Abstract
Objective. Hypertension associates with subarachnoid hemorrhage from saccular intracranial aneurysm (sIA-SAH) when compared to matched controls or general population. Few series compare hypertension in unruptured sIA versus sIA-SAH, so its impact on the sIA disease remains uncertain.
Methods. Kuopio sIA Database (www.uef.fi/ns) contains all cases of unruptured and ruptured sIAs admitted to Kuopio University Hospital from its Eastern Finnish catchment population. We compared the age-adjusted incidence of drug-treated hypertension in 467 unruptured and 1053 ruptured sIA patients admitted to Kuopio University Hospital from 1995 to 2007, using the national registry of prescribed medicines.
Results. Antihypertensive medication was more frequent in the unruptured (73% versus 62%) with higher age-adjusted incidence. At sIA diagnosis, the sIA-SAH group had more often untreated hypertension (29% versus 23%). The size of unruptured sIAs increased with age at sIA diagnosis, independently of hypertension. Multiple sIAs, familial sIA, and sIA-SAH were not associated with hypertension in multivariate analysis. Results indicate that drug-treated hypertension associates with the formation of sIAs rather than their growth or rupture.
Conclusion. Hypertension is highly prevalent in the carriers of unruptured sIAs when compared to those with ruptured sIA. Hypertension may associate with the sIA formation, and may predispose to the rupture of sIA if untreated.
Key messages
Hypertension is highly prevalent in carriers of unruptured sIAs.
Hypertension may associate with the formation of sIA.
Untreated hypertension may predispose to the rupture of sIA.
Introduction
About 3% of the general population develops saccular intracranial aneurysms (sIAs) during life at the forks of intracranial extracerebral arteries (Citation1,Citation2). Most do not rupture and are too small to cause neurological symptoms by compression. If diagnosed during life, most unruptured sIAs are incidental findings in neuroimaging for other causes or screening for familial sIAs. The course of the sIA disease involves formation (Citation3–5), further growth (Citation4–7), and rupture (Citation8) of the sIA pouch. About 95% of aneurysmal subarachnoid hemorrhages are caused by the rupture of the sIA wall (sIA-SAH), a devastating form of stroke that affects the working age population (Citation9). The annual incidence of SAH is 4–7 per 100,000 worldwide (Citation10) but over twice as high in Finland and in Japan (Citation10). The sIA disease is a complex trait, affected by age, female sex, smoking, hypertension, and excess drinking (Citation11), and by genomic factors (Citation12,Citation13) with at least 10% of ruptured sIA-SAH patients having a family history (Citation14,Citation15).
Hypertension, a complex trait that also affects the arterial wall (Citation16), is a strong risk factor for brain infarction and intracerebral hemorrhage (Citation17). Antihypertensive medication is essential in their primary and secondary prevention (Citation18). Hypertension is significant in the formation and growth of sIAs in animal models (Citation19) as well as in small clinical series (Citation3–5). Hypertension strongly associates with angiographically verified sIA-SAH when compared to matched controls or the general population () (Citation20–25). Few series compare hypertension in unruptured sIA carriers against sIA-SAH cases or unaffected controls (Citation20,Citation26–28).
Table I. Previous studies on the association of hypertension and angiographically verified saccular intracranial aneurysms since 2000.
Kuopio sIA Database (www.uef.fi/ns) contains all cases of unruptured and ruptured sIAs admitted to the Kuopio University Hospital (KUH) from a defined Eastern Finnish catchment population since 1980 (Citation15). We have studied the phenotype (Citation15), familial form (Citation14,Citation15), outcome (Citation29), concomitant diseases (Citation30,Citation31), and genomics of the sIA disease (Citation12,Citation13). Here we compare the incidence of drug-treated hypertension in 467 unruptured and 1053 ruptured sIA patients with first diagnosis between 1995 and 2007. We analyze whether hypertension associates with the formation or further growth of sIA rather than rupture.
Materials and methods
Catchment population of Kuopio University Hospital
During the study period from 1995 to 2007, only Neurosurgery of KUH provided full-time acute and elective neurosurgical services for the KUH catchment population in Eastern Finland (Citation31).
