Abstract
Objectives. To describe the relationship of plasma apelin levels with blood pressure in a coastal Chinese population.
Methods. This cross-sectional study included a total of 1031 subjects from the coastal areas of China. One-way analysis of variance (ANOVA) and linear trend test, Pearson's correlation analysis, as well as multivariate linear regression analysis were used to evaluate the association between plasma apelin levels and blood pressure.
Results. Plasma apelin levels dropped with increasing quartiles of systolic blood pressure (SBP), diastolic blood pressure (DBP), and mean arterial blood pressure (MABP) (all P < 0.001). SBP, DBP, and MABP values decreased as the apelin levels increased within the quartiles. After adjusting for age and gender, the significant differences in SBP, DBP, and MABP between the groups within the apelin quartiles remained (all P < 0.05). A significant negative correlation between SBP, DBP, as well as MABP and apelin levels was observed (all P < 0.01); even after adjusting for cardiovascular confounding factors, this negative correlation remained (all P < 0.001).
Conclusion. A negative correlation between plasma apelin levels and blood pressure was found in this 1000-population-based epidemiological study. Apelin may become a potential therapeutic target of anti-hypertensive treatment.
Key messages
The association of plasma apelin levels with blood pressure was observed in this cross-sectional study which included 1031 subjects.
Plasma apelin levels dropped with increasing blood pressure, and a negative correlation was found between apelin levels and blood pressure.
This study demonstrated an association between apelin levels and blood pressure, but whether apelin has a blood pressure regulative effect still needs to be verified in future fundamental research.
Introduction
Apelin is a recently discovered cardiovascular bioactive peptide which is widely distributed in various parts of the body, including the endothelial cells (Citation1–4). To date, apelin is believed to be the only endogenous ligand for the APJ receptor, also known as angiotensin II type 1 receptor (AT1)-associated protein (Citation3). It has been proposed that apelin is secreted as a pre-protein, an immature peptide, which is cleaved by protease to form several shorter active peptides, namely apelin-36, apelin-17, apelin-13, and apelin-12. These peptides all possess the ability to bind with APJ receptors, but with distinct biological functions due to their various lengths (Citation3,Citation5). The cardiovascular effects of the apelin-APJ system are inverse to the effects of the renin–angiotensin system (RASS). Apelin plays a potent role in blood pressure regulation, which has been confirmed by many studies both in animal models (Citation6,Citation7) and in human (Citation8,Citation9). Tatemoto et al. demonstrated that the administration of apelin-12, -13, and -36 transiently lowered the blood pressure of Wistar-Kyoto (WKY) rats, and apelin-12 exhibited the strongest activity compared to apelin-13 and apelin-36 on lowering blood pressure. The hypotensive effect of apelin-12 was abolished in the presence of nitrite oxide (NO) synthase inhibitor, suggesting that apelin may reduce blood pressure via a NO-dependent mechanism (Citation10). Ishida et al. have proven that the blood pressure-lowering response to apelin was abolished in APJ-deficient mice, which indicates that APJ exerts the hypotensive effect of apelin in vivo (Citation11). In human studies, it has been found that apelin caused NO-dependent vasodilation in man without apparent effect on venous tone (Citation9). Systemic intravenous apelin-13 infusion on patients with chronic stable heart failure reduced mean arterial pressure and peripheral vascular resistance index irrespective of sodium depletion or angiotensin II co-infusion (Citation12). Despite all the above-mentioned findings, to our knowledge, no clinical data have been published in terms of the relationship between plasma apelin levels and blood pressure. Therefore, our study focused on determining the association between plasma apelin levels, blood pressure, and cardiovascular risk factors in a population of 1000 from the coastal areas of China, as revealed by a cross-sectional study.
