Abstract
Background. The BASE-ACS trial demonstrated an outcome of the titanium-nitride-oxide-coated bioactive stents (BAS) statistically non-inferior to that of the everolimus-eluting stents (EES) at 12-month follow-up in patients presenting with acute coronary syndrome (ACS). We performed a post hoc analysis of the BASE-ACS trial with particular focus on stent-oriented versus patient-oriented outcome at 24-month follow-up.
Methods. A total of 827 patients with ACS were randomly assigned to receive either BAS (417) or EES (410). Stent-oriented outcome was defined as a composite of cardiac death, target vessel-related non-fatal myocardial infarction, or ischemia-driven target lesion revascularization. Patient-oriented outcome was defined as a composite of all-cause death, any non-fatal myocardial infarction, or any revascularization.
Results. Clinical follow-up for 24 months was completed in 406 (97.4%) patients in the BAS group and in 398 (97.1%) in the EES group. Stent-oriented outcome at 24-month follow-up occurred at similar frequencies in the two stent groups (10.1% for BAS versus 11.2% for EES, P = 0.53). Likewise, patient-oriented outcome at 24-month follow-up was similar in the two groups (16.3% versus 19.8%, respectively, P = 0.2).
Conclusions. In patients presenting with ACS, the rates of both stent-oriented and patient-oriented outcomes at 24-month follow-up in the BAS group were similar to those in the EES group.
Trial registration: ClinicalTrials.gov identifier: NCT00819923.
Key messages
For patients presenting with acute coronary syndrome who underwent early percutaneous coronary intervention, the rate of patient-oriented outcome in patients receiving the titanium-nitride-oxide-coated bioactive stents was similar to that in patients receiving everolimus-eluting stents at 24-month follow-up.
The rate of stent-oriented outcome at 24-month follow-up was also similar in the two stent groups.
The rate of stent-oriented outcome was substantially lower than that of patient-oriented outcome.
Introduction
Over the past decade, the introduction of first-generation drug-eluting stents (DES) has revolutionized the practice of coronary intervention, reducing the rates of target lesion revascularization by one-half to two-thirds at long-term follow-up (Citation1,Citation2). A further step forward was taken with the design of second-generation DES. In this context, everolimus-eluting stents (EES) significantly reduced late lumen loss (assessed by quantitative coronary angiography) as compared with paclitaxel-eluting stents, with non-inferior rates of a composite outcome of safety and efficacy (Citation3).
Accumulating evidence from registries and meta-analyses has questioned the long-term safety of first-generation DES, raising concerns about a higher risk of late—and very late—stent thrombosis (ST) (Citation4–6). According to the FDA-convened Circulatory System Device Panel, this long-term risk was chiefly encountered with the use of DES for off-label indications, such as patients with more complex clinical and lesion characteristics (Citation7). These patients quite possibly have a worse long-term clinical outcome attributed to their higher-risk clinical and angiographic profile—as for instance diabetes mellitus and chronic total coronary occlusion—rather than stent-oriented events. Hence, in order to obtain a comprehensive evaluation of overall cardiovascular outcome, composite end-points of randomized clinical trials should focus not only on stent-oriented events, but also on patient-oriented clinical events (Citation8).
The safety of titanium-nitride-oxide-coated bioactive stents (BAS) has been established in several reports from real-world unselected populations (Citation9,Citation10), as well as from randomized clinical trials in patients with acute coronary syndrome (ACS) (Citation11,Citation12). In the multicenter randomized controlled BASE-ACS trial, BAS proved non-inferior to EES in patients presenting with ACS at 12-month follow-up (Citation12). We performed a post hoc analysis of the BASE-ACS trial with particular focus on stent-oriented versus patient-oriented outcome at 24-month follow-up.
