Abstract
Background. Some studies showed an anti-atherogenic effect of TNF-α blockers on lipid profile, but these data have been challenged.
Objective. To perform a meta-analysis on lipid profile changes induced by TNF-α blocker treatment.
Methods. Prospective studies on rheumatic patients receiving TNF-α blockers and providing before-and-after treatment values of triglycerides (TGs), total cholesterol (TC), HDL-cholesterol (HDLc), LDL-cholesterol (LDLc), and atherogenic index (AI) were included. Standardized mean differences (SMD) in lipid profile were analyzed at short-term (2–12 weeks), middle-term (13–24 weeks), and long-term (25–52 weeks) assessments.
Results. Thirty articles (1707 patients) were included. TNF-α blockers determined an increase in TC at short-term, middle-term, and long-term assessments (SMD: 0.20 mmol/L [95% CI: 0.04, 0.35]; SMD: 0.27 mmol/L [95% CI: 0.08, 0.46]; SMD: 0.22 mmol/L [95% CI: 0.01, 0.43]). HDLc increased only at the short-term assessment (SMD: 0.19 mmol/L [95% CI: 0.10, 0.28]), and TGs achieved a significant increase at the long-term assessment (SMD: 0.19 mmol/L [95% CI: 0.04, 0.34]). LDLc and AI were not affected by TNF-α blocker treatment.
Conclusions. Slight but significant increases in TC occurred without any significant change in LDLc and AI. Changes in HDLc and TGs were not consistent among the different time point assessments. These quantitative changes in lipid profile do not seem to be able to explain cardiovascular risk improvement reported in patients receiving TNF-α blockers. Further studies on other mechanisms are needed to address this issue.
Key words::
Key messages
TNF-α blockers determine a slight consistent increase in TC levels, a transient increase in HDLc, and a progressive rise in TGs. LDLc and AI are not affected by TNF-α blocker treatment.
Most of the reported changes in lipid profile are slight, transient, or fluctuating and are unlikely to explain cardiovascular risk improvement in rheumatic patients receiving TNF-α blockers. Thus, some further mechanisms should be investigated.
Introduction
Patients with rheumatic diseases, such as rheumatoid arthritis (RA), psoriatic arthritis (PsA), and ankylosing spondylitis (AS), exhibit an increased risk of cardiovascular (CV) events and an increased CV mortality (Citation1). In these patients, immune-mediated chronic systemic inflammation interacts with traditional CV risk factors (Citation2,Citation3).
Tumor necrosis factor-α (TNF-α) has a central role in the pathophysiology of chronic inflammation in major arthritides (Citation2,Citation4) and is also involved in the induction and in the maintenance of the atherosclerotic process (Citation5–7). Further supporting this link, the treatment with TNF-α blockers, besides inducing a significant reduction of the inflammatory burden, has been found to reduce the CV risk (Citation1). However, with only few exceptions—represented by studies on changes in some markers of CV risk in PsA patients (Citation8,Citation9)—most of this evidence derives from studies on RA (Citation5,Citation10).
Some data showed that the treatment with TNF-α blockers is able to normalize platelet hyper-reactivity, (Citation11–13), to reduce subclinical atherosclerosis (Citation14–18), and to improve insulin resistance in rheumatic patients (Citation19–21). Some studies also showed that TNF-α blockers are able to induce anti-atherogenic changes in lipid profile (Citation22). However, these data have been challenged, and this issue needs to be further addressed (Citation23).
Thus, the aim of our systematic review and meta-analysis of the literature is to assess changes in lipid profile after short-, middle-, and long-term treatment with TNF-α blockers in patients with rheumatic diseases.
Methods
A protocol for this review reporting specific objectives, criteria for study selection, approach to assess study quality, outcomes, and statistical methods was prospectively developed.
Search strategy and selection criteria
A systematic research of the literature published until January 2013 was performed using MEDLINE, Cochrane library, EMBASE, Scopus, and Web of Science databases to identify all articles evaluating lipid profile in patients with rheumatic diseases treated with TNF-α blockers.
The search strategy was developed without any publication year or language restriction and used the medical subject headings and text words presented in the online-only Data Supplement. In addition, abstracts of 2009–2012 European League Against Rheumatism (EULAR) and American College of Rheumatology (ACR) congresses were manually reviewed. The full text of relevant abstracts was retrieved. In addition, references of retrieved articles were manually scanned for other relevant articles.
In case of missing data, study authors were contacted by e-mail to try to retrieve original data. Two independent authors (M.N.D.D.M. and P.A.) analyzed each article and performed the data extraction independently. In case of disagreement, a third investigator was consulted (F.D.). Discrepancies were resolved by consensus. Selection results were reported according to PRISMA flow chart (Supplementary Figure 1 to be found online at http://informahealthcare.com/doi/abs/10.3109/07853890.2013.874661).
Data collection and assessment of risk of bias
To be included in the analysis, a study had to have enrolled patients with one of the major arthritides (AR, PsA, AS) treated with TNF-α blockers and to provide values (means with standard deviation) before and after treatment of at least one variable among the following: triglycerides (TGs), total cholesterol (TC), HDL-cholesterol (HDLc), LDL-cholesterol (LDLc), and atherogenic index (AI, corresponding to the TC/HDLc ratio). Studies evaluating patients with chronic inflammatory bowel diseases and with psoriasis without arthritis were not included to obtain data with higher homogeneity. Given the characteristics of the included studies, the evaluation of the methodological quality of each study was performed with the Newcastle–Ottowa Scale (NOS), which is specifically developed to assess quality of non-randomized observational studies (Citation24). Results of the quality assessment are reported in Supplementary Table I to be found online at http://informahealthcare.com/doi/abs/10.3109/07853890.2013.874661.