Materials and methods
Study population
In the period between July 2011 and November 2011, 1031 subjects from the Lianjiang and Xiapu coastal counties in Fujian Province were selected by a random sampling method. Inclusion criteria were as follows: subjects were eligible if they were over the age of 30 (date of birth before 1 January 1982) and had resided for more than 5 years in the local area. Exclusion criteria included diagnosed secondary hypertension, acute myocardial infarction, atrial fibrillation, chronic heart failure, infectious disease (high-sensitivity C-reactive protein (hs-CRP) > 10 mg/L in our study), hepatic function insufficiency, pulmonary hypertension, hematuria or urinary tract infection, malignant tumor, acquired immune deficiency syndrome (AIDS), pregnancy, current taking of medication that can influence plasma apelin levels, as well as denial or non-cooperation for any reasons. The study was conducted according to the tenets of the Declaration of Helsinki, and the institutional review board approved the study protocol. Written informed consent was obtained from each participant.
Data collection
The questionnaire survey covered the information of age, gender, smoking or drinking, snoring, history of related diseases (hypertension, coronary heart disease, heart failure, arrhythmias, diabetes, stroke, hepatic disease, etc.), drug treatment, and family history of hypertension.
Physical examination
Body weight and body height were measured without shoes on and in light clothing. Body mass index (BMI) was calculated using the formula: weight (kg)/height (m)2. Waist circumference (WC) was measured at the middle point between the costal margin and iliac crest. Blood pressure in the right arm was measured twice in the sitting position, using a manual sphygmomanometer, after the subjects had been resting for 30 min, and the mean of the two readings was used for analysis.
Laboratory measurements
A venous blood sample was drawn after 8–12 hours of overnight fasting for laboratory tests. Triglycerides (TG) and total cholesterol (TC) were measured by the GPO-PAP and CHOD-PAP method. High-density lipoprotein cholesterol (HDL-C) and low-density lipoprotein cholesterol (LDL-C) were estimated by the direct method. Uric acid (UA) was measured by the uricase ultraviolet method. The glucose oxidase electrode method was applied for determining fasting blood glucose (FBG) levels. The picric acid method was applied to measure serum creatinine (Scr) on a Beckman DxC800 analyzer (Beckman Coulter, Fullerton, CA, USA). Levels of hs-CRP and urine microalbumin (mALB) were measured by immune scatter turbidimetry using a special protein analysis instrument (Siemens Healthcare Diagnostics, GmbH, Berlin, Germany). Hemoglobin A1c (HbA1c) levels were estimated by high-performance liquid chromatography (HA-8160, ARKRAY, Kyoto, Japan). Urine creatinine (CR) was measured by picric acid method (LX20 automatic biochemical analyzer, Beckman Kurt, California, USA).
For measurement of apelin-12 levels, a 3 mL venous blood sample was obtained in EDTA and heparin-containing tubes after 8–12 hours of overnight fasting. After the tubes were shaken in an upside-down position a couple of times, blood samples were centrifuged at 3000 rpm for 10 min. Approximately 0.4 mL plasma was extracted and stored at − 70°C until further use. Apelin-12 levels were determined by a commercially available enzyme immunoassay (Phoenix Pharmaceuticals, Berlingame, CA, USA) according to the manufacturer's instructions. The antibody used in this apelin assay cross-reacts 100% with apelin-12, −13, and −36. The assay therefore includes all of the above peptides if present in the plasma. The sensitivity of detection of the apelin-12 enzyme-linked immunosorbent assay (ELISA) assay was 60 pg/mL, and the intra-assay and inter-assay differences were < 10% and < 15%, respectively.
Definitions of variables
According to Standards of Medical Care in Diabetes—2011 (Citation13), diabetes was defined as fasting plasma glucose (FPG) ≥ 7.0 mmol/L (126 mg/dL) or HbA1c ≥ 6.5% or previously diagnosed diabetes and/or treatment with hypoglycemia agents. Apelin levels were divided into quartiles: the lowest quartile (Q1) corresponding to < 164.8 pg/mL, the second quartile (Q2) corresponding to 164.8–220.0 pg/mL, the third quartile (Q3) corresponding to 220.0–283.1 pg/mL, and the highest quartile (Q4) corresponding to > 283.1 pg/mL. There were 258, 258, 258, and 257 cases in quartiles Q1, Q2, Q3, and Q4, respectively.