Material and methods
Patient selection and study design
The design of the original trial has been previously reported (Citation12). Briefly, the BASE-ACS (randomized comparison of titanium-nitride-oxide-coated bioactive stent with everolimus-eluting stent in acute coronary syndrome) trial is a prospective multicenter single-blinded randomized controlled clinical trial, with the chief aim to evaluate non-inferiority in clinical outcomes of Titan2® (Hexacath, Paris, France) BAS as compared with Xience V (Abbott Vascular, Santa Clara, CA, USA) EES in patients presenting with the whole spectrum of ACS. The study enrolled a total of 827 patients above 18 years, presenting with ACS, with at least one significant de novo lesion (defined as at least 50% diameter stenosis by visual estimation) in a native coronary artery or coronary bypass graft. Chief exclusion criteria were limited to unprotected left main disease or aorto-ostial lesions, intolerance to the study medications, planned surgery within 12 months of the index procedure, and life expectancy less than 12 months. Enrolled patients were randomly assigned in a 1:1 fashion to receive either BAS or EES. The operators were by necessity aware of the assigned study stent, whereas patients and the staff involved in follow-up assessment were blinded to the allocated stent type.
Ethical issues
The study was initiated by the investigators and conducted according to the ethical guidelines of the 1964 Declaration of Helsinki, as revised in 2002. An informed written consent was obtained from every patient after full explanation of the study protocol. The study protocol was approved by the Ethics Committees of the co-ordinating center, Satakunta Central Hospital, and the other participating hospitals. The BASE-ACS trial is registered with ClinicalTrials.gov, number NCT00819923.
Pharmacological interventions
Patients already maintained on aspirin received no additional aspirin loading dose. Those not maintained on aspirin were pre-treated with aspirin at a loading dose of 250 mg orally, or 250– 500 mg intravenously during percutaneous coronary intervention (PCI), and continued thereafter at a daily dose of 75– 150 mg indefinitely. Oral clopidogrel was initiated at a loading dose of at least 300 mg before or immediately after the procedure and continued thereafter at a daily dose of 75 mg. According to the protocol, patients in either group were prescribed oral clopidogrel for a minimum of 6 months and, thereafter, for extended periods (maximum 12 months) according to the operator's discretion. During the procedure, low-molecular-weight heparin (enoxaparin sodium) or unfractionated heparin was administered intravenously in the standard dosage recommended by the guidelines. Use of periprocedural glycoprotein IIb/IIIa inhibitors or bivalirudin was left up to the operator's discretion.
Study end-points and definitions
The diagnostic criteria for non-ST-segment elevation ACS and ST-segment elevation myocardial infarction (MI) have been previously described in detail (Citation12). The primary end-point was the first occurrence of major adverse cardiac events (MACE), defined as a composite of cardiac death, non-fatal MI, or ischemia-driven target lesion revascularization (TLR) at 12-month follow-up. Secondary end-points included all-cause death, a composite of cardiac death or non-fatal MI, and definite ST at 12-month follow-up. Cardiac death was defined as death from cardiovascular causes or any death without another known cause. Stent-oriented outcome was defined as a composite of cardiac death, target vessel-related non-fatal MI, or ischemia-driven TLR. Patient-oriented outcome was defined as a composite of all-cause death, any non-fatal MI, or any revascularization. Any revascularization included all TLR (ischemia-driven and non-ischemia-driven), all target vessel revascularization (ischemia-driven and non-ischemia-driven), and any non-target vessel revascularization, by percutaneous or surgical means. This post hoc analysis of stent-oriented and patient-oriented outcomes was not pre-specified in the original study protocol published in ClinicalTrials.gov, number NCT00819923. ST was adjudicated according to the criteria of definite and probable stent thrombosis described by the Academic Research Consortium (ARC) (Citation8). An independent Clinical Events Committee whose members were blinded to stent group allocation throughout the study period adjudicated all clinical events for analysis.
