minocha J, Idakoji I, Riaz A, Karp J, Gupta R, Chrisman HB, et al. Improving inferior vena cava filter retrieval rates: impact of a dedicated inferior vena cava filter clinic. J Vasc Interv Radiol. 2010; 21:1847–51.
- Young T, Tang H, Hughes R. Vena caval filters for the prevention of pulmonary embolism. Cochrane Database Syst Rev. 2010;(2): CD006212.
- Walsh J. Safety and effectiveness of inferior vena cava filters used to protect against pulmonary embolus [PDF]. San Francisco: California Technology Assessment Forum; 2011. Available from: http://www.ctaf.org/assessments/safety-and-effectiveness-inferior-vena-cava-filters-used-protect-against-pulmonary (accessed 21 April 2012).
- Hajduk B, Tomkowski WZ, Malek G, Davidson BL. Vena cava filter occlusion and venous thromboembolism risk in persistently anticoagulated patients: a prospective, observational cohort study. Chest. 2010;137:877–82.
- Ray CE Jr, Prochazka A. The need for anticoagulation following inferior vena cava filter placement: systematic review. Cardiovasc Intervent Radiol. 2008;31:316–24.