Statistical analysis
All the data were analyzed by the SPSS 17.0 statistical software (SPSS, Inc., Chicago, IL, USA). Skewed distributed variables were taken as approximately normal distribution after logarithmic transformation was conducted. We used one-way analysis of variance (ANOVA) to estimate plasma apelin levels associated with the presence versus absence of cardiovascular risk categorical variables (e.g. gender, diabetes) or increasing quartiles of continuous variables (e.g. age, BMI, systolic blood pressure (SBP), diastolic blood pressure (DBP), mean arterial blood pressure (MABP), LDL-C, HDL-C, HbA1c, hs-CRP, and mALB), and linear trend test was conducted. The differences of SBP, DBP, and MABP within the apelin quartiles were compared by ANOVA followed by least significant difference-t (LSD-t) between two groups. Covariance analysis was used to compare differences between SBP, DBP, and MABP within the apelin quartiles, whereas gender and age were considered as covariants. Pearson's correlation analysis was applied to evaluate the linear correlation between SBP, DBP, as well as MABP and the apelin levels. Multivariate linear regression analysis was applied to assess the independent association between the apelin levels and blood pressure. Model 1 was not adjusted for potential confounding variables; Model 2 was adjusted for age and gender; Model 3 was adjusted as Model 2 and additionally for BMI, LDL-C, hs-CRP, diabetes, and mALB. Differences and correlations were considered significant at P < 0.05.
Results
A total of 1301 subjects aged between 30 and 79 years (mean age, 55.1 ± 10.9 years) and comprising 416 males and 615 females were included in this study. Plasma apelin levels in all the population, male subjects, and female subjects were 227.42 ± 80410 pg/mL, 220.57 ± 78870 pg/mL, and 232.06 ± 81170 pg/mL, respectively. Apelin-12 levels of male subjects were significantly lower than those of female subjects (P < 0.05).
shows the association of cardiovascular risk factors with plasma apelin levels. Decreased apelin levels were associated with increasing age (P = 0.042). Apelin levels of subjects with diabetes were lower than those of non-diabetic subjects (P = 0.028). Apelin levels dropped as the quartiles of SBP, DBP, and MABP increased (all P < 0.001). Decreased apelin levels were also associated with increasing BMI, hs-CRP, and mALB levels (all P < 0.05). However, apelin levels were not associated with LDL-C, HDL-C, FPG, and HbA1c quartiles (P > 0.05).
Table I. The association of cardiovascular risk factors with plasma apelin-12 levels.
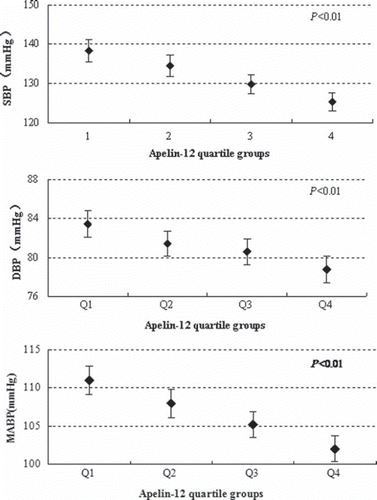
There were significant differences in SBP, DBP, and MABP between the groups within the apelin Q1–Q4 quartiles (all P < 0.05). SBP values in the apelin quartiles Q1–Q4 were 138.39 ± 22.72 mmHg, 134.53 ± 22.39 mmHg, 129.78 ± 19.18 mmHg, and 125.31 ± 18.12 mmHg, respectively, whereas the corresponding DBP values were 83.45 ± 11.03 mmHg, 81.42 ± 10.75 mmHg, 80.60 ± 10.76 mmHg, and 78.77 ± 11.48 mmHg, respectively, and the MABP values were 110.96 ± 15.26 mmHg, 108.00 ± 15.16 mmHg, 105.19 ± 13.68 mmHg, 102.02 ± 13.78 mmHg. shows that SBP, DBP, and MABP values dropped with increasing apelin levels within the quartiles. Significant differences in SBP were observed between the groups within apelin-12 Q1–Q4 quartiles (all P < 0.05). DBP values of Q2, Q3, and Q4 within the apelin quartiles were significantly lower than those in Q1 (all P <0.05), and the DBP of Q4 was lower than that of Q2 (P < 0.05). However, no significant difference in DBP was observed between Q3 and Q2, and between Q3 and Q4 (both P > 0.05). As shown in , significant differences in MABP were observed between the groups within apelin Q1–Q4 quartiles (all P < 0.05). Considering that the above-mentioned confounding factors might influence the association between blood pressure and apelin levels, a covariance analysis was conducted for controlling the effects of these confounding factors. Even after adjusting for confounding factors, the significant differences in SBP, DBP, and MABP between the groups within the apelin quartiles remained (F value = 17.50 for SBP, 6.26 for DBP, 15.04 for MABP; all P < 0.05).