Statistical analysis
All data were analyzed on the basis of the intention-to-treat principle. The trial was powered for testing non-inferiority of BAS as compared with EES for the primary composite end-point. The criterion for non-inferiority was considered to have been met if the upper limit of the one-sided 95% confidence interval for the difference between groups was not more than 5 percentage points higher than the observed rate of the primary composite end-point in the group receiving EES. Assuming a 12-month rate of the primary composite end-point of 9.2% for EES, we calculated that enrollment of at least 400 patients per group would yield at least 90% power to detect non-inferiority for the primary composite end-point (1-sided α significance level 0.05). Superiority testing was pre-specified if the criterion for non-inferiority with respect to the primary composite end-point was met. Hazard ratios (HR) and 95% confidence intervals (CI) were derived from univariate Cox models for the comparisons between the groups. Survival curves for time-to-event variables were constructed on the basis of all available follow-up data with the use of Kaplan–Meier estimates and were compared with the use of the log-rank test. Continuous variables were presented as means ± SD, while categorical variables were described with absolute and relative (percentage) frequencies. Comparisons between the two groups were performed using the unpaired t test for continuous, and the Pearson chi-square test or Fisher's exact test for categorical variables. All tests were 2-sided, and a probability value of P < 0.05 was considered statistically significant. All data were analyzed with the use of SPSS version 16 (Citation13), and SAS system for Windows version 9.1 (SAS Institute Inc., Cary, NC, USA).
Results
Baseline clinical, angiographic, and procedural data
Between January 2009 and September 2010, a total of 827 patients were enrolled in 14 centers, in five European and one Southeast Asian countries. Patients were randomly assigned to receive either BAS (417 patients, 480 lesions) or EES (410 patients, 484 lesions). The baseline clinical, angiographic, and procedural characteristics of the two groups were well matched (). The frequency of complex lesions (types B and C) was similar in the two groups (P = 0.39).
Table I. Baseline clinical, angiographic, and procedural characteristics of the two individual study groups.
Clinical outcome
Clinical follow-up for 24 months was completed in 406 (97.4%) patients in the BAS group, and 398 (97.1%) in the EES group. Clinical outcome data are presented in . BAS was non-inferior to EES with respect to the primary composite end-point of MACE at 24-month follow-up (11.3% versus 12.7%, respectively; hazard ratio (HR) for BAS 0.94; 95% confidence interval (CI) 0.77–1.15; P = 0.53 for superiority; P = 0.001 for non-inferiority) (). Stent-oriented outcome at 24-month follow-up occurred at similar frequencies in the two stent groups (10.1% for BAS versus 11.2% for EES; HR for BAS 0.94; 95% CI 0.76–1.17; P = 0.53) (). Likewise, patient-oriented outcome at 24-month follow-up occurred at similar frequencies in the two stent groups (16.3% versus 19.8%, respectively; HR for BAS 0.89; 95% CI 0.76–1.05; P = 0.2) ().
Figure 1. Kaplan–Meier estimates for the primary end-point, stent-oriented and patient-oriented outcomes, in the two study groups, over 24 months of follow-up. Kaplan–Meier curves show the cumulative incidence of major adverse cardiac events (the primary end-point), a composite of cardiac death, non-fatal MI, or ischemia-driven TLR (A); stent-oriented outcome, a composite of cardiac death, target vessel-related non-fatal MI, or ischemia-driven TLR (B); patient-oriented outcome, all-cause death, any non-fatal MI, or any revascularization (C). BAS = bioactive stents; EES = everolimus-eluting stents; MI = myocardial infarction; TLR = target lesion revascularization.
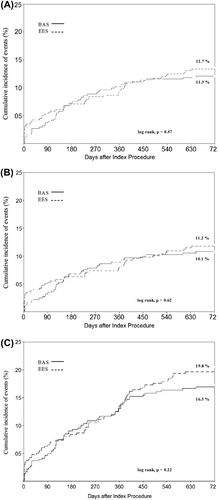
Table II. Clinical events in the two individual study groups at 24-month follow-up.