The KUH area contains four central hospitals with neurological units of their own. From 1995 to 2007, the geographic area remained the same, but the population decreased from 880,914 to 851,066. The median age increased from 38 to 42 years in males and from 41 to 45 years in females, and the proportion of males remained unchanged at 49%.
Kuopio Intracranial Aneurysm Database (www.uef.fi/ns)
All cases of SAH diagnosed by spinal tap or CT in the KUH catchment area have been acutely admitted to KUH for angiography and treatment if not moribund or very aged. Cases with unruptured intracranial aneurysms (IAs) and no SAH have also had neurosurgical consultation for elective occlusion. They were detected as incidental findings in neuroimaging for other causes, as symptomatic or by screening sIA family members (). The findings were confirmed by four-vessel catheter angiography, magnetic resonance angiography (MRA), or computed tomography angiography (CTA). The exact numbers of rejected cases are not available. KUH Neurosurgery maintains a database of all cases of unruptured and ruptured intracranial aneurysms admitted to the KUH since 1980. The database has been prospective since 1990. The database is run by a full-time nurse, who interviews new cases and codes variables with detailed information, including family history. The criterion for sIA family is at least two affected first-degree relatives (Citation15). Clinical data from the hospital periods and follow-up visits are entered. The purchases of medications prescribed to these patients from 1995 to 2008, as well as cancers and other diagnosed diseases, and causes of death have been entered from national registries.
Table II. Indications for diagnostic neuroimaging in 467 unruptured sIA patients in 1995–2007 from Eastern Finland.
Study population
The cohort consisted of 467 unruptured and 1053 ruptured consecutive sIA patients, fulfilling the following criteria: 1) a citizen of Finland and resident of the KUH catchment area at first diagnosis of sIA disease between 1 January 1995 and 31 December 2007; 2) admission alive to KUH; 3) verification of sIAs by angiography; 4) other intracranial vascular malformations (fusiform, traumatic, or mycotic aneurysm, moyamoya disease, arteriovenous malformation) excluded by angiography; and 5) autosomal polycystic kidney disease and Marfan's syndrome excluded.
Antihypertensive medication
The diagnosis of hypertension was made based on purchased antihypertensive drugs. The Social Insurance Institution of Finland maintains a nationwide registry of all prescribed medicines purchased since 1994. Purchases by the 1520 sIA patients between 1 January 1994 and 31 December 2008 were obtained from the registry and linked to the database. The patient recruitment period from 1 January 1995 to 31 December 2007 allows data on the purchased drugs for at least 1 year before and after the sIA diagnosis. Antihypertensive as well as antidiabetic and statin medications were classified according to the anatomic therapeutic chemical (ATC) classification system. In Finland, these drug groups are sold by prescription only.
Variables
The variables used in the analyses were ():
Table III. Clinical characteristics of 1520 sIA patients in 1995–2007 from Eastern Finland.
For all sIA patients:
sIA disease carrier (gender; age at first sIA diagnosis; sporadic versus familial sIA patient; use and starting age of antihypertensive, antidiabetic, and statin medications; current or former smoking versus none, missing in 1 unruptured and 288 sIA-SAH patients)
sIA disease (location and diameter of the primary sIA; one versus two or more sIAs)
occlusive therapy (microsurgical versus endovascular versus other versus none)
For sIA-SAH patients only:
4. condition on admission to KUH (Hunt and Hess Scale; intracerebral hemorrhage; intraventricular hemorrhage; acute hydrocephalus)
5. outcome at 12 months (Glasgow Outcome Scale)
Review of relevant literature
A PubMed search on 27 November 2013 for articles published in English after 1 January 2001 with the terms (subarachnoid* hemorrhage) AND (hypertension OR “blood pressure”) gave 825 hits. Only the following were included: 1) case-control studies with 2) angiographically verified IAs, 3) age- and sex-matched controls, and 4) multivariate analysis of risk factors. Another search with the terms aneurysm* AND (intracranial OR cerebral) AND unruptured AND (risk factor* OR etiology OR hypertension OR “blood pressure”) gave 530 hits. Only studies with angiographically verified IAs were included. The reference lists of relevant articles were checked for further relevant publications. The most recent study was included from a single cohort ().