Data regarding sample size, major clinical and demographic variables, and values of TC, LDLc, HDLc, TGs, and AI before and after initiation of TNF-α blocker treatment were extracted. After evaluating the length of observation intervals reported in each included study, lipid profile changes were stratified at three time points: short-term (2–12 weeks), middle-term (13–24 weeks), and long-term (25–52 weeks). For studies with two values for the same time point (e.g. data at 25 and 52 weeks), only the latest data were used. For homogeneity, data obtained after the 52 weeks of treatment have been separately discussed but not included in the analysis. Studies not reporting standard deviations or standard errors were excluded.
Statistical analysis
Statistical analysis of pre–post mean lipid levels (with standard deviations) was performed with the Review Manager software (Version 5.2, The Cochrane Collaboration, Copenhagen, Denmark), and it was expressed as standard mean difference (SMD) with 95% confidence intervals (95% CI).
With the exception of the atherogenic index which was reported as an absolute number, all the other values have been expressed as millimoles per liter (mmol/L). When expressed as milligrams per deciliter (mg/dL), values have been converted by using the formula: 1 mg/dL = 0.0259 mmol/L.
Analysis of standardized mean differences was carried out, and random effects model was used to take into account the heterogeneity among the studies. The appropriateness of pooling data across studies was assessed with the use of the I2 test for heterogeneity. I2 values of 0% indicate no heterogeneity, 25% low, 25%–50% moderate, and 50% high heterogeneity (Citation25). In case of statistically significant heterogeneity among studies, we planned to remove one study at a time to identify the source of heterogeneity.
Publication bias was assessed by the Egger test and represented graphically by funnel plots of the standard difference in means versus the standard error. Visual inspection of funnel plot asymmetry was performed to address for possible small-study effect, as well as Egger's test to address publication bias, over and above any subjective evaluation. A P < 0.10 was considered statistically significant (Citation26).
Sensitivity analyses
We repeated sensitivity analyses by including using high-quality studies (NOS score ≥ 7).
Subgroup analyses
We planned to perform separate subgroup analyses of studies including only one type of rheumatic disease or reporting on patients receiving only one TNF-α blocker.
Meta-regression analyses
We hypothesized that changes in lipid levels may be affected by differences in baseline characteristics of patients included in different studies (mean age, percentage of male patients) or by the use of concomitant therapies in different studies (percentage of patients treated with corticosteroids or methotrexate). To assess the possible effect of such variables in explaining the different results, observed across studies, we performed a meta-regression analysis after implementing a regression model with changes in lipid levels as dependent variable (y) and the above-mentioned variables as independent variables (x). This analysis was performed with STATA 11.1 (Stata Corp, Austin, TX, USA).
Results
After excluding duplicate results, the search retrieved 1668 articles. Overall 1492 articles were excluded because they were off the topic, 146 were excluded because they were reviews/comments/case reports or they lacked the data of interest. In addition, two letters-to-the-Editor were excluded (Citation27,Citation28) because it was not possible to exclude that data reported in these letters had been partially reported in a previously published full-length paper (Citation29).
Thus, 30 articles (1707 patients) were included in the final analysis () (Citation6,Citation17,Citation29–56).
Table I. Characteristics of included studies.
In detail, 29 studies with data on TC (1627 patients), 28 studies with data on HDLc (1565 patients), 18 studies with data on LDLc (992 patients), 26 studies with data on TGs (1267 patients), and 14 studies with data on AI (550 patients) were evaluated.
Study characteristics
shows the characteristics of the studies included in the meta-analysis. Twenty articles reported on patients receiving only one type of anti-TNF-α drug (3 with adalimumab, 3 with etanercept, and 14 with infliximab), and other 10 studies included patients receiving different types of TNF-α blockers. Overall, we analyzed data about 290 patients receiving adalimumab, 686 under etanercept, and 731 under infliximab. The follow-up varied from 2 weeks to 2 years, and the number of patients from 6 to 292. The mean age of study participants ranged from 32.6 to 56.0 years, and 35.1% of all study participants were male. All included studies but one (Citation35) reported data of patients starting for the first time a treatment with TNF-α blockers (TNF-α blocker-naïve patients). In total, a concomitant use of corticosteroids or of methotrexate was reported by 82.6% and by 92% of studies, while 22.2% of studies included patients who used statins. Median quality score of studies, assessed using NOS, was 7. With the exception of only one study (Citation32), all included studies were non-randomized observational cohort studies. Results were stratified as short-, middle-, and long-term according to the length of follow-up (see Methods section). In only one case (Citation36), also results at 18 and 24 months were available, but, as specified in the methods section, only data till 12 months were included in the analysis.
Short-term study outcomes (2 weeks to 3 months)
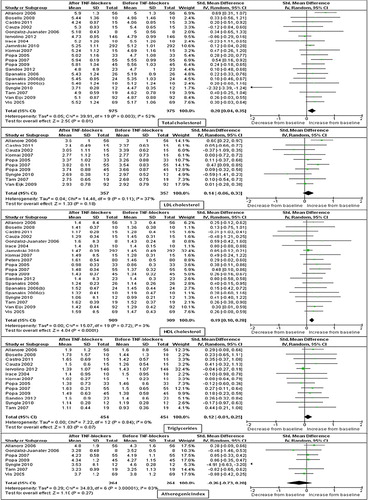
As reported in , we found a significant increase in TC levels (SMD: 0.20 mmol/L; 95% CI: 0.04, 0.35; P = 0.01; I2 = 52%) in the 20 studies (975 patients) evaluating this variable. Heterogeneity among these studies was significant (I2 = 52%; P = 0.003). Removing from the analysis the study performed by Syngle et al. (Citation49), we confirmed a significant increase in TC levels with a low heterogeneity among the studies (SMD: 0.21 mmol/L; 95% CI: 0.11, 0.30; P < 0.001; I2 = 5%). Changes in LDLc levels were evaluated in 10 studies (357 patients) for a resulting not significant increase in the LDLc levels (SMD: 0.14 mmol/L; 95% CI: –0.06, 0.33; P = 0.18; I2 = 37%). HDLc levels were evaluated in 20 studies (909 patients), and a significant increase was reported (SMD: 0.19 mmol/L; 95% CI: 0.10, 0.28; P < 0.001; I2 = 0%) after treatment with TNF-α blockers. Levels of TGs (evaluated in 13 studies, 454 patients) were marginally significantly increased (SMD: 0.12 mmol/L; 95% CI: –0.01, 0.25; P = 0.07; I2 = 0%). No significant changes were found in the seven studies (264 patients) reporting on AI (SMD: –0.26; 95% CI: –0.73, 0.20; P = 0.27). Heterogeneity among studies was highly significant (I2 = 83%; P < 0.00001). After excluding from the analysis the study performed by Single et al. (Citation49), similar results with no heterogeneity were found (SMD: 0.04; 95% CI: –0.13, 0.22; P = 0.62; I2 = 0%).