The rate of non-fatal MI at 24-month follow-up was significantly lower in the BAS group as compared with the EES group (2.9% versus 7.1%, respectively, P = 0.005). Moreover, there was a trend toward a lower rate of ARC-definite ST at 24-month follow-up in patients who received the BAS as compared with those who received the EES (1.0% versus 2.7%, respectively, P = 0.054) (). During the first 12 months of follow-up, all ARC-definite ST events (12 events) occurred while the patients were on clopidogrel, whereas during the period from 12- to 24-month follow-up, ARC-definite ST events (3 events) occurred after the patients stopped clopidogrel. At 12-month follow-up, 51.3% of patients in the BAS group were still maintained on clopidogrel as compared with 68.3% of patients in the EES group (P < 0.001). Yet, at 24-month follow-up, only 2.6% of patients in the BAS group and 3.7% in the EES group were still on clopidogrel therapy. The mean duration of clopidogrel use was 8.7 ± 3.6 versus 10.2 ± 3.0 months in the BAS and EES groups, respectively (P < 0.001).
Table III. Stent thrombosis in the two individual study groups according to ARC definition at 24-month follow-up.
Discussion
Major findings
The current post hoc analysis of the BASE-ACS trial demonstrated that in patients presenting with the whole spectrum of ACS and undergoing early PCI, the composite incidence of stent- oriented outcome (cardiac death, target vessel-related non-fatal MI, or ischemia-driven TLR) at 24-month follow-up in patients receiving BAS was similar to that in patients receiving EES. Moreover, the composite incidence of patient-oriented outcome (all-cause death, any non-fatal MI, or any revascularization) at 24-month follow-up was also similar between the two groups. Yet, incidence of stent-oriented outcome was far below that of patient-oriented outcome.
Clinical outcome of the BASE-ACS trial at 24 months
In the BASE-ACS trial, the hazard ratio for MACE associated with BAS as compared with EES at 12-month follow-up was 1.04 (P = 0.81) (Citation12). This hazard ratio decreased to 0.94 at 24-month follow-up (P = 0.53). Among the individual components of MACE, we observed a decrease of the hazard ratio associated with BAS from 12- to 24-month follow-up for both cardiac death (from 1.49 to 1.14) and ischemia-driven TLR (from 1.17 to 1.03) (Citation12). The hazard ratio for non-fatal MI associated with BAS remained unchanged. During the period of follow-up from 12 to 24 months, more cardiac death and ischemia-driven TLR events occurred in the EES group than in the BAS group. The excess cardiac death events in the EES group as compared with the BAS group during this period of follow-up can be explained by more events of ST in the EES group between 12 and 24 months (), during which time the vast majority of patients in either group were not maintained on clopidogrel therapy. The excess ischemia-driven TLR events in the EES group as compared with the BAS group during this period of follow-up can be viewed as a ‘catch-up phenomenon’. Yet, the lack of angiographic follow-up in the current study may have influenced the relative rates of TLR between the two groups. It is well known that angiographic follow-up increases the absolute difference in TLR between stent groups beyond that which would otherwise be observed with clinical follow-up alone. However, with clinical follow-up, the results are more likely to reflect real-life practice, avoiding unnecessary re-interventions for clinically ‘silent’ angiographic lesions.
The rates of clinical events associated with EES at 24-month follow-up in the current trial were slightly higher than those reported in the 2-year report of the RESOLUTE All Comers trial (stent-related outcome 11.2% versus 10.7%, non-fatal MI 7.1% versus 5%, and ischemia-driven TLR 7.1% versus 5.1%) (Citation14). The clinical event rates were further lower in the 2-year outcome of the COMPARE (MACE 7.4%, non-fatal MI 3.9%, and ischemia-driven TLR 2.6%) and the SPIRIT IV (MACE 7.1%, non-fatal MI 2.5%, and ischemia-driven TLR 4.5%) trials (Citation15,Citation16). Cardiac death at 2-year follow-up was slightly higher (2.2%) in both the RESOLUTE All Comers and COMPARE trials, and lower (0.9%) in the SPIRIT IV trial (Citation14–16). The exclusive enrollment of patients presenting with ACS in the current trial, in contrast to the all-comer populations of the other three trials, might explain the difference in the rates of adverse events among the trials at 24-month follow-up. Yet, in a 2-year report of pooled data from the SPIRIT and the COMPARE trials, the event rates associated with EES in patients with ACS were lower than the current trial: MACE 8.7%, non-fatal MI 4%, ischemia-driven TLR 4.7% (Citation17). On the other hand, the 2-year cardiac event rates associated with BAS in the current trial were similar to those in the 2-year report of the TITAX AMI trial which enrolled patients with acute MI who underwent early PCI: MACE 11.3% versus 11.2%, cardiac death or non-fatal MI 4.8% versus 5.1%, and ischemia-driven TLR 7.4% versus 9.3%, respectively (Citation18).