Statistical analysis
Univariate analyses were performed using the Mann–Whitney U test or the chi-square test. Multivariate analyses were performed using linear regression () and binomial logistic regression (). P < 0.05 was considered significant. In the analyses relating the sIA diameter to the age at diagnosis, cases with missing sIA diameter or smoking status as well as symptomatic unruptured sIAs were excluded ().To account for differences of ages at sIA diagnosis between the unruptured and ruptured groups, age was standardized to the joint age distribution of both age groups. Standardized rates and confidence intervals were calculated according to Fay and Feuer (Citation32). The incidences of hypertension in the unruptured and ruptured groups were calculated for 10-year age intervals, and the confidence intervals in each age group were calculated as exact central Poisson confidence intervals (Citation33). Age standardization was done using R statistical computing environment, and other analyses were made using SPSS 19 for Mac.
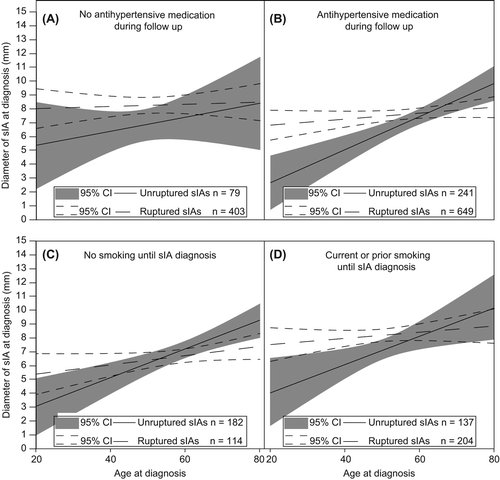
Figure 2. Correlation of the age at diagnosis with the diameter of unruptured saccular intracranial aneurysms (sIAs) and ruptured ones (sIA-SAH). Regression lines and 95% confidence intervals (CIs) are shown for unruptured (continuous lines) and ruptured (dotted lines) patients. A and B: The patients without (A) versus with (B) antihypertensive medication during follow-up. The sIA diameter data were available for 320 unruptured sIA patients and 1052 ruptured sIA patients. C and D: The patients without (C) versus with (D) history of smoking (former or current) until the sIA diagnosis. The sIA diameter data and smoking data were available for 319 (182 in C and 137 in D) unruptured sIA patients and 318 (114 in C and 204 in D) ruptured sIA patients. The unruptured sIAs that caused symptoms were removed from the analysis.
Ethical aspects
The study was approved by the Ethics Committee of the KUH. Data fusion from the national registries was performed with the approval from the Ministry of Social Affairs and Health of Finland.
Results
Study cohort
The entire cohort consisted of 1520 consecutive sIA patients, first diagnosed between 1995 and 2007 (). There were 467 unruptured sIA cases (25% familial) and 1053 sIA-SAH cases (11% familial), with total follow-up times of 3074 and 6130 years, respectively. Of the 467 unruptured sIA cases, 92% were incidental findings (median 6 mm) in neuroimaging, while 8% caused neurological symptoms mainly by their size (median 16 mm) (). The cumulative mortality rate at 12 months was 3.9% for the unruptured cases and 8.5% for the sIA-SAH cases in good condition on admission (Hunt and Hess Scale 1–2) but 42.5% for the sIA-SAH cases in poor condition (Hunt and Hess Scale 3–5).
Hypertension in unruptured versus sIA-SAH patients
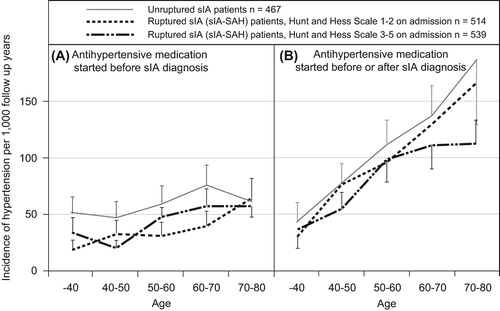
During the observation period for drug use (1994 to 2008), antihypertensive medication was used by 73% of the unruptured sIA patients in contrast to 62% of the sIA-SAH patients (). The medication was started before the sIA diagnosis more often in the unruptured group (57% versus 33%) (). The age-adjusted incidence of hypertension was higher in the unruptured group, 94.5 (95% CI 87.6–102) versus 72.5 (95% CI 68.5–76.8) per 1000 follow-up years (). The incidence was calculated separately for the sIA-SAH cases with poor condition on admission (Hunt and Hess Scale 3–5) because their high mortality reduced the percentage of antihypertensive medications started after the rupture (, ). Antihypertensive treatment was started after the sIA diagnosis in 23% of the unruptured and 29% of the sIA-SAH patients (P < 0.05).