Middle-term study outcomes (4–6 months)
As reported in , the significant increase in TC levels found in the short term was also confirmed in the 12 studies (367 patients) evaluating this variable in the middle term (SMD: 0.27 mmol/L; 95% CI: 0.08, 0.46; P = 0.005). Heterogeneity among these studies was marginally significant (I2 = 37%; P = 0.09). Similar findings with no heterogeneity were found after the exclusion of the study by Seriolo et al. (Citation29) (SMD: 0.18 mmol/L; 95% CI: 0.02, 0.33; P = 0.02; I2 = 0%). There was a marginally significant increase in HDLc (13 studies with 446 patients; SMD: 0.12 mmol/L; 95% CI: –0.01, 0.25; P = 0.08; I2 = 0%) and in levels of TGs (nine studies with 307 patients; SMD: 0.15 mmol/L; 95% CI: –0.01, 0.30; P = 0.07; I2 = 0%), whereas no significant changes in LDLc (seven studies with 263 patients; SMD: 0.11 mmol/L; 95% CI: –0.06, 0.27; P = 0.22; I2 = 0%) and AI (eight studies with 296 patients; SMD: 0.06; 95% CI: –0.10, 0.22; P = 0.43; I2 = 0%) were found.
Long-term study outcomes (7–12 months)
As reported in , a significant increase in TC was reported (SMD: 0.22 mmol/L; 95% CI: 0.01, 0.43; P = 0.04; I2 = 64%) in the 10 studies (613 patients) evaluating this variable in the long term. A significant heterogeneity (I2 = 64%, P = 0.003) was found among these studies. However, a one-at-a-time study omission, although leading to a progressive reduction of heterogeneity, did not change these results (SMD: 0.12 mmol/L; 95% CI: 0.00, 0.25; P = 0.05, I2 = 35%). In line with short- and middle-term findings, no significant changes were found in eight studies (331 patients) evaluating LDLc levels (SMD: 0.16 mmol/L; 95% CI: –0.05, 0.36; P = 0.13). These studies showed a slightly significant heterogeneity (I2 = 44%; P = 0.09), and, after excluding the study by Garces et al. (Citation43), similar results with a lower heterogeneity were found (SMD: 0.10 mmol/L; 95% CI: –0.10, 0.29; P = 0.33, I2 = 27%). HDLc levels were evaluated in nine studies (417 patients) which showed no significant increase in the HDLc levels (SMD: 0.11 mmol/L; 95% CI: –0.07, 0.29; P = 0.21) with a significant heterogeneity (I2 = 43%; P = 0.08). However, a one-at-a-time study omission did not reduce the heterogeneity. Evaluating 10 studies (591 patients), a significant increase in levels of TGs was found (0.19 mmol/L; 95% CI: 0.04, 0.34; P = 0.02; I2 = 32%). No changes were found in the six studies (237 patients) reporting on AI (SMD: 0.05; 95% CI: –0.12, 0.22; P = 0.57; I2 = 0%). Only one study (Citation36) reported data about lipid profile at 18 and 24 months. Of interest, also this study confirmed that no significant changes occurred in TC, HDLc, and LDLc levels. In contrast, a clear trend towards increased levels was reported for TGs.
An assessment, over time, of absolute mean values at the three pre-defined time points (Supplementary Figure 2 to be found online at http://informahealthcare.com/doi/abs/10.3109/07853890.2013.874661) confirmed that TC exhibited consistently increased values as compared with baseline during treatment with TNF-α blockers, HDLc showed only a trend toward an increase, and TGs showed a progressive increase over time.
Despite the stratification of data in short, middle, and long term, the included studies somehow differed in the three time points. However, evaluating mean lipid profile changes versus baseline at each exact assessment time (expressed in weeks), we found results consistent with those reported above ( to be found online at http://informahealthcare.com/doi/abs/10.3109/07853890.2013.874661).
Publication bias
Publication bias was found for TC levels at short-, middle-, and long-term (Egger = 0.003, 0.09, and 0.003, respectively), for LDLc and HDLc at long-term (Egger = 0.09 and 0.08, respectively), and for AI values at short-term assessment (Egger < 0.0001). No bias was found for the other assessments. Of interest, the funnel plot visual analysis showed that, in all cases, bias was due to four studies (Citation29,Citation43,Citation49,Citation53) declared of low quality and by one study (Citation34) including exclusively subjects under a concomitant steroid and DMARDs treatment.
Sensitivity analysis
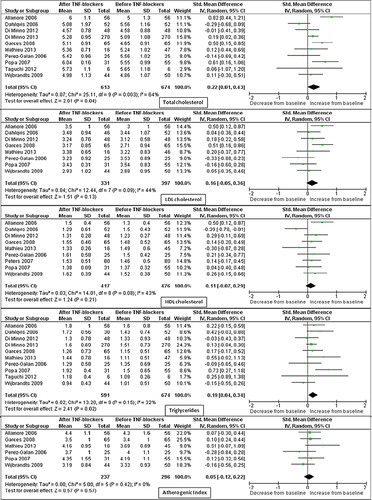
Based on study quality assessment, 17 studies were classified as ‘high quality’, and all the analyses were repeated by including only these studies (Citation6,Citation17,Citation33,Citation34,Citation36–40,Citation42,Citation45–48, Citation50,Citation51,Citation54).
