Abstract
Background. There are limited data regarding clinical presentation and management of Staphylococcus aureus central line- associated bloodstream infection (CLABSI) in immunocompromised cancer patients.
Methods. In this review, we evaluated 299 patients with 304 episodes of S. aureus-CLABSI between 2005 and 2011.
Findings. By multivariate analysis, the major predictors of complicated S. aureus-CLABSI were septic shock, catheter site inflammation, presence of peripherally inserted central catheter, anti-cancer chemotherapy within 10 days, and persistent bacteremia beyond 72 hours (P ≤ 0.02). A total of 67% of the cases were defined as complicated. In the subset of patients who were uncomplicated on presentation, patients receiving antimicrobials ≥ 14 days had similar rates of relapse, attributable mortality, and development of complications compared to those receiving shorter-course therapy. By competing risk analysis, removal of the catheter within 3 days of the onset of bacteremia was associated with a lower relapse rate at 90 days (P = 0.024).
Interpretation. The majority of S. aureus-CLABSI in cancer patients are complicated and require prolonged course of antimicrobial treatment. Early removal of the catheter within the first 3 days is associated with better course. In patients with prompt removal of the catheter and no evidence of a complicated course, treatment beyond 2 weeks may not be necessary.
Key messages
For uncomplicated S. aureus-CLABSI, early removal of the catheter is associated with a better outcome.
Introduction
Central venous catheters (CVCs) are essential in the management of cancer patients for administering chemotherapy, blood products, and necessary intravenous drugs. However, the leading complication of CVCs is central line-associated bloodstream infection (CLABSI), which is associated with substantial morbidity and mortality, especially in cancer patients (Citation1). National surveillance reports have shown that Staphylococcus aureus is the second leading cause of CLABSI (Citation2). The mortality rate in cancer patients with S. aureus-CLABSI is 25%–30%, and more than 50% of patients develop hematogenous complications (Citation3). Moreover, the presentation and outcome of any infection, especially S. aureus-CLABSI, vary according to risk factors.
Despite the significant impact of S. aureus-CLABSI in cancer patients, there are limited data regarding its appropriate management (Citation3–6). For example, the latest Infectious Diseases Society of America (IDSA) guidelines of intravascular catheter-related infections acknowledged the paucity of evidence to determine the optimal duration for the treatment of S. aureus-CLABSI derived from large randomized clinical trials (Citation7). The guidelines recommend removal of the catheter, without specifying the timing, and also suggested a prolonged antimicrobial therapy for S. aureus-CLABSI in cancer patients (Citation7). A shorter duration (minimum of 14 days) was suggested to treat non-immunocompromised and non-neutropenic patients (Citation7).
Ghanem et al. evaluated 91 cancer patients with S. aureus catheter-related bacteremias and suggested a prolonged duration of treatment (more than 2 weeks of anti-staphylococcal intravenous therapy) given the high rate of complications but failed to specify the duration of treatment or the timing of the catheter removal (Citation3). Hence, the management of cancer patients, particularly those with neutropenia, has not been well studied. To address this knowledge gap, we sought to define the clinical manifestations of S. aureus-CLABSI, to identify predictors of a complicated course, and to evaluate the management as far as optimal duration of treatment and timing of catheter removal in a large cohort of cancer patients, including patients with neutropenia.
Methods
Study design and patients
We conducted a retrospective chart review of all patients who had S. aureus-CLABSI between January 2005 and December 2011 at The University of Texas MD Anderson Cancer Center (Houston, TX, USA). Staphylococcus aureus-CLABSI episodes were selected from a database of S. aureus bacteremia that is prospectively maintained by infection control personnel. The Institutional Review Board at MD Anderson approved this study and waived the requirement for obtaining informed consent.
Data collection
Clinical and microbiological data were retrieved from the comprehensive electronic medical records with a follow-up period of 3 months following the onset of the bacteremia. Patients with polymicrobial bacteremias were excluded. We also excluded patients with evidence of S. aureus pneumonia at the time of bacteremia since it would be difficult to establish a causal relationship.