Figure 1. Incidence of hypertension in 467 unruptured saccular intracranial aneurysm (sIA) patients and 1053 ruptured ones (sIA-SAH) by the 10-year age groups and their 95% confidence intervals. A: Patients with antihypertensive medication started before the sIA diagnosis. Follow-up time ends at the start of antihypertensive medication or the time of sIA diagnosis. B: Patients with antihypertensive medication started before or after the sIA diagnosis. Follow-up time ends at the start of antihypertensive medication, death, or 31 December 2008.
Smoking by unruptured versus sIA-SAH patients
Current or former smokers until sIA diagnosis were identified in 45% of the unruptured sIA cases (62% males, 32% females) and in 42% of the sIA-SAH cases (55% males, 32% females; data missing in 27%) ().
Hypertension and smoking versus familial sIA disease
Familial sIA disease was carried by 25% of the unruptured group (23% males, 26% females) and by 11% of the sIA-SAH group (16% males, 12% females) (). In multivariate analysis, hypertension or smoking was not associated with the familial sIA disease in either group (data not shown).
Hypertension and smoking versus multiple sIAs
Multiple sIAs (two or more) were diagnosed in 25% of the unruptured group (26% males, 25% females) and in 28% of the sIA-SAH group (25% males, 30% females) (). In multivariate analysis, hypertension or smoking was not associated with multiple sIAs (data not shown).
Hypertension and smoking versus size of sIA at first diagnosis
The size at diagnosis of the incidentally found unruptured sIAs in 428 patients () increased with age (), independently of gender, familial sIA disease, hypertension, or smoking in multivariate analysis (0.10 mm/year; 95% CI 0.048–0.154; P < 0.001). In comparison, the size of the ruptured sIAs did not increase significantly with the age at first diagnosis (0.03 mm/year; 95% CI –0.001 to 0.054; P = 0.06).
Discussion
Essential findings
We studied the incidence of hypertension in 467 unruptured sIA patients as compared to 1053 sIA-SAH patients, first diagnosed between 1995 and 2007 and admitted to KUH, which solely serves a defined Eastern Finnish catchment population. Only 10% of the unruptured group did not present with hypertension, smoking, or familial sIA disease, independent risk factors for the sIA disease (Citation11). In total, 73% of the unruptured sIA patients and 62% of the sIA-SAH patients had purchased antihypertensive medication between 1994 and 2008 (Citation31), at least 1 year before or after the sIA diagnosis. The lower overall percentage of hypertension in sIA-SAH patients may partially relate to their higher 12-month mortality. The age-adjusted incidence of hypertension was higher in the unruptured sIA group than in the sIA-SAH group (). The size of unruptured sIAs increased with the age at sIA diagnosis (), independently of hypertension. Multiple sIAs, familial sIA disease, or sIA-SAH were not associated with hypertension in multivariate analysis either. Firstly, our findings indicate that hypertension is strongly associated with the formation of sIA. Secondly, untreated hypertension may predispose to the rupture of the sIA wall (Citation27), because at the time of sIA diagnosis the sIA-SAH patients had significantly more often untreated hypertension (see the columns ‘AHTM started after sIA diagnosis’ in ).