Figure 1. Systolic blood pressure, diastolic blood pressure, and mean arterial blood pressure within apelin-12 quartiles. Data were shown as means and 95% confidence interval; apelin-12 levels were divided into quartiles: the lowest quartile (Q1): < 164.8 pg/mL; the second quartile (Q2): 164.8–220.0 pg/mL; the third quartile (Q3): 220.0–283.1 pg/mL; the highest quartile (Q4): > 283.1 pg/mL.
Pearson linear correlation analysis showed a significant negative correlation between SBP, DBP, as well as MABP and apelin levels (correlation coefficient –0.24 for SBP, –0.15 for DBP, and –0.23 for MABP; all P < 0.01).
Multivariate linear regression analysis () showed a significant negative correlation between SBP, DBP, as well as MABP and apelin levels (Model 1) (standard β = –0.103 for SBP, –4.436 for DBP, –9.336 for MABP; all P < 0.001). This negative correlation between SBP, DBP, as well as MABP and apelin levels remained after adjusting for age and gender (Model 2) (standard β = –0.094 for SBP, –4.124 for DBP, and –8.590 for MABP; all P < 0.001), and after further adjusting for BMI, LDL-C, hs-CRP, diabetes, mALB (Model 3) (standard β = –0.087 for SBP, –3.667 for DBP, and –7.881 for MABP; all P < 0.001).
Table II. Multivariate linear regression analysis of the association with apelin-12 levels and blood pressure.
Discussion
Apelin, a novel cardiovascular bioactive peptide and a circulatory paracrine hormone, is extensively distributed in a variety of tissues, including heart, lungs, kidney, central nervous system, and fatty tissues but is mainly restricted to vascular endothelium and vascular smooth muscular cells (Citation1). Many animal and human studies have confirmed that apelin can serve as a peripheral vasodilator (Citation14) and plays a role in blood pressure regulation (Citation6–9).
This is a cross-sectional study with 1031 subjects randomly selected from the coastal areas of China and aims to explore the association of plasma apelin levels with blood pressure and cardiovascular risk factors. We demonstrated that plasma apelin levels are associated with age, gender, presence or absence of diabetes, SBP, DBP, MABP, BMI, and LDL-C. Then the relationship between plasma apelin levels and blood pressure was further investigated. The SBP, DBP, and MABP values dropped with increasing apelin levels within the quartiles, and even after confounding factors were adjusted the significant differences in SBP, DBP, and MABP between the groups within the apelin quartiles remained. Furthermore, linear correlation analysis also indicated a negative linear correlation between SBP, DBP, as well as MABP and apelin levels, and this negative correlation remained after confounding factors were controlled.