Definitions
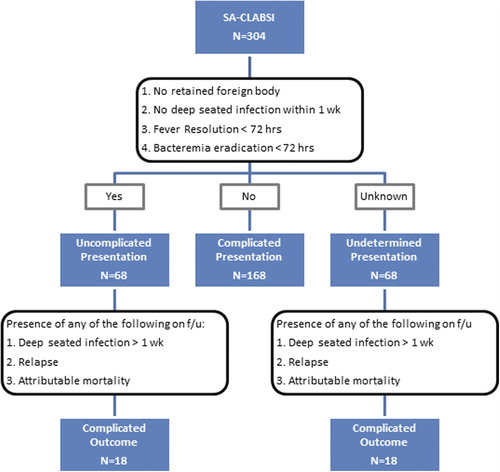
CLABSI was defined per the Centers for Disease Control and Prevention criteria (Citation8). Specifically, patients with a CVC that was present at least 48 hours before the onset of bacteremia were considered to have S. aureus-CLABSI if they had at least one positive blood culture for S. aureus and they had no identifiable source of infection other than the catheter (Citation8). Staphylococcus aureus-CLABSI episodes were considered uncomplicated at presentation if the patient lacked all of the following criteria: 1) retained foreign body (other than the CVC); 2) evidence of infective endocarditis or deep-seated infection within the first 7 days of bacteremia; 3) persistent fever or bacteremia beyond 72 hours of the initiation of adequate therapy (). For this classification, we only included the episodes with documented repeat blood cultures obtained within the first 72 hours of bacteremia onset. If the patient did not have any of the factors listed and had no repeat blood cultures drawn, then the episode was labeled as undetermined on presentation. Sepsis and septic shock were defined per established guidelines (Citation9). A complicated outcome was defined as the occurrence of any of the following during the 3-month follow-up period: deep-seated infection, relapse, or attributable mortality. Deep-seated infections included any of the following complications that occurred during the follow-up period and were confirmed by radiology and/or culture result with the same antibiogram: endocarditis, septic thrombosis, septic arthritis, deep-tissue abscess, epidural abscess, psoas abscess, meningitis, osteomyelitis, and septic pulmonary emboli. Attributable mortality was defined as clinical or microbiological evidence of infection at the time of death. A new episode of S. aureus bacteremia was considered a relapse if the organism had the same antibiogram as the first isolate and the bacteremia occurred within the 3-month follow-up.
Statistical analysis
Continuous variables were compared between groups using the Wilcoxon rank-sum test, and the association between the categorical variables and the groups was assessed by Fisher's exact test. To assess the binary outcome (complicated course), multivariate logistic regression was used to assess the effects of potentially important risk factors. For each fitted logistic regression model, non-significant variables in univariate analyses were eliminated using a cut-off P value of < 0.1.
In addition, the cumulative incidence curves of relapse were estimated and compared between patients who got early catheter removal and those who did not in a competing risk analysis using death as a competing event. All tests were two-sided tests with a significance level of 0.05. The competing risk analyses were performed using the statistical software R version 2.15.0 (R Development Core Team, 2008), and all other statistical analyses were performed using SAS version 9.3 (SAS Institute Inc., Cary, NC, USA).
Results
Patients’ demographic and clinical data
The charts of 762 patients with indwelling CVCs who had S. aureus bacteremia were reviewed. We identified 299 patients with 304 episodes of S. aureus-CLABSI. Patients’ characteristics and clinical presentation are outlined in . At the time of presentation, fever was absent in nearly one-third of the patients, and only 28% had signs of inflammation at the catheter site. Furthermore, 38% of the afebrile patients and 31% of the patients without catheter site inflammation had received steroids within the last week prior to the bacteremia. Of all the S. aureus isolates recovered, 47% were methicillin-resistant, and 51% had a vancomycin minimum inhibitory concentration (MIC) of 2 μg/mL. Transthoracic echocardiography (TTE) was performed for half of the episodes (51%), and transesophageal echocardiography (TEE) was subsequently performed for 29 episodes (10%). Follow-up blood cultures within 72 hours of bacteremia were available for 177 episodes (58%). On presentation, 168 episodes were complicated (). The remaining episodes were classified as uncomplicated (68 episodes) or undetermined presentation (68 episodes), with a complicated course later identified in 36 episodes on follow-up. A total of 67% of S. aureus-CLABSI in our cancer population was eventually defined as complicated (either on presentation or upon follow-up). Relapse occurred in 28 episodes, and a total of 148 deep-seated infections were identified in 146 patients ().