Of interest, results of sensitivity analyses showed that most changes in lipid profile, while confirmed in the short term, are progressively lost at middle- and long-term follow-up ( to be found online at http://informahealthcare.com/doi/abs/10.3109/07853890.2013.874661).
Subgroup analyses
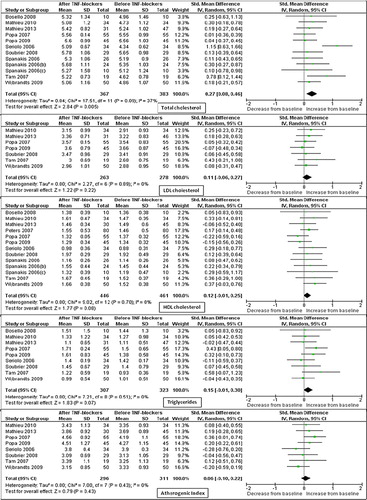
Further stratification was performed according to type of rheumatic disease and to type of TNF-α blocker (). The stratification according to disease type showed that most of the results are derived from studies on RA. In line with this finding, in studies on rheumatoid arthritis all the parameters considered, except AI, were significantly increased at the short-term assessment and TC was significantly increased at middle-term assessment. All the other parameters at middle- and long-term assessment were not significantly different. Conversely, in studies on AS and PsA, results are sparse and based on a very limited number of studies and patients (if any). Similarly, most of studies were on patients exclusively treated with infliximab, and patients treated with this compound seem to have a transitory rise in the HDLc levels and an increase in the levels of TGs at all time points.
Table II. Subgroup analyses according to disease type (Panel A) and drug type (Panel B).
Meta-regression analyses
Regression models did not show any clear trend (regression coefficient with a P value always > 0.2) when we analyzed the possible relationship across the studies between differences in baseline characteristics or in the use of concomitant therapies and changes in lipid levels.
Discussion
Results of the present meta-analysis of studies investigating changes in lipid levels induced by a treatment with TNF-α blockers in rheumatic patients suggest that these drugs are associated with a significant increase in TC, consistently confirmed at short-, middle-, and long-term assessments. As to HDLc, the significant increase found at the short-term assessment was not confirmed at the middle- and long-term assessments. Levels of TGs showed a progressive increase over time, achieving statistical significance at the long-term assessment. In contrast, LDLc and AI were not affected by TNF-α blocker treatment.
At variance with a previous meta-analysis (Citation57), we tried to assess the effects of TNF-α blockers on lipid profile in different types of rheumatic diseases. Although there is no evidence suggesting that effects of TNF-α blockers on lipid profile should differ based on underlying rheumatic disease, most of the included studies were on RA patients, and only five studies specifically reported on PsA and five on AS. Thus, in RA patients, TC, HDLc, LDLc, and levels of TGs were significantly increased at short-term assessment and TC was significantly increased also at middle-term assessment. All the other parameters were not significantly different from baseline. On the other hand, when we considered patients with PsA and AS, due to the relatively low number of studies and of enrolled patients, we were unable to find any statistically significant difference in lipid profile as compared to baseline.
Similarly, the subgroup analysis for the different types of TNF-α blocker drugs showed that most of the results derive from studies on patients receiving infliximab, and that the few available data on patients under etanercept or adalimumab do not lead to definite results.
Moreover, little is known about the influence of the other biologic agents (i.e. anti-IL6, anti-CD20) on lipid profile. Anti-IL6 (tocilizumab) seems to induce pro-atherogenic lipid changes (Citation58,Citation59), while contrasting results have been reported for anti-CD20 (rituximab) (Citation60–62). Thus, further specifically designed prospective studies are needed in order to establish the effect of these increasingly used drugs on lipid profile.
Although underlying mechanisms are not fully addressed, the cardioprotective effect of TNF-α blockers has been suggested by several studies. However, the evaluation of changes in each lipid fraction (HDLc, LDLc, and TGs) predicts the CV risk better than the overall assessment of TC values.
LDLc, which is the major atherogenic lipoprotein, has been considered as the primary cholesterol-lowering therapy target for the reduction of CV risk (Citation63). In the present meta-analysis, we did not find any significant change of LDLc levels after treatment with TNF-α blockers. On the other hand, low levels of HDLc being a strong predictor of the CV risk, the increase in HDLc reported in this meta-analysis might suggest a cardioprotective effect of TNF-α blockers (Citation64).
However, this hypothesis is hampered by the evidence that changes in HDLc were mild, limited to the short-term assessment, and not associated with significant changes in the atherogenic index (i.e. TC/HDLc ratio).
In addition, we found that, moving from short term to middle and long term, TNF-α blocker treatment induced a significant increase in the levels of TGs. This effect is likely to be secondary to the effect of TNF-α blockers on insulin resistance (Citation65). Indeed, by improving insulin resistance, TNF-α blockers induce the uptake of glucose in muscle and fat cells and its conversion to glycogen and triglycerides (Citation66).
However, it is relevant to highlight that most of the reported changes are transient or fluctuating and potentially biased by the presence of confounding factors.
Indeed, although the meta-regression analyses did not show any significant influence of concomitant therapies on the results, we could not exclude that the relative lack of effect on the middle and long term may be partially due to the confounding effect of a concomitant therapy with statins, steroids, and/or methotrexate. In particular, some studies suggest that a treatment with traditional DMARDs may induce effects on lipid profile similar to those reported in our meta-analysis (Citation67–69).