The systemic administration of apelin induced a significant decrease in SBP in wild-type but not APJ-deficient mice, and almost no vasorelaxation action caused by apelin was observed in APJ-deficient mice. On the contrary, after the injection of angiotensin II, APJ-deficient mice exhibited an increase in SBP and the sensitivity to angiotensin II-induced vasopressor response, which indicates that apelin-APJ plays a role in blood pressure regulation as a counter-regulatory component against angiotensin II (Citation11). In Spontaneously hypertensive rat (SHR), the up-regulation of apelin-APJ exerts a counter-regulatory effect against the pressor action of angiotensin II-AT1, leading to a reduction of AT1 expression and an elevation of serum NO levels (Citation15,Citation16). Very recent studies have demonstrated that lower plasma apelin levels were observed in hypertensive populations (Citation17). Also, apelin genetic polymorphisms in hypertension were proven to be associated with onset age of hypertension (Citation19,Citation20). In our study, a negative linear correlation between apelin levels and blood pressure was found. Given that apelin-APJ plays a counter-regulatory role against the activity of angiotensin II-AT1, the balance between apelin-APJ and angiotensin II may be involved in this negative linear correlation, yet the specific mechanism needs to be elucidated in future studies.
It should be noted that we not only took into consideration well-recognized hypertensive risk factors, such as current smoking, obesity, dyslipidemia, and diabetes, but also several novel independent risk factors of hypertension, which were recently reported in prospective studies, such as mALB (Citation21), and hs-CRP (Citation22,Citation23). After these above-mentioned confounding factors were controlled by multivariate linear regression, a negative correlation was still found between apelin levels and blood pressure. Our findings suggested that plasma apelin levels dropped as the blood pressure increased. A negative correlation was observed between plasma apelin levels and blood pressure, and this negative correlation remained after cardiovascular confounding factors were controlled.
Study limitations
Several limitations of this study should be noted. First of all, because it is a cross-sectional study, the findings cannot be used to establish a conclusive cause-and-effect relationship between blood pressure and apelin levels. Also, the balance between apelin-APJ and RASS play pivotal roles in blood pressure regulation. However, the level of angiotensin II has not been measured to elucidate the interactive mechanism of apelin and angiotensin II. In addition, considering that the participation of subjects was voluntary, a selective bias is inevitable in this study.
Conclusion
In conclusion, a negative correlation between plasma apelin levels and blood pressure was observed in this 1000-population-based epidemiological study, which indicates that apelin, a newly discovered peptide, may have an association with blood pressure, and the apelin-APJ system may become a potential therapeutic target of anti-hypertensive treatment.
Acknowledgements
Pengli Zhu, Feng Huang, and Fan Lin contributed equally to the study.
Declaration of interest: Our study was supported by the Fujian Major Program of Basic Science Project Foundation entitled ‘Epidemiologic survey of hypertension and prehypertension intervention research in HaiDao County of Fujian Province’ (2010Y0013) and the Fujian Medical Innovation Project entitled ‘The association of plasma apelin levels and apelin polymorphism with hypertension and hypertensive vascular injury’ (2012-CX-1). The authors have no conflicts of interest to declare in relation to this article.
References
- Charles CJ. Putative role for apelin in pressure/volume homeostasis and cardiovascular disease. Cardiovsc Hematol Agents Med Chem. 2007;5:1–10.
- Tatemoto K, Hosoya M, Habata Y, Fujii R, Kakegawa T, Zou MX, et al. Isolation and characterization of a novel endogenous peptide ligand for the human APJ receptor. Biochem Biophys Res Commun. 1998;251:471–6.
- Lee DK, Cheng R, Nguyen T, Fan T, Kariyawasam AP, Liu Y, et al. Characterization of apelin, the ligand for the APJ receptor. J Neurochem. 2000;74:34–41.
- Kleinz MJ, Davenport AP. Immunocytochemical localization of the endogenous vasoactive peptide apelin to human vascular and endocardial endothelial cells. Regul Pept. 2004;118:119–25.
- Hosoya M, Kawamata Y, Fukusumi S, Fujii R, Habata Y, Hinuma S, et al. Molecular and function characteristic of APJ: tissue distribution of mRNA and interaction with the endogenous ligand apelin. J Biol Chem. 2000;275:21061–7.
- Cheng X, Cheng XS, Pang CC. Venous dilator effect of apelin, an endogenous peptide ligand for the orphan APJ receptor, in conscious rats. Eur J Pharmacol. 2003;470:171–5.