Table I. Patients’ characteristics and clinical presentation.
Table II. Clinical course and outcome of S. aureus-CLABSI.
S. aureus-CLABSI complicated course
Almost half of S. aureus-CLABSI episodes were identified with a complicated course with relapse, deep-seated infection, and attributable mortality (). By univariate analysis there was no association between the development of relapse, deep-seated infection, and attributable mortality and any of the following: underlying malignancy, diabetes, steroid use, methicillin susceptibility, MIC of vancomycin, fever, neutropenia, platelet count, route of antibiotic administration, the time to the initiation of antibiotic therapy, a vancomycin-based regimen, a linezolid-based regimen, and persistent fever beyond 72 hours (data not shown). Only septic shock, peripherally inserted central catheters (PICCs), local inflammation at catheter site, anti-cancer chemotherapy administration within 10 days after the onset of bacteremia, and persistent bacteremia beyond 72 hours were statistically significant for a complicated course in both univariate and multivariate logistic regression (P ≤ 0.02) ().
Table III. Risk factors for complicated S. aureus-CLABSI course on multivariate analysis.
Uncomplicated S. aureus-CLABSI subpopulation
Among the episodes with documented repeat blood cultures within 72 hours, a total of 68 episodes were uncomplicated at the time of presentation (). The median duration of treatment in this population was 15 days (range 4–55 days). During the follow-up period, a complicated course was observed in 18 episodes as follows: 9 relapses, 4 attributable mortalities, and 6 deep-seated infections (2 deep-tissue abscesses, 2 septic thrombosis, 1 osteomyelitis, and 1 septic pulmonary emboli). One episode had both osteomyelitis and attributable mortality. In an attempt to evaluate the impact of a prolonged therapy on outcome in this subpopulation of patients with uncomplicated presentation, we compared patients treated for 14 days or less to those treated beyond 14 days. In this subpopulation, there was no statistically significant difference between the two treatment groups with respect to relapse, attributable mortality, or development of a complicated course (P > 0.99, P = 0.33, and P = 0.94, respectively). Further analysis showed no statistically significant difference with respect to outcome in patients treated for 10–14 days compared with those treated longer than 14 days (data not shown).
Catheter management
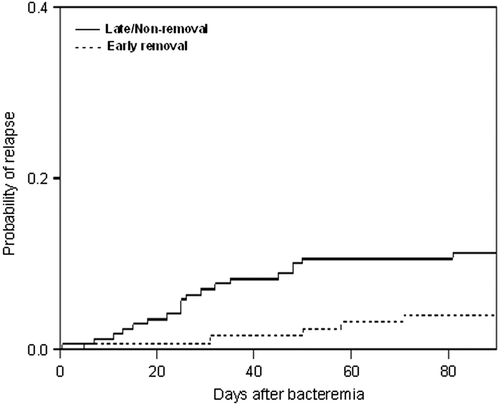
The CVC was removed or exchanged within 7 days of the onset of bacteremia in 66% of the episodes of S. aureus-CLABSI. A higher rate of relapse was observed in patients whose CVC was retained beyond 3 days compared to those whose CVC was removed or exchanged within the first 3 days from the onset of bacteremia (12.7% versus 4.7%; P = 0.017).
Furthermore, by competing risk analysis using death as a competing event, as patients who die could not relapse after death, patients whose CVC was removed beyond 3 days or retained had a higher probability for relapse compared to those whose CVC was removed within the first 3 days from the onset of bacteremia (P = 0.024) ().
Discussion
This is the largest study evaluating S. aureus-CLABSI in cancer patients, including neutropenics, addressing multiple aspects of management in this patient population including clinical presentation, risk factors for a complicated course, optimal duration of therapy, and timing of catheter removal.