Hypertension and sIA disease
One would expect that independent risk factors of the sIA disease would predispose to younger age, and larger size and multiple sIAs at diagnosis. Smoking predisposes to SAH (Citation11,Citation34), even showing synergy with hypertension (Citation35), and smoking is a risk factor for de novo sIA formation verified angiographically (Citation3–5). The mechanisms by which hypertension would affect the formation, further growth, or rupture of the sIA pouch have remained uncertain. In previous angiographically verified sIA series, hypertension has strongly associated with sIA-SAH when compared to matched controls or general population (), suggesting that hypertension would predispose the sIA wall to rupture. However, few series have compared unruptured sIA patients to controls or to sIA-SAH patients (). In a case–control study of 250 aneurysmal SAH patients (25% hypertensive) and 206 unruptured IA patients (38% hypertensive), hypertension was not a significant risk factor for rupture (Citation27). In a Japanese case–control study, 53% of the 858 ruptured and 58% of the 285 unruptured sIA patients had a history of hypertension until the sIA diagnosis as compared to 30% in 798 controls (Citation20). The author concluded that hypertension associates with the formation of sIAs rather than their rupture.
Hypertension in unruptured sIA patients
In the UCAS study, 43.4% of the 5720 unruptured sIA patients (91% incidental; mean age 62.5 years; 68% women; no previous SAH) had a history of hypertension before the angiographic sIA diagnosis (Citation36). Hypertension did not significantly associate with subsequent rupture of the sIAs (HR 1.41; 95% CI 0.96–2.07). In the ISUIA study, 41% of the 4060 angiographically verified unruptured sIA patients had a history of hypertension before the angiographic diagnosis (Citation37). Hypertension was not a significant risk factor for the sIA rupture either. In a recent study of 206 unruptured IA patients and 574 controls, 38% and 19% had a history of hypertension, respectively. Hypertension was a significant risk factor for unruptured sIA in multivariate analysis (OR 2.9; 1–4.6) (Citation28). In a recent study of 263 patients with 384 conservatively treated small anterior circulation sIAs, hypertension was significantly associated with subsequent sIA rupture in follow-up (P < 0.001; HR, 2.6; 95% CI 2.1–3.3) (Citation38). In our study, hypertension was detected in 52% before the sIA diagnosis, tallying with previous studies. Hypertension was detected in further 23% after the sIA diagnosis, a trend unnoticed previously. In the national FINRISK study, only 56.2% to 74.5% of participants in the KUH catchment area were aware of their hypertension (Citation39).
Hypertension and unruptured sIA growth
In animal models, hypertension is essential both in the formation and further growth of sIAs (Citation19). Few clinical series have correlated hypertension to the growth of unruptured sIAs between two angiographies. Hypertension was significantly associated with growth in a retrospective series of 53 sIAs (Citation5), but not in a retrospective series of 165 patients (Citation7) and a prospective series of 87 patients (Citation4). The size of unruptured sIAs increases with the age at diagnosis (Citation15), probably due to longer exposure to modifiable risk factors. In our study, the size of unruptured sIAs increased with age at diagnosis, irrespective of hypertension or smoking.
Hypertension and familial sIA disease
The sIA disease is a complex trait, and at least 10% of cases are familial (Citation14,Citation15), 25% of the unruptured and 11% of the ruptured patients in the present series. Possible genomic or epigenomic mechanisms behind the familial form are unknown. A putative sIA risk locus at 5q26 associates with high systolic blood pressure, but leading hypertension risk loci did not associate with the sIA disease in Finland (Citation13). In the Nordic Twin Cohort, the concordance for SAH diagnoses from national hospital discharge registers was only 3.1% in monozygotic twins, but data for unruptured cases was unavailable (Citation40). Korja et al. suggested that familial clustering of modifiable risk factors, e.g. smoking, high blood pressure, and heavy alcohol consumption, significantly contribute to familial SAH (Citation40). In our series, hypertension or smoking were not associated with familial sIA among the unruptured or ruptured patients.
Hypertension and multiple sIAs
Hypertension was significantly associated with the presence of multiple (two or more) intracranial aneurysms in 392 ruptured patients (OR 1.9, 95% CI 1.5–3.5) (Citation41), but not in 298 ruptured and 121 unruptured patients (Citation42), nor in 266 ruptured patients (Citation43). In our study, hypertension or smoking were not associated with multiple sIAs.