Stent-oriented versus patient-oriented outcome
Primary and secondary composite end-points in late-breaking randomized controlled trials comparing coronary stents have encompassed a wide range of adverse outcome, ranging from patient-oriented events such as all-cause mortality, to device-specific events such as definite ST. Over the years, randomized trials have gradually shifted from including non-stent-related complications to particularly focusing on stent-oriented ones. This apparently sound shift would rationally avoid attributing to the device some consequences of other disease process that would otherwise artificially worsen the outcome of the index stent, as for instance cancer death. However, this trend would clearly ignore the total burden of cardiovascular—and non-cardiovascular—disease, which is heavily instrumental in determining the ultimate patient prognosis, especially in patients with substantially higher levels of risk. Furthermore, comparisons of composite end-points between the many randomized trials of coronary stents available to date have been difficult due to the lack of consensus definitions of end-points and the frequent overlap between the individual components of composite end-points. Hence, it is a quite reasonable approach to report individually both the most specific stent-oriented outcome and the most ‘generic’ patient-oriented outcome in each study, paving the way for uniform consensus definitions of both sets of composite end-points, and allowing for comparisons between trials at both levels of outcome.
The importance of patient-oriented outcome
Interestingly, the current report demonstrated that the rate of patient-oriented adverse events was substantially higher than that of stent-oriented events, with an approximate doubling of event rates for patient-oriented outcome. A similar proportion of stent-oriented versus patient-oriented outcome was observed in the 2-year report of the RESOLUTE All Comers trial (Citation14). This underscores the prime role played by stent-unrelated patient co-morbidities in drawing the final landscape of patient prognosis following PCI and stenting. Patient-oriented outcome liberally included any non-cardiac death, any MI not related to the target vessel, and any revascularization not related to the target lesion. Thus, it would honestly reflect the extent and severity of disease in the coronary tree outside the treated lesion, the prevalence of vulnerable plaques other than the treated one, and, quite possibly, the intrinsic predisposition of patients for atherosclerotic disease progression (Citation19). Likewise, it would mirror the total risk profile of patients which would augment cardiovascular adverse outcome, such as diabetes mellitus and chronic renal insufficiency, or separately compromise patients’ survival, such as cancer and other lethal illnesses (Citation19,Citation20).
Clinical implications
The finding that roughly half the events that occurred following PCI was unrelated to the index stent would highlight the indispensable value of secondary prevention measures during long-term follow-up. In this sense, strict control of modifiable risk factors and optimization of medical management of patients following invasive coronary procedures might seem even more important than the mere choice between the alternative coronary devices. Additionally, premature discontinuation of clopidogrel is widely acknowledged as the most important predictor of late ST following DES implantation (Citation5). In the current study, 3.7% of patients in the EES group were still maintained on dual anti-platelet therapy at 24-month follow-up. This figure is lower than those reported from the RESOLUTE All Comers and COMPARE trials, in which the proportion of patients in the EES arm receiving dual anti-platelet therapy at 24-month follow-up were 18% and 13%, respectively (Citation14,Citation15). The substantial drop out of patients in the EES group off the dual anti-platelet drug coverage may bear association with the trend toward a higher 24-month rate of ARC-definite ST in the EES group in the current study, as compared with the BAS group. Furthermore, although only 51% of patients in the BAS group were on clopidogrel, as compared with 68% of patients in the EES group, at 12 months (P <0.001), the rate of cardiac death or non-fatal MI was not higher in the BAS group; indeed there was a trend toward a lower rate of such events in the BAS group at 24 months (P = 0.056). This quite interesting finding may possibly imply that a shorter duration of dual anti-platelet therapy (less than 12 months) in patients with ACS who receive BAS might be sufficient.