Interestingly, the clinical presentation of S. aureus-CLABSI in the cancer population may be similar to what has been previously reported in non-cancer patients, where local inflammation is uncommon and clinical findings are unreliable for the diagnosis of CLABSI (Citation7,Citation10). Nearly one-third of our cancer patients lacked fever at presentation, and nearly 72% of episodes did not present with inflammation at the catheter site. It is important to note that 26% of the latter group had neutropenia. Thus, physicians caring for patients with cancer need to have a high index of suspicion in order to make a timely diagnosis of S. aureus-CLABSI. We speculate that the immunocompromised state of cancer patients inhibits their ability to mount an adequate inflammatory response, thereby making their clinical presentation more subtle (Citation11). In addition, steroid use in this population can suppress the inflammatory response. In fact, in this study, 38% of the afebrile patients and 31% of the patients with no catheter site inflammation had received steroids. Nevertheless, catheter site inflammation was a predictor of a complicated course on both univariate and multivariate analysis. Therefore, cancer patients who, despite their low immunity, show local site inflammation should be considered at high risk of developing complications as even subtle signs of inflammation may suggest a high burden of disease.
Our study showed that persistent bacteremia beyond 72 hours is a predictor of complicated outcome. This finding is consistent with the results of a large study by Fowler et al., who showed that a positive blood culture at 48–96 hours is a strong predictor of complicated S. aureus bacteremia (Citation12).
Another strong predictor of a complicated course in the Fowler et al. study was a persistent fever at 72 hours (Citation12), which was not demonstrated in our study. The implication is that, in our cancer patient population, one should be critical in evaluating fever and its absence, since fever can be caused by non-infectious causes like tumor fever and necrosis, and it can be masked by various agents such as steroids. Moreover, given their suppressed immunity, cancer patients may not be able to mount an adequate inflammatory response (Citation11). Therefore, our findings suggest that in cancer patients with S. aureus-CLABSI, fever (and its persistence) may not be the best parameter to predict outcome and hence we need to rely on the resolution of bacteremia rather than defervescence.
In our study, we found that administration of anti-cancer chemotherapy within 10 days after the onset of bacteremia was also a risk factor for the development of a complicated course. We observed that 87% of those patients received anti-cancer chemotherapy within the first 48 hours following the bacteremia. In most of these patients, chemotherapy could have been administered before obtaining the result of the blood culture or after the patient became afebrile following few days of adequate antimicrobial therapy. This is an important medical challenge that we face with cancer patients, whereby there are no recommendations as to when anti-cancer chemotherapy should be administered after the onset of S. aureus bacteremia and initiation of appropriate antimicrobial therapy. Therefore, before administering anti-cancer chemotherapy to cancer patients with suspected or proven S. aureus-CLABSI, especially those who are receiving steroids, it may be prudent to defer anti-cancer chemotherapy for 10 days and make sure that the repeated blood cultures are negative.
Our findings also highlighted PICC lines as a risk factor for complicated courses. One explanation could be that available PICCs are not antibiotic-impregnated. Another explanation is related to the mechanical complications associated with their insertion, including PICC-related venous thrombosis (Citation13–18). Wilson et al. showed a higher risk of venous thrombosis with PICCs compared with CVCs in patients admitted to a neurological intensive care unit who were unlikely to receive anticoagulation (OR 6.3, 95% CI 1.5–26.7; P = 0.003) (Citation17). Therefore, given the tendency of PICC lines to be more frequently associated with thrombosis and septic thrombosis of the veins in the setting of CLABSI, it is not surprising that they are independently associated with a more complicated course.
Because the majority of our patient population with S. aureus-CLABSI ultimately developed a complicated course, treating them for a long duration might be prudent (4 weeks of systemic antimicrobials). This recommendation is consistent with a previous study at our institution that suggested the need for a prolonged course of therapy given the high risk for deep-seated infections in immunocompromised patients (Citation3). However, a subset of patients who have no evidence of complications at presentation or a subsequent complicated course could be treated for a short course of antimicrobial therapy (14 days). The 2-week cut-off in treating S. aureus bacteremia had been addressed in many studies (Citation18–22), but all had a low number of cancer patients. More than two decades ago, Raad et al. suggested that antimicrobial treatment duration of 14 days should be adequate for uncomplicated CLABSI occurring in low-risk patients (Citation5). Based on our findings as well as several other studies that support this finding, we would suggest that in the subset population of patients who have no evidence of complications at presentation and have no risk factors for a complicated course (such as septic shock, PICC line, anti-cancer chemotherapy administered within 10 days of the bacteremia, catheter site inflammation, and persistent bacteremia beyond 72 hours) treatment duration beyond 2 weeks may not be necessary.
