Abstract
Background. The FinPAC trial showed that the strategy of uninterrupted oral anticoagulation (OAC) was non-inferior to interrupted OAC for the primary outcome of bleeding and thromboembolic complications in patients undergoing cardiac rhythm management device (CRMD) implantation.
Methods. We conducted a post hoc analysis of the FinPAC data to explore the incidence and predictors of significant (> 100 cm2) pocket hematoma after CRMD implantation among the study population (n = 447). A total of 213 patients were on OAC, 128 were on aspirin, and 106 on no antithrombotic therapy.
Results. The incidence of significant pocket hematoma during hospital stay was significantly higher among patients using OAC (5.6%) and aspirin (5.5%) than in those with no antithrombotic medications (0.9%), but only one patient (0.8%) in the aspirin group needed revision of hematoma. Two patients (0.9%) in the OAC group and one (0.8%) in the aspirin group needed blood products. In multivariable regression analysis, no pre- procedural features predicted the significant hematoma in any of the groups.
Conclusions. Clinically significant pocket hematoma is a rare complication after CRMD implantation in patients with ongoing therapeutic OAC. The incidence of significant pocket hematoma formation is similar in patients using OAC and those using aspirin.
Trial registration: ClinicalTrials.gov identifier: NCT00479362.
Key messages
Significant pocket hematomas are rare after pacing device implantation performed during therapeutic oral anticoagulation.
The bleeding risk of therapeutic peri-procedural anticoagulation is similar to that of aspirin therapy.
Patients at a moderate-to-high risk of thromboembolism should undergo cardiac rhythm management device implantation without interruption of oral anticoagulation.
Introduction
Many patients (12%–45%) undergoing implantation of cardiac rhythm management device (CRMD) use long-term oral anticoagulation (OAC) (Citation1,Citation2). The optimal strategy for perioperative management of OAC therapy is controversial. The current practice guidelines recommend transient discontinuation of OAC and ‘bridging’ with heparin in patients at moderate-to-high risk of thromboembolic events before surgical procedures (Citation3). Accordingly, heparin bridging is the standard-of-care for CRMD implantation in many centers. However, several reports indicate that compared with uninterrupted OAC, the strategy of heparin bridging may increase rather than decrease the risk of pocket hematoma and other perioperative bleeding complications in patients undergoing CRMD implantation (Citation1,Citation4–7).
The result of the multicenter randomized FinPAC trial showed that the strategy of uninterrupted OAC was non-inferior to interrupted OAC without heparin bridging for the primary outcome of any pocket hematoma in patients undergoing CRMD implantation (Citation8). We conducted a post hoc analysis of the FinPAC data to explore the predictors of significant pocket hematoma formation after CRMD implantation in patients with an indication for long-term anticoagulation therapy. In addition, we compared the incidence of complications to control groups with ongoing aspirin treatment or no antithrombotic treatment during implantation.
Material and methods
Patient selection and study design
The FinPAC trial is a part of an ongoing study program in Western Finland assessing thrombotic and bleeding complications associated with various cardiac procedures in patients using long-term OAC (Citation9–11). The FinPAC trial was aimed to evaluate the safety of uninterrupted antithrombotic therapy in patients undergoing CRMD implantation. The inclusion and exclusion criteria of the trial have been previously described in detail (Citation8). In short, we excluded patients with known coagulation disorder or bleeding diathesis, mechanical heart valves or other absolute contraindication to interrupt warfarin, international normalized ratio (INR) not in therapeutic range at randomization, and significant anemia (hemoglobin < 100 gm/L). The main study group comprised 213 patients on long-term chronic OAC, who were randomized on a 1:1 basis in two groups. In the first group, no pause in the OAC was used, whereas in the other group OAC was discontinued 2 days before the CRMD implantation (with no heparin bridging). In addition to the main study group, we enrolled 128 patients on long-term aspirin and 106 patients with no antithrombotic therapy during the same study period at the same centers, in order to compare the magnitude of complications in these three patient groups. Patients in the aspirin group continued on aspirin (dose 100 mg daily in 116 patients) throughout the peri-procedural period. CHA2DS2VASc and HAS BLED scores were calculated to evaluate the individual risks for thromboembolic and bleeding events, respectively.