Conclusion
In patients presenting with ACS who underwent early PCI, the rate of patient-oriented outcome in patients receiving BAS was similar to that in patients receiving EES at 24-month follow-up. Moreover, the rate of stent-oriented outcome at 24-month follow-up was also similar in the two stent groups. Yet, the rate of stent-oriented outcome was substantially lower than that of patient-oriented outcome.
Study limitations
It should be noted that any comparison between stent-oriented and patient-oriented outcomes is hypothesis-generating, and was not pre-specified a priori in the study protocol. Moreover, any death of unknown cause was by default classified as a cardiac death, even if it was indeed non-cardiac in origin. Furthermore, although the current trial was well-powered to detect non-inferiority of BAS as compared with EES concerning the primary composite end-point of total MACE at 12-month follow-up, it was not adequately powered to address the individual components of safety or efficacy. Additionally, although ischemia-driven TLR was a component of MACE, the investigators have no available data about the methods of ischemia detection in the individual participating centers. Finally, the fact that TLR was ischemia-driven may have underestimated the actual rates of in-stent restenosis; however, it would avoid unnecessary re-interventions in borderline restenotic lesions due to the ‘oculostenotic reflex’ and undue patient anxiety.
Declaration of interest: The authors declare that they have no conflict of interests.
The study was supported by grants from the Finnish Foundation for Cardiovascular Research, Helsinki, Finland. This work was also supported by an unrestricted institutional grant from Hexacath, Paris, France; however, the company had no role in study design, data collection, data analysis and interpretation, or manuscript writing.
References
- Stone GW, Moses JW, Ellis SG, Schofer J, Dawkins KD, Morice MC, et al. Safety and efficacy of sirolimus- and paclitaxel-eluting coronary stents. N Engl J Med. 2007;356:998–1008.
- Morice MC, Serruys PW, Barragan P, Bode C, Van Es GA, Stoll HP, et al. Long-term clinical outcomes with sirolimus-eluting coronary stents: five-year results of the RAVEL trial. J Am Coll Cardiol. 2007;50:1299–304.
- Stone GW, Midei M, Newman W, Sanz M, Hermiller JB, Williams J, et al.; SPIRIT III Investigators. Comparison of an everolimus-eluting stent and a paclitaxel-eluting stent in patients with coronary artery disease: a randomized trial. JAMA. 2008;299:1903–13.
- Nordmann AJ, Briel M, Bucher HC. Mortality in randomized controlled trials comparing drug-eluting vs. bare metal stents in coronary artery disease: a meta-analysis. Eur Heart J. 2006;27:2784–814.
- Iakovou I, Schmidt T, Bonizzoni E, Ge L, Sangiorgi GM, Stankovic G, et al. Incidence, predictors, and outcome of thrombosis after successful implantation of drug-eluting stents. JAMA. 2005;293:2126–30.
- Lagerqvist B, James SK, Stenestrand U, Lindback J, Nilsson T, Wallentin L; SCAAR Study Group. Long-term outcomes with drug-eluting stents versus bare-metal stents in Sweden. N Engl J Med. 2007;356:1009–19.
- Pinto Slottow TL, Waksman R. Overview of the 2006 Food and Drug Administration Circulatory System Devices Panel meeting on drug-eluting stent thrombosis. Catheter Cardiovasc Interv. 2007;69:1064–74.
- Cutlip DE, Windecker S, Mehran R, Boam A, Cohen DJ, van Es GA, et al.; Academic Research Consortium. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. 2007; 115:2344–51.