Weaknesses and strengths of the present study
In the present study, blood pressure was not monitored prospectively, but the hypertension diagnosis was based on the purchase of prescribed antihypertensive medicines. Higher 12-month mortality of sIA-SAH patients may have reduced the incidence of hypertension detectable after sIA-SAH. Of the unruptured sIA cases, 92% seemed incidental findings, but hypertension may have affected indications for neuroimaging.
There are several strengths that derive from the Scandinavian health care system. Finland is divided into exclusive catchment areas for the five university hospitals, allowing cohorts that are unselected and minimally biased. Accurate population statistics and stable populations ensure that few patients are lost to follow-up. Our series contains angiographically verified sIA cases only, while several series have included other forms of intracranial aneurysms and non-aneurysmal cases of SAH, or were based on archival ICD codes only. We carefully analyzed the reasons for diagnostic imaging in unruptured cases. In Finland, antihypertensive medications are sold by prescription only, and the purchases are available in the national registry. In previous studies, hypertension diagnosis was often made based on previous history or interview, and the hypertension status was not updated during follow-up after the initial sIA diagnosis.
Suggested further research
Saccular intracranial aneurysms form during life at the branching sites of intracranial extracerebral arteries, in the so-called medial raphe (Citation44), the seal between the medial arterial walls of the two daughter branches when they originate from the mother branch. Fetal development of branching tubular trees in various organs, branching morphogenesis, has not been elucidated in intracranial extracerebral arteries.
Two obvious clinical questions arise. Firstly, can the formation of sIAs be prevented by antihypertensive medication? Hardly, if hypertension is an inborn complex trait that affects the arterial walls and the medial raphe area, in particular, long before the rise of measured blood pressure values that finally triggers the start of medication. Possibly, if the sIA pouch formation is actually caused by elevated blood pressure, but even then it is demanding to design reliable population-based studies, and none exist, so far. Unruptured sIAs are in most cases incidental findings in neuroimaging for other reasons. Secondly, is it reasonable, cost-effective, and ethically sound to screen unruptured sIAs by MRA or CTA after fresh diagnoses of hypertension?
Acknowledgements
J.E.J. and M.v.u.z.F. jointly directed this work. A.L. collected and analyzed data, wrote manuscript; M.K. analyzed data, edited manuscript. A.Ri. collected data. T.K. collected data, edited manuscript. A.Ro. collected data, edited manuscript. J.R. collected data, edited manuscript. J.H. collected data, edited manuscript. J.G.E edited manuscript. J.E.J. analyzed data, edited manuscript. M.v.u.z.F. analyzed data, edited manuscript. We thank Associate Professor Seppo Juvela for valuable comments.
Declaration of interest: The authors report no conflicts of interest. This research was funded by the University of Eastern Finland, EVO funding of Kuopio University Hospital, Sakari Sohlberg foundation, North Savo Regional Fund of Finnish Cultural Foundation, and Emil Aaltonen Foundation.
References
- Vlak MH, Algra A, Brandenburg R, Rinkel GJ. Prevalence of unruptured intracranial aneurysms, with emphasis on sex, age, comorbidity, country, and time period: a systematic review and meta-analysis. Lancet Neurol. 2011;10:626–36.
- Ronkainen A, Miettinen H, Karkola K, Papinaho S, Vanninen R, Puranen M, et al. Risk of harboring an unruptured intracranial aneurysm. Stroke. 1998;29:359–62.
- Tonn J, Hoffmann O, Hofmann E, Schlake HP, Sörensen N, Roosen K.“De novo” formation of intracranial aneurysms: who is at risk?Neuroradiology. 1999;41:674–9.
- Juvela S, Poussa K, Porras M. Factors affecting formation and growth of intracranial aneurysms: a long-term follow-up study. Stroke. 2001; 32:485–91.
- Wermer MJH, van der Schaaf IC, Velthuis BK, Algra A, Buskens E, Rinkel GJ; ASTRA Study Group. Follow-up screening after subarachnoid haemorrhage: frequency and determinants of new aneurysms and enlargement of existing aneurysms. Brain. 2005;128:2421–9.
- Matsubara S, Hadeishi H, Suzuki A, Yasui N, Nishimura H. Incidence and risk factors for the growth of unruptured cerebral aneurysms: observation using serial computerized tomography angiography. J Neurosurg. 2004;101:908–14.