In addition, the inter-relationship between inflammation and lipid homeostasis could be more complex and not completely explained by quantitative changes in lipid profile. For instance, some recent data suggest that TNF-α blocker treatment is able to induce qualitative changes in lipid composition (Citation70,Citation71). Moreover, a recent study reporting on children with juvenile idiopathic arthritis suggests that effects of etanercept on lipid levels could be influenced by other potential age-related determinants. Indeed, at variance with most of other studies, they reported a significant reduction in TC, LDLc, and TGs in their study sample (Citation72).
Overall, considering that such a slight quantitative lipid profile change is unlikely to explain CV risk improvement in rheumatic patients receiving TNF-α blockers, some further mechanisms should be taken into account (Citation1,Citation73,Citation74). Moreover, also the long-term effect of TNF-α blockers on the levels of TGs should be considered. Recent studies have shown an independent effect of triglycerides on the risk of myocardial infarction, ischemic heart disease, and stroke, particularly in women (Citation75,Citation76).
In line with these differences in the CV risk among male and female subjects, a stratification of results according to gender could have been interesting. However, none of the evaluated studies reported data allowing for this stratification.
Our study has some potential limitations. First, studies included in our meta-analysis have different inclusion and exclusion criteria, and most of the patients included in the analysis were receiving one or more co-medications.
Since meta-analysis is performed on aggregate data and some information is missing from each study, the multivariate approach allowed for the adjustment for some—but not all—potential confounders. For example, in addition to differences in age and gender, also the impact of smoking habit on lipid levels should have been addressed. It is known that smoking habit is associated with modification in levels of TGs, HDL, and LDL (Citation77). However, studies reported in the present meta-analysis did not report information about this covariate and, in turn, did not allow for a subanalysis.
Thus, although results of meta-regression analyses did not show any influence of baseline characteristics and/or co-medication on lipid levels, caution is necessary in overall results interpretation. Second, consistency of the results among different studies may be low since lipid values were analyzed in different laboratories. However, to overcome this potential limitation we assessed the effect of TNF-α blockers on lipid profile using the standardized mean difference, which expresses the size of the effect in each study relative to the variability observed. Finally, presence of publication bias may affect the validity of our results. The funnel plots appeared asymmetric for a few of the evaluated end-points suggesting the presence of publication bias for these parameters. However, the exclusion of studies affected by bias and the sensitivity analysis confirmed and refined our results.
In conclusion, these data consistently show that, during a 12-month treatment with TNF-α blockers, a slight but significant increase in TC occurred without any significant change in LDLc and the atherogenic index. Changes in HDLc were significant only at the short term assessment, whereas TGs levels showed a progressive increase over time, achieving a significant increase at the long-term assessment. Whereas results of our meta-analysis are focused on quantitative changes in TC, HDLc, LDLc, TGs, and AI, further studies specifically assessing qualitative changes in lipid profile (i.e. pro-inflammatory HDLc, small dense LDLc, or oxidative LDLc levels) are needed to better evaluate the potential cardioprotective effects of TNF-α blockers.
Supplementary Tables I and II
Download PDF (443.5 KB)Acknowledgements
M.N.D.D.M. and F.D. conceived and designed the study, performed statistical analysis, interpreted results, and drafted the manuscript; R.P., A.D.M., R.L., and P.A. acquired clinical data and drafted the manuscript. All authors read and approved the final version of the manuscript.
M.N.D.D.M. and P.A. had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
All the members of the CaRRDs study group* were involved in literature search, organization of the work, and editing of the manuscript. All authors revised and approved the present version of this manuscript and approved the mention of their names in the article.
The authors want to thank Professor Solbritt Rantapää Dahlqvist for promptly providing original study data when contacted by e-mail.
Declaration of interest: The authors report no conflicts of interest. No funding and economic support has been received for this study.
References
- Di Minno MN, Iervolino S, Lupoli R, Russolillo A, Coppola A, Peluso R, et al. Cardiovascular risk in rheumatic patients: the link between inflammation and atherothrombosis. Semin Thromb Hemost. 2012;38:497–505.
- Sattar N, McCarey DW, Capell H, McInnes IB. Explaining how “high-grade” systemic inflammation accelerates vascular risk in rheumatoid arthritis. Circulation. 2003;108:2957–63.
- del Rincon ID, Williams K, Stern MP, Freeman GL, Escalante A. High incidence of cardiovascular events in a rheumatoid arthritis cohort not explained by traditional cardiac risk factors. Arthritis Rheum. 2001;44:2737–45.
- van Kuijk AW, Reinders-Blankert P, Smeets TJ, Dijkmans BA, Tak PP. Detailed analysis of the cell infiltrate and the expression of mediators of synovial inflammation and joint destruction in the synovium of patients with psoriatic arthritis: implications for treatment. Ann Rheum Dis. 2006;65:1551–7.
- Dixon WG, Symmons DP. What effects might anti-TNF-α treatment be expected to have on cardiovascular morbidity and mortality in rheumatoid arthritis? A review of the role of TNF-alpha in cardiovascular pathophysiology. Ann Rheum Dis. 2007;66:1132–6.
- Popa C, van den Hoogen FH, Radstake TR, Netea MG, Eijsbouts AE, den Heijer M, et al. Modulation of lipoprotein plasma concentrations during long-term anti-TNF-α therapy in patients with active rheumatoid arthritis. Ann Rheum Dis. 2007;66:1503–7.
- Libby P. Changing concepts of atherogenesis. J Intern Med. 2000;247: 349–58.
- Mazzoccoli G, Notarsanto I, de Pinto GD, Dagostino MP, De Cata A, D’Alessandro G, et al. Anti-tumor necrosis factor-α therapy and changes of flow-mediated vasodilatation in psoriatic and rheumatoid arthritis patients. Intern Emerg Med. 2010;5:495–500.
- Tam LS, Li EK, Shang Q, Tomlinson B, Li M, Leung YY, et al. Tumour necrosis factor alpha blockade is associated with sustained regression of carotid intima-media thickness for patients with active psoriatic arthritis: a 2-year pilot study. Ann Rheum Dis. 2011; 70:705–6.