In our study, early removal of the catheter within the first 3 days of bacteremia onset was associated with a lower relapse rate on both univariate and multivariate analysis. Many previous studies have emphasized the importance of prompt catheter removal in S. aureus-CLABSI (Citation23–26). However, very few have sought to determine the optimal time for catheter removal. The latest Infectious Diseases Society of America guidelines recommended removal of the catheter in S. aureus-CLABSI but did not specify the timing (Citation7). A previous study at our institution looked at a cut-off of 48 hours and did not predict a correlation with the development of intravascular versus extravascular complications (Citation3). Fowler et al. showed that each day's delay of catheter removal from the onset of symptoms was associated with an increased risk for hematogenous complication (OR 1.14; 95% CI 1.06–1.21) in non-neutropenic patients with catheter-associated S. aureus bacteremia (Citation26). However, in patients with cancer, especially those who are neutropenic or are receiving steroids, evaluating the time of the onset of symptoms is difficult because most of the patients are not able to mount an adequate inflammatory reaction in response to infection. Our study suggests that in cancer patients with S. aureus-CLABSI, removing the catheter within 3 days from the onset of bacteremia seems to be a reasonable management target that may improve the outcome and decrease the risk of relapse.
The current study has several limitations. Because of the retrospective design of the study, the patients were not treated according to a defined protocol. Thus, echocardiography, venous duplex ultrasonography, and follow-up blood cultures were not performed in all patients, and the true incidence of complications may therefore have been underestimated. The use of steroids in cancer patients is a confounder for the evaluation of fever and its resolution. Nonetheless, this is the largest study of S. aureus-CLABSI in cancer patients, and it provides new insight into its management.
In cancer patients with S. aureus-CLABSI, the majority developed complications and hence required a prolonged course of treatment (4 weeks). Risk factors for a complicated course are septic shock, PICC lines, catheter site inflammation, administration of anti-cancer chemotherapy within 10 days of the bacteremia, and persistence of bacteremia for more than 72 hours. However, in the small subset of patients with uncomplicated S. aureus-CLABSI at presentation and who did not have any of the risk factors for a complicated course, treatment duration beyond 2 weeks may not be necessary. Removal of the catheter within 3 days of the onset of the bacteremia may be associated with a better outcome. A large prospective randomized clinical trial is needed to validate further these findings.
Declaration of interest: The authors report no conflicts of interest.
References
- Wisplinghoff H, Cornely OA, Moser S, Bethe U, Stützer H, Salzberger B, et al. Outcomes of nosocomial bloodstream infections in adult neutropenic patients: a prospective cohort and matched case-control study. Infect Control Hosp Epidemiol. 2003;24:905–11.
- Sievert DM, Ricks P, Edwards JR, Schneider A, Patel J, Srinivasan A, et al. Antimicrobial-resistant pathogens associated with healthcare- associated infections: summary of data reported to the national healthcare safety network at the centers for disease control and prevention, 2009–2010. Infect Control Hosp Epidemiol. 2013;34:1–14.
- Ghanem GA, Boktour M, Warneke C, Pham-Williams T, Kassis C, Bahna P, et al. Catheter-related Staphylococcus aureus bacteremia in cancer patients: high rate of complications with therapeutic implications. Medicine (Baltimore). 2007;86:54–60.
- Raad I, Narro J, Khan A, Tarrand J, Vartivarian S, Bodey GP. Serious complications of vascular catheter-related Staphylococcus aureus bacteremia in cancer patients. Eur J Clin Microbiol Infect Dis. 1992;11:675–82.
- Raad II, Sabbagh MF. Optimal duration of therapy for catheter-related Staphylococcus aureus bacteremia: a study of 55 cases and review. Clin Infect Dis. 1992;14:75–82.
- Park KH, Cho OH, Lee SO, Choi SH, Kim YS, Woo JH, et al. Outcome of attempted Hickman catheter salvage in febrile neutropenic cancer patients with Staphylococcus aureus bacteremia. Ann Hematol. 2010;89:1163–9.