- Burns JD, Huston J, Layton KF, Piepgras DG, Brown RD. Intracranial aneurysm enlargement on serial magnetic resonance angiography: frequency and risk factors. Stroke. 2009;40:406–11.
- van Gijn J, Kerr RS, Rinkel GJE. Subarachnoid haemorrhage. Lancet. 2007;369:306–18.
- Feigin VL, Lawes CM, Bennett DA, Barker-Collo SL, Parag V. Worldwide stroke incidence and early case fatality reported in 56 population-based studies: a systematic review. Lancet Neurol. 2009;8:355–69.
- de Rooij NK, Linn FHH, van der Plas JA, Algra A, Rinkel GJE. Incidence of subarachnoid haemorrhage: a systematic review with emphasis on region, age, gender and time trends. J Neurol Neurosurg Psychiatry. 2007;78:1365–72.
- Feigin VL, Rinkel GJE, Lawes CMM, Algra A, Bennett DA, van Gijn J, et al. Risk factors for subarachnoid hemorrhage: an updated systematic review of epidemiological studies. Stroke. 2005;36:2773–80.
- Yasuno K, Bilguvar K, Bijlenga P, Low S-K, Krischek B, Auburger G, et al. Genome-wide association study of intracranial aneurysm identifies three new risk loci. Nat Genet. 2010;42:420–5.
- Gaál EI, Salo P, Kristiansson K, Rehnström K, Kettunen J, Sarin A-P, et al. Intracranial aneurysm risk locus 5q23. 2 is associated with elevated systolic blood pressure. PLoS Genet. 2012;8: doi e1002563.
- Ronkainen A, Hernesniemi J, Puranen M, Niemitukia L, Vanninen R, Ryynänen M, et al. Familial intracranial aneurysms. Lancet. 1997; 349:380–4.
- Huttunen T, von und zu Fraunberg M, Frösen J, Lehecka M, Tromp G, Helin K, et al. Saccular intracranial aneurysm disease: distribution of site, size, and age suggests different etiologies for aneurysm formation and rupture in 316 familial and 1454 sporadic Eastern Finnish patients. Neurosurgery. 2010;66:631–8.
- Ehret GB, Caulfield MJ. Genes for blood pressure: an opportunity to understand hypertension. Eur Heart J. 2013;34;951–61.
- O’Donnell MJ, Xavier D, Liu L, Zhang H, Chin SL, Rao-Melacini P, et al. Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the INTERSTROKE study): a case-control study. Lancet. 2010;376:112–23.
- Goldstein LB, Bushnell CD, Adams RJ, Appel LJ, Braun LT, Chaturvedi S, et al. Guidelines for the primary prevention of stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42:517–84.
- Aoki T, Nishimura M. The development and the use of experimental animal models to study the underlying mechanisms of CA formation. J Biomed Biotechnol. 2011;2011:535921.
- Inagawa T. Risk factors for the formation and rupture of intracranial saccular aneurysms in Shimane, Japan. World Neurosurg. 2010;73:155–64.
- Ruiz-Sandoval JL, Cantú C, Chiquete E, León-Jiménez C, Arauz A, Murillo-Bonilla LM, et al. Aneurysmal subarachnoid hemorrhage in a Mexican multicenter registry of cerebrovascular disease: the RENAMEVASC study. J Stroke Cerebrovasc Dis. 2009;18:48–55.
- Shiue I, Arima H, Hankey GJ, Anderson CS, Group FTA. Modifiable lifestyle behaviours account for most cases of subarachnoid haemorrhage: a population-based case–control study in Australasia. J Neurol Sci. 2012;313:92–4.
- Okamoto K, Horisawa R. The joint effect of oxidative stress and antioxidants on the risk of an aneurysmal rupture subarachnoid hemorrhage: a case-control study in Japan. Ann Epidemiol. 2007;17: 359–63.
- Ohkuma H. Risk factors for aneurysmal subarachnoid hemorrhage in Aomori, Japan. Stroke. 2002;34:96–100.
- Kubota M, Yamaura A, Ono J. Prevalence of risk factors for aneurysmal subarachnoid haemorrhage: results of a Japanese multicentre case control study for stroke. Br J Neurosurg. 2001;15:474–8.