- Peters MJ, Symmons DP, McCarey D, Dijkmans BA, Nicola P, Kvien TK, et al. EULAR evidence-based recommendations for cardiovascular risk management in patients with rheumatoid arthritis and other forms of inflammatory arthritis. Ann Rheum Dis. 2010;69:325–31.
- Di Minno MN, Iervolino S, Peluso R, Scarpa R, Di Minno G. Platelet reactivity and disease activity in subjects with psoriatic arthritis. J Rheumatol. 2012;39:334–6.
- Mac Mullan PA, Peace AJ, Madigan AM, Tedesco AF, Kenny D, McCarthy GM. Platelet hyper-reactivity in active inflammatory arthritis is unique to the adenosine diphosphate pathway: a novel finding and potential therapeutic target. Rheumatology (Oxford). 2010;49:240–5.
- Pamuk GE, Vural O, Turgut B, Demir M, Pamuk ON, Cakir N. Increased platelet activation markers in rheumatoid arthritis: are they related with subclinical atherosclerosis?Platelets. 2008;19:146–54.
- Di Minno MN, Peluso R, Iervolino S, Lupoli R, Russolillo A, Tarantino G, et al. Hepatic steatosis, carotid plaques and achieving MDA in psoriatic arthritis patients starting TNF-α blockers treatment: a prospective study. Arthritis Res Ther. 2012;14:R211.
- Gonzalez-Juanatey C, Vazquez-Rodriguez TR, Miranda-Filloy JA, Gomez-Acebo I, Testa A, Garcia-Porrua C, et al. Anti-TNF-α- adalimumab therapy is associated with persistent improvement of endothelial function without progression of carotid intima-media wall thickness in patients with rheumatoid arthritis refractory to conventional therapy. Mediators Inflamm. 2012;2012:674265.
- Di Minno MN, Iervolino S, Peluso R, Scarpa R, Di Minno G; CaRRDs Study Group. Carotid intima-media thickness in psoriatic arthritis: differences between tumor necrosis factor-α blockers and traditional disease-modifying antirheumatic drugs. Arterioscler Thromb Vasc Biol. 2011;31:705–12.
- Di Minno MN, Iervolino S, Peluso R, Russolillo A, Lupoli R, Scarpa R, et al. Hepatic steatosis and disease activity in subjects with psoriatic arthritis receiving tumor necrosis factor-α blockers. J Rheumatol. 2012;39:1042–6.
- Del Porto F, Laganà B, Lai S, Nofroni I, Tinti F, Vitale M, et al. Response to anti-tumour necrosis factor alpha blockade is associated with reduction of carotid intima-media thickness in patients with active rheumatoid arthritis. Rheumatology (Oxford). 2007;46:1111–15.
- Channual J, Wu JJ, Dann FJ. Effects of tumor necrosis factor-α blockade on metabolic syndrome components in psoriasis and psoriatic arthritis and additional lessons learned from rheumatoid arthritis. Dermatol Ther. 2009;22:61–73.
- Miranda-Filloy JA, Llorca J, Carnero-López B, González-Juanatey C, Blanco R, González-Gay MA. TNF-alpha antagonist therapy improves insulin sensitivity in non-diabetic ankylosing spondylitis patients. Clin Exp Rheumatol. 2012;30:850–5.
- Stagakis I, Bertsias G, Karvounaris S, Kavousanaki M, Virla D, Raptopoulou A, et al. Anti-tumor necrosis factor therapy improves insulin resistance, beta cell function and insulin signaling in active rheumatoid arthritis patients with high insulin resistance. Arthritis Res Ther. 2012;14:R141.
- Popa CD, Arts E, Fransen J, van Riel PL. Atherogenic index and high-density lipoprotein cholesterol as cardiovascular risk determinants in rheumatoid arthritis: the impact of therapy with biologicals. Mediators Inflamm. 2012;2012:785946.
- Schimmel EK, Yazici Y. Increased lipid levels but unchanged atherogenic index in rheumatoid arthritis patients treated with biologic disease modifying antirheumatic drugs: published experience. Clin Exp Rheumatol. 2009;27:446–51.
- Wells G, Shea B. Data extraction for nonrandomised systematic reviews. University of Ottowa, Ottowa. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm (accessed on 5 November 2013).
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60.
- Sterne JA, Egger M, Smith GD. Systematic reviews in health care: investigating and dealing with publication and other biases in meta-analysis. BMJ. 2001;323:101–5.
- Seriolo B, Paolino S, Ferrone C, Cutolo M. Effects of etanercept or infliximab treatment on lipid profile and insulin resistance in patients with refractory rheumatoid arthritis. Clin Rheumatol. 2007;26:1799–800.
- Seriolo B, Paoliono S, Ferrone C, Cutolo M. Comments on the original article by Soubrier et al. Effects of anti-tumor necrosis factor therapy on lipid profile in patients with rheumatoid arthritis. Joint Bone Spine. 2009;76:117–18.
- Seriolo B, Paolino S, Sulli A, Fasciolo D, Cutolo M. Effects of anti-TNF-α treatment on lipid profile in patients with active rheumatoid arthritis. Ann N Y Acad Sci. 2006;1069:414–19.
- Cauza E, Cauza K, Hanusch-Enserer U, Etemad M, Dunky A, Kostner K. Intravenous anti TNF-alpha antibody therapy leads to elevated triglyceride and reduced HDLc-cholesterol levels in patients with rheumatoid and psoriatic arthritis. Wien Klin Wochenschr. 2002; 114:1004–7.
- Irace C, Mancuso G, Fiaschi E, Madia A, Sesti G, Gnasso A. Effect of anti TNFalpha therapy on arterial diameter and wall shear stress and HDLc cholesterol. Atherosclerosis. 2004;177:113–18.
- Popa C, Netea MG, Radstake T, Van der Meer JW, Stalenhoef AF, van Riel PL, et al. Influence of anti-tumour necrosis factor therapy on cardiovascular risk factors in patients with active rheumatoid arthritis. Ann Rheum Dis. 2005;64:303–5.