- Lee DK, Saldivia VR, Nguyen T, Cheng R, George SR, O’Dowd BF. Modification of the terminal residue of apelin-13 antagonizes its hypotensive action. Endocrinology. 2005;146:231–6.
- Salcedo A, Garijo J, Monge L, Fernández N, Luis García-Villalón A, Sánchez Turrión V, et al. Apelin effects in human splanchnic arteries. Role of nitric and prostanoids. Regul Pept. 2007;144:50–5.
- Japp AG, Gruden NL, Amer DA, Li VK, Goudie EB, Johnston NR, et al. Vascular effects of apelin in vivo in man. J Am Coll Cardiol. 2008;52:908–13.
- Tatemoto K, Takayama K, Zou MX, Kumaki I, Zhang W, Kumano K, et al. The novel peptide apelin lowers blood pressure via a nitric oxide-dependent mechanism. Regul Pept. 2001;99:87–92.
- Ishida J, Hashimoto T, Hashimoto Y, Nishiwaki S, Iguchi T, Harada S, et al. Regulatory roles for APJ, a seven-transmembrane receptor related to angiotensin-type 1 receptor in blood pressure in vivo. J Biol Chem. 2004;279:26274–9.
- Barnes GD, Alam S, Carter G, Pedersen CM, Lee KM, Hubbard TJ, et al. Sustained cardiovascular actions of APJ agonism during renin-angiotensin system activation and in patients with heart failure. Circ Heart Fail. 2013;6:482–91.
- American Diabetes Association. Standards of medical care in diabetes—2011. Diabetes Care. 2011;34:S11–61.
- Jia YX, Lu ZF, Zhang J, Pan CS, Yang JH, Zhao J, et al. Apelin activates L-arginine/nitric oxide synthase/nitric oxide pathway in rat aortas. Peptides. 2007;28:2023–9.
- Zhong JC, Huang DY, Liu GF, Jin HY, Yang YM, Li YF, et al. Effects of all-trans retinoic acid on orphan receptor APJ signaling in spontaneously hypertensive rats. Cardiovasc Res. 2005;65:743–50.
- Zhong JC, Huang DY, Yang YM, Li YF, Liu GF, Song XH, et al. Upregulation of angiotension-converting enzyme 2 by all-trans retinoic acid in spontaneously hypertensive rats. Hypertension. 2004;44:907–12.
- Sonmez A, Celebi G, Erdem G, Tapan S, Genc H, Tasci I, et al. Plasma apelin and ADMA levels in patients with essential hypertension. Clin Exp Hypertens. 2010;32:179–83.
- Strazynska A, Bryl W, Hoffmann K, Pupek-Musialik D. The estimation of concentration of apelin, insulin, fasting glucose and anthropometric factors in young hypertensive patients. J Hypertens. 2010;28 (e-Supplement A):e187.
- Li WW, Niu WQ, Zhang Y, Wu S, Gao PJ, Zhu DL. Family-based analysis of apelin and AGTRL1 gene polymorphisms with hypertension in Han Chinese. J Hypertens. 2009;27:1194–201.
- Niu W, Wu S, Zhang Y, Li W, Ji K, Gao P, et al. Validation of genetic association in apelin-AGTRL1 system with hypertension in a larger Han Chinese population. J Hypertens. 2010;28:1854–61.
- Wang W, Lee ET, Fabsitz RR, Devereux R, Best L, Welty TK, et al. A longitudinal study of hypertension risk factors and their relation to cardiovascular disease: the Strong Heart Study. Hypertension. 2006;47:403–9.
- Sung KC, Suh JY, Kim BS, Kang JH, Kim H, Lee MH, et al. High sensitivity C-reactive protein as an independent risk factor for essential hypertension. Am J Hypertens. 2003;16:429–33.
- Andrievskaia EM, Ivleva AIa, Minina ES, Lagutina OE. [Predictive value C-reactive protein assessment level in women with arterial hypertension]. Kardiologiia. 2011;51:32–8.