- You SH, Kong DS, Kim JS, Jeon P, Kim KH, Roh HK, et al. Characteristic features of unruptured intracranial aneurysms: predictive risk factors for aneurysm rupture. J Neurol Neurosurg Psychiatry. 2010;81:479–84.
- Vlak MH, Rinkel GJ, Greebe P, Algra A. Risk of rupture of an intracranial aneurysm based on patient characteristics: a case-control study. Stroke. 2013;44:1256–9.
- Vlak MH, Rinkel GJ, Greebe P, Algra A. Independent risk factors for intracranial aneurysms and their joint effect: a case-control study. Stroke. 2013;44:984–7.
- Huttunen T, von und zu Fraunberg M, Koivisto T, Ronkainen A, Rinne J, Sankila R, et al. Long-term excess mortality of 244 familial and 1502 sporadic one-year survivors of aneurysmal subarachnoid hemorrhage compared with a matched Eastern Finnish catchment population. Neurosurgery. 2011;68:20–7.
- Huttunen T, Riihinen A, Pukkala E, von und zu Fraunberg M, Koivisto T, Ronkainen A, et al. Increased relative risk of lung cancer in 2,904 patients with saccular intracranial aneurysm disease in Eastern Finland. Neuroepidemiology. 2012;38:93–9.
- Lindgren AE, Kurki MI, Riihinen A, Koivisto T, Ronkainen A, Rinne J, et al. Type 2 diabetes and risk of subarachnoid hemorrhage in 1542 carriers of saccular intracranial aneurysm in eastern Finland. Diabetes Care. 2013;36:2020–6.
- Fay MP, Feuer EJ. Confidence intervals for directly standardized rates: a method based on the gamma distribution. Stat Med. 1997;16: 791–801.
- Garwood F. Fiducial limits for the Poisson distribution. Biometrika. 1936;28:437–42.
- Juvela S, Porras M, Poussa K. Natural history of unruptured intracranial aneurysms: probability of and risk factors for aneurysm rupture. J Neurosurg. 2000;93:379–87.
- Lindekleiv H, Sandvei MS, Romundstad PR, Wilsgaard T, Njølstad I, Ingebrigtsen T, et al. Joint effect of modifiable risk factors on the risk of aneurysmal subarachnoid hemorrhage: a cohort study. Stroke. 2012;43:1885–9.
- , UCAS Japan InvestigatorsMorita A, Kirino T, Hashi K, Aoki N, Fukuhara S, et al. The natural course of unruptured cerebral aneurysms in a Japanese cohort. N Engl J Med. 2012;366:2474–82.
- Wiebers DO, Whisnant JP, Huston J, Meissner I, Brown RD, Piepgras DG, et al. Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet. 2003;362:103–10.
- Guresir E, Vatter H, Schuss P, Platz J, Konczalla J, Du Mesnil de Rochement R, et al. Natural history of small unruptured anterior circulation aneurysms: a prospective cohort study. Stroke. 2013;44: 3027–31.
- Kastarinen M, Antikainen R, Peltonen M, Laatikainen T, Barengo NC, Jula A, et al. Prevalence, awareness and treatment of hypertension in Finland during 1982–2007. J Hypertens. 2009;27:1552–9.
- Korja M, Silventoinen K, McCarron P, Zdravkovic S, Skytthe A, Haapanen A, et al. Genetic epidemiology of spontaneous subarachnoid hemorrhage: Nordic Twin Study. Stroke. 2010;41:2458–62.
- Ellamushi HE, Grieve JP, Jäger HR, Kitchen ND. Risk factors for the formation of multiple intracranial aneurysms. J Neurosurg. 2001; 94:728–32.
- Qureshi AI, Suarez JI, Parekh PD, Sung G, Geocadin R, Bharwaj A, et al. Risk factors for multiple intracranial aneurysms. Neurosurgery. 1998; 43:22–6.
- Juvela S. Risk factors for multiple intracranial aneurysms. Stroke. 2000;31:392–7.
- Stehbens WE. Medial raphes (“defects”) in prenatal cerebral arteries. Stroke. 1996;27:1916–17.