- Vis M, Nurmohamed MT, Wolbink G, Voskuyl AE, de Koning M, van de Stadt R, et al. Short term effects of infliximab on the lipid profile in patients with rheumatoid arthritis. J Rheumatol. 2005;32:252–5.
- Allanore Y, Kahan A, Sellam J, Ekindjian OG, Borderie D. Effects of repeated infliximab therapy on serum lipid profile in patients with refractory rheumatoid arthritis. Clin Chim Acta. 2006;365:143–8.
- Gonzalez-Juanatey C, Llorca J, Sanchez-Andrade A, Garcia-Porrua C, Martin J, Gonzalez-Gay MA. Short-term adalimumab therapy improves endothelial function in patients with rheumatoid arthritis refractory to infliximab. Clin Exp Rheumatol. 2006;24:309–12.
- Dahlqvist SR, Engstrand S, Berglin E, Johnson O. Conversion towards an atherogenic lipid profile in rheumatoid arthritis patients during long-term infliximab therapy. Scand J Rheumatol. 2006;35:107–11.
- Spanakis E, Sidiropoulos P, Papadakis J, Ganotakis E, Katsikas G, Karvounaris S, et al. Modest but sustained increase of serum high density lipoprotein cholesterol levels in patients with inflammatory arthritides treated with infliximab. J Rheumatol. 2006;33:2440–6.
- Pérez-Galán MJ, Salvatierra-Ossorio J, Cáliz-Cáliz R, Guzmán-Ubeda MA. Influence of tumor necrosis alpha blockade with infliximab on lipid profile in patients with active rheumatoid arthritis. Med Clin (Barc). 2006;126:757.
- Tam LS, Tomlinson B, Chu TT, Li TK, Li EK. Impact of TNF inhibition on insulin resistance and lipids levels in patients with rheumatoid arthritis. Clin Rheumatol. 2007;26:1495–8.
- Peters MJ, Vis M, van Halm VP, Wolbink GJ, Voskuyl AE, Lems WF, et al. Changes in lipid profile during infliximab and corticosteroid treatment in rheumatoid arthritis. Ann Rheum Dis. 2007;66:958–61.
- Komai N, Morita Y, Sakuta T, Kuwabara A, Kashihara N. Anti-tumor necrosis factor therapy increases serum adiponectin levels with the improvement of endothelial dysfunction in patients with rheumatoid arthritis. Mod Rheumatol. 2007;17:385–90.
- Soubrier M, Jouanel P, Mathieu S, Poujol D, Claus D, Dubost JJ, et al. Effects of anti-tumor necrosis factor therapy on lipid profile in patients with rheumatoid arthritis. Joint Bone Spine. 2008;75:22–4.
- Garcês SP, Parreira Santos MJ, Vinagre FM, Roque RM, da Silva JA. Anti-tumour necrosis factor agents and lipid profile: a class effect?. Ann Rheum Dis. 2008;67:895–6.
- Bosello S, Santoliquido A, Zoli A, Di Campli C, Flore R, Tondi P, et al. TNF-alpha blockade induces a reversible but transient effect on endothelial dysfunction in patients with long-standing severe rheumatoid arthritis. Clin Rheumatol. 2008;27:833–9.
- Popa C, van Tits LJ, Barrera P, Lemmers HL, van den Hoogen FH, van Riel PL, et al. Anti-inflammatory therapy with tumour necrosis factor alpha inhibitors improves high-density lipoprotein cholesterol antioxidative capacity in rheumatoid arthritis patients. Ann Rheum Dis. 2009;68:868–72.
- van Eijk IC, de Vries MK, Levels JH, Peters MJ, Huizer EE, Dijkmans BA, et al. Improvement of lipid profile is accompanied by atheroprotective alterations in high-density lipoprotein composition upon tumor necrosis factor blockade: a prospective cohort study in ankylosing spondylitis. Arthritis Rheum. 2009;60:1324–30.
- Wijbrandts CA, van Leuven SI, Boom HD, Gerlag DM, Stroes EG, Kastelein JJ, et al. Sustained changes in lipid profile and macrophage migration inhibitory factor levels after anti-tumour necrosis factor therapy in rheumatoid arthritis. Ann Rheum Dis. 2009;68:1316–21.
- Mathieu S, Dubost JJ, Tournadre A, Malochet-Guinamand S, Ristori JM, Soubrier M. Effects of 14 weeks of TNF alpha blockade treatment on lipid profile in ankylosing spondylitis. Joint Bone Spine. 2010;77:50–2.
- Syngle A, Vohra K, Sharma A, Kaur L. Endothelial dysfunction in ankylosing spondylitis improves after tumor necrosis factor-alpha blockade. Clin Rheumatol. 2010;29:763–70.
- Jamnitski A, Visman IM, Peters MJ, Dijkmans BA, Voskuyl AE, Nurmohamed MT. Beneficial effect of 1-year etanercept treatment on the lipid profile in responding patients with rheumatoid arthritis: the ETRA study. Ann Rheum Dis. 2010;69:1929–33.
- Castro KR, Aikawa NE, Saad CG, Moraes JC, Medeiros AC, Mota LM, et al. Infliximab induces increase in triglyceride levels in psoriatic arthritis patients. Clin Dev Immunol. 2011;2011:352686.
- Taguchi H, Nishi K, Suzuki T, Okano Y. Anti-atherosclerotic effects of etanercept in rheumatoid arthritis patients. Nihon Rinsho Meneki Gakkai Kaishi. 2012;35:183–7.
- Mathieu S, Pereira B, Couderc M, Rabois E, Dubost JJ, Soubrier M. No significant changes in arterial stiffness in patients with ankylosing spondylitis after tumour necrosis factor alpha blockade treatment for 6 and 12 months. Rheumatology (Oxford). 2013;52:204–9.