- Mermel LA, Allon M, Bouza E, Craven DE, Flynn P, O’Grady NP, et al. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;49:1–45.
- O’Grady NP, Alexander M, Burns LA, Dellinger EP, Garland J, Heard SO, et al. Summary of recommendations: Guidelines for the Prevention of Intravascular Catheter-related Infections. Clin Infect Dis. 2011;52:1087–99.
- Annane D, Bellissant E, Cavaillon JM. Septic shock. Lancet. 2005; 365:63–78.
- Safdar N, Maki DG. Inflammation at the insertion site is not predictive of catheter-related bloodstream infection with short-term, noncuffed central venous catheters. Crit Care Med. 2002;30:2632–5.
- Sickles EA, Greene WH, Wiernik PH. Clinical presentation of infection in granulocytopenic patients. Arch Intern Med. 1975;135:715–19.
- Fowler VG, Olsen MK, Corey GR, Woods CW, Cabell CH, Reller LB, et al. Clinical identifiers of complicated Staphylococcus aureus bacteremia. Arch Intern Med. 2003;163:2066–72.
- Chemaly RF, de Parres JB, Rehm SJ, Adal KA, Lisgaris MV, Katz-Scott DS, et al. Venous thrombosis associated with peripherally inserted central catheters: a retrospective analysis of the Cleveland Clinic experience. Clin Infect Dis. 2002;34:1179–83.
- Grove JR, Pevec WC. Venous thrombosis related to peripherally inserted central catheters. J Vasc Interv Radiol. 2000;11:837–40.
- Lobo BL, Vaidean G, Broyles J, Reaves AB, Shorr RI. Risk of venous thromboembolism in hospitalized patients with peripherally inserted central catheters. J Hosp Med. 2009;4:417–22.
- Fearonce G, Faraklas I, Saffle JR, Cochran A. Peripherally inserted central venous catheters and central venous catheters in burn patients: a comparative review. J Burn Care Res. 2010;31:31–5.
- Wilson TJ, Stetler WR, Fletcher JJ. Comparison of catheter-related large vein thrombosis in centrally inserted versus peripherally inserted central venous lines in the neurological intensive care unit. Clin Neurol Neurosurg. 2013;115:879–82.
- Graham DR, Keldermans MM, Klemm LW, Semenza NJ, Shafer ML. Infectious complications among patients receiving home intravenous therapy with peripheral, central, or peripherally placed central venous catheters. Am J Med. 1991;91:95S–100S.
- Jernigan JA, Farr BM. Short-course therapy of catheter-related Staphylococcus aureus bacteremia: a meta-analysis. Ann Intern Med. 1993;119:304–11.
- Ehni WF, Reller LB. Short-course therapy for catheter-associated Staphylococcus aureus bacteremia. Arch Intern Med. 1989;149: 533–6.
- Mylotte JM, McDermott C, Spooner JA. Prospective study of 114 consecutive episodes of Staphylococcus aureus bacteremia. Rev Infect Dis. 1987;9:891–907.
- Raad II, Bodey GP. Infectious complications of indwelling vascular catheters. Clin Infect Dis. 1992;15:197–208.
- Dugdale DC, Ramsey PG. Staphylococcus aureus bacteremia in patients with Hickman catheters. Am J Med. 1990;89:137–41.
- Malanoski GJ, Samore MH, Pefanis A, Karchmer AW. Staphylococcus aureus catheter-associated bacteremia. Minimal effective therapy and unusual infectious complications associated with arterial sheath catheters. Arch Intern Med. 1995;155:1161–6.
- Fowler VG, Sanders LL, Sexton DJ, Kong L, Marr KA, Gopal AK, et al. Outcome of Staphylococcus aureus bacteremia according to compliance with recommendations of infectious diseases specialists: experience with 244 patients. Clin Infect Dis. 1998;27:478–86.
- Fowler VG, Justice A, Moore C, Benjamin DK, Woods CW, Campbell S, et al. Risk factors for hematogenous complications of intravascular catheter-associated Staphylococcus aureus bacteremia. Clin Infect Dis. 2005;40:695–703.