- Mosseri M, Miller H, Tamari I, Plich M, Hasin Y, Brizines M, et al. The titanium-NO stent: results of a multicenter registry. EuroIntervention. 2006;2:192–6.
- Karjalainen PP, Ylitalo A, Airaksinen KEJ, Nammas W. Five-year clinical outcome of titanium nitride-oxide-coated bioactive stent implantation in a real world population: a comparison with paclitaxel-eluting stents: The PORI registry. J Interv Cardiol. 2011;24:1–8.
- Tuomainen PO, Ylitalo A, Niemelä M, Kervinen K, Pietilä M, Sia J, et al. Five-year clinical outcome of titanium-nitride-oxide-coated bioactive stents versus paclitaxel-eluting stents in patients with acute myocardial infarction: long-term follow-up from the TITAX AMI trial. Int J Cardiol.2012 Dec 3 [Epub ahead of print].
- Karjalainen PP, Niemelä M, Airaksinen JK, Rivero-Crespo F, Romppanen H, Sia J, et al. A prospective randomised comparison of titanium-nitride-oxide-coated bioactive stents with everolimus- eluting stents in acute coronary syndrome: the BASE-ACS trial. EuroIntervention. 2012;8:306–15.
- Buehl A, Zoefel P. SPSS 11: Introduction in modern data analysis, 8th ed. Munich, Germany: Addison-Wesley; 2002.
- Silber S, Windecker S, Vranckx P, Serruys PW; RESOLUTE All Comers Investigators. Unrestricted randomised use of two new generation drug-eluting coronary stents: 2-year patient-related versus stent-related outcomes from the RESOLUTE All Comers trial. Lancet. 2011; 377:1241–7.
- Smits PC, Kedhi E, Royaards KJ, Joesoef KS, Wassing J, Rademaker-Havinga TA, et al. 2-year follow-up of a randomized controlled trial of everolimus- and paclitaxel-eluting stents for coronary revascularization in daily practice. COMPARE (Comparison of the everolimus eluting XIENCE-V stent with the paclitaxel eluting TAXUS LIBERTÉ stent in all-comers: a randomized open label trial).J Am Coll Cardiol. 2011; 58:11–18.
- Stone GW, Rizvi A, Sudhir K, Newman W, Applegate RJ, Cannon LA, et al.; SPIRIT IV Investigators. Randomized comparison of everolimus- and paclitaxel-eluting stents: 2-year follow-up from the SPIRIT (Clinical Evaluation of the XIENCE V Everolimus Eluting Coronary Stent System) IV trial. J Am Coll Cardiol. 2011;58:19–25.
- Planer D, Smits PC, Kereiakes DJ, Kedhi E, Fahy M, Xu K, et al. Comparison of everolimus- and paclitaxel-eluting stents in patients with acute and stable coronary syndromes: pooled results from the SPIRIT (A Clinical Evaluation of the XIENCE V Everolimus Eluting Coronary Stent System) and COMPARE (A Trial of Everolimus-Eluting Stents and Paclitaxel-Eluting Stents for Coronary Revascularization in Daily Practice) Trials. JACC Cardiovasc Interv. 2011;4:1104–15.
- Karjalainen PP, Ylitalo A, Niemelä M, Kervinen K, Mäkikallio T, Pietilä M, et al. Two-year follow-up after percutaneous coronary intervention with titanium-nitride-oxide-coated stents versus paclitaxel-eluting stents in acute myocardial infarction. Ann Med. 2009;21:1–9.
- Stone GW, Maehara A, Lansky AJ, de Bruyne B, Cristea E, Mintz GS, et al.; PROSPECT Investigators. A prospective natural-history study of coronary atherosclerosis. N Engl J Med. 2011;364:226–35.
- Leon MB, Allocco DJ, Dawkins KD, Baim DS. Late clinical events after drug-eluting stents: the interplay between stent-related and natural history-driven events. JACC Cardiovasc Interv. 2009;2:504–12.