- Sandoo A, van Zanten JJ, Toms TE, Carroll D, Kitas GD. Anti-TNF-α therapy transiently improves high density lipoprotein cholesterol levels and microvascular endothelial function in patients with rheumatoid arthritis: a pilot study. BMC Musculoskelet Disord. 2012;13:127.
- Di Minno MN, Peluso R, Iervolino S, Lupoli R, Russolillo A, Scarpa R, et al. Obesity and the prediction of minimal disease activity: a prospective study in psoriatic arthritis. Arthritis Care Res (Hoboken). 2013;65:141–7.
- Iervolino S, Di Minno MN, Peluso R, Lofrano M, Russolillo A, Di Minno G, et al. Predictors of early minimal disease activity in patients with psoriatic arthritis treated with tumor necrosis factor-α blockers. J Rheumatol. 2012;39:568–73.
- Daïen CI, Duny Y, Barnetche T, Daurès JP, Combe B, Morel J. Effect of TNF inhibitors on lipid profile in rheumatoid arthritis: a systematic review with meta-analysis. Ann Rheum Dis. 2012;71:862–8.
- Strang AC, Bisoendial RJ, Kootte RS, Schulte DM, Dallinga-Thie GM, Levels JH, et al. Pro-atherogenic lipid changes and decreased hepatic LDL receptor expression by tocilizumab in rheumatoid arthritis. Atherosclerosis. 2013;229:174–81.
- Al-Shakarchi I, Gullick NJ, Scott DL. Current perspectives on tocilizumab for the treatment of rheumatoid arthritis: a review. Patient Prefer Adherence. 2013;7:653–66.
- Raterman HG, Levels H, Voskuyl AE, Lems WF, Dijkmans BA, Nurmohamed MT. HDL protein composition alters from proatherogenic into less atherogenic and proinflammatory in rheumatoid arthritis patients responding to rituximab. Ann Rheum Dis. 2013; 72:560–5.
- Kerekes G, Soltész P, Dér H, Veres K, Szabó Z, Végvári A, et al. Effects of rituximab treatment on endothelial dysfunction, carotid atherosclerosis, and lipid profile in rheumatoid arthritis. Clin Rheumatol. 2009;28:705–10.
- Mathieu S, Pereira B, Dubost JJ, Lusson JR, Soubrier M. No significant change in arterial stiffness in RA after 6 months and 1 year of rituximab treatment. Rheumatology (Oxford). 2012;51:1107–11.
- Third Report of the National Cholesterol Education Program (NCEP). Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–421.
- Bass KM, Newschaffer CJ, Klag MJ, Bush TL. Plasma lipoprotein levels as predictors of cardiovascular death in women. Arch Intern Med. 1993;153:2209–16.
- Russolillo A, Iervolino S, Peluso R, Lupoli R, Di Minno A, Pappone N, et al. Obesity and psoriatic arthritis: from pathogenesis to clinical outcome and management. Rheumatology (Oxford). 2013;52:62–7.
- Lambert JE, Parks EJ. Postprandial metabolism of meal triglyceride in humans. Biochim Biophys Acta. 2012;1821:721–6.
- Boers M, Nurmohamed MT, Doelman CJ, Lard LR, Verhoeven AC, Voskuyl AE, et al. Influence of glucocorticoids and disease activity on total and high density lipoprotein cholesterol in patients with rheumatoid arthritis. Ann Rheum Dis. 2003;62:842–5.
- Georgiadis AN, Papavasiliou EC, Lourida ES, Alamanos Y, Kostara C, Tselepis AD, et al. Atherogenic lipid profile is a feature characteristic of patients with early rheumatoid arthritis: effect of early treatment–a prospective, controlled study. Arthritis Res Ther. 2006;8:R82.
- Park YB, Choi HK, Kim MY, Lee WK, Song J, Kim DK, et al. Effects of antirheumatic therapy on serum lipid levels in patients with rheumatoid arthritis: a prospective study. Am J Med. 2002;113:188–93.
- Hahn BH, Grossman J, Chen W, McMahon M. The pathogenesis of atherosclerosis in autoimmune rheumatic diseases: roles of inflammation and dyslipidemia. J Autoimmun. 2007;28:69–75.
- Kiortsis DN, Mavridis AK, Filippatos TD, Vasakos S, Nikas SN, Drosos AA. Effects of infliximab treatment on lipoprotein profile in patients with rheumatoid arthritis and ankylosing spondylitis. J Rheumatol. 2006;33:921–3.
- De Sanctis S, Marcovecchio ML, Gaspari S, Del Torto M, Mohn A, Chiarelli F, et al. Etanercept improves lipid profile and oxidative stress measures in patients with juvenile idiopathic arthritis. J Rheumatol. 2013;40:943–8.
- Daien CI, Fesler P, Du Cailar G, Daien V, Mura T, Dupuy AM, et al. Etanercept induces a decrease in left ventricular mass in patients with rheumatoid arthritis. Ann Rheum Dis. 2011;3:120.
- Di Minno MN, Iervolino S, Peluso R, Di Minno A, Ambrosino P, Scarpa R. Haemostatic and fibrinolytic changes are related to inflammatory conditions in patients with psoriatic arthritis. Effect of different treatments. J Rheumatol. [in press]
- Nordestgaard BG, Benn M, Schnohr P, Tybjaerg-Hansen A. Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. JAMA. 2007;298:299–308.
- Freiberg JJ, Tybjaerg-Hansen A, Jensen JS, Nordestgaard BG. Nonfasting triglycerides and risk of ischemic stroke in the general population. JAMA. 2008;300:2142–52.
- Schuitemaker GE, Dinant GJ, van der Pol GA, van Wersch JW. Relationship between smoking habits and low-density lipoprotein-cholesterol, high-density lipoprotein-cholesterol, and triglycerides in a hypercholesterolemic adult cohort, in relation to gender and age. Clin Exp Med. 2002;2:83–8.