Device implantation and perioperative management
All devices were implanted according to the current clinical practice guidelines (Citation12). After prophylactic antibiotic, an incision was made and dissected down to the fascia of the pectoral muscle under local anesthesia. Leads were implanted under fluoroscopic guidance via subclavian or axillary vein puncture, or cephalic vein cut-down. The pocket was expanded with blunt dissection. Electrocautery was used when necessary to ensure adequate hemostasis. All devices were implanted above the pectoral muscle fascia.
Ethical issues
This investigator-driven study was conducted according to the guidelines of the 1964 Declaration of Helsinki, as revised in 2002. The study protocol was approved by the ethics committees of the participating centers. Informed written consent was obtained from every patient after full explanation of the study protocol. The FinPAC trial is registered with ClinicalTrials.gov under the identifier: NCT00479362.
Study definitions and end-points
All patients were evaluated at discharge and 1 month after the index procedure. The primary outcome measure of this substudy was the formation of a significant pocket hematoma (> 100 cm2 in area) and other bleeding complications. Major bleeding was defined as any bleeding or pocket hematoma that required an additional intervention. Pocket exploration was performed if the hematoma progressively enlarged causing pain or threat to the suture line.
Statistical analysis
Continuous variables were reported as the mean ± standard deviation. Categorical variables were described with absolute and relative (percentage) frequencies. Baseline clinical, procedural, and laboratory measures were tested for correlation with the occurrence of a significant pocket hematoma in univariate analyses. Multivariable regression analysis was performed to identify the independent predictors of a significant pocket hematoma in patients on long-term OAC and in those on aspirin. Significant pocket hematoma was entered as the dependent variable, and those variables correlating with significant pocket hematoma in univariate analyses were entered as the covariates. Statistical analysis was performed using SPSS statistical software (SPSS v. 17.0.1, SPSS Inc., Chicago, IL, USA).
Results
Baseline characteristics
The baseline clinical, procedural, and laboratory data of the three groups are shown in and . The main indication for OAC was atrial fibrillation. The HAS BLED score was higher in patients using aspirin, compared with those using OAC (1.9 ± 0.9 versus 1.4 ± 0.8, respectively); yet, the CHA2DS2VASc score was comparable in these two groups. The route of access was comparable between the three groups. Patients using OAC were more likely to undergo cardiac resynchronization therapy (P = 0.013); however, patients using aspirin had more leads inserted during the procedure (P < 0.001).
Table I. Baseline clinical and procedural characteristics of the study groups.
Table II. Laboratory data of the study groups.
The incidence of significant pocket hematoma
Significant hematomas and other bleeding complications are summarized in . There was no significant difference in the incidence of significant pocket hematoma between patients using OAC and those using aspirin; 12 out of 213 patients on long-term OAC (5.6%) and 7 out of 128 (5.5%) on long-term aspirin had significant pocket hematoma. In contrast, only 1 out of 106 patients (0.9%) with no antithrombotic medications had significant pocket hematoma. One patient (0.8%) in the aspirin group needed revision of hematoma, whereas in the OAC and control group no revisions were needed. Blood product transfusion was needed by two patients (0.9%) in the OAC group, one (0.8%) in the aspirin group, while no patient in the control group needed blood products. The lowest postoperative hemoglobin level was 88 g/L in the OAC group, 99 g/L in the aspirin group, and 96 g/L in patients with no antithrombotics. One patient with OAC had a stroke 3 days after the procedure (). His INR was 1.7 at the time of the stroke. No mortality was observed in the three groups during 30-day follow-up. Hospital stay was comparable among all patient groups, and only a minority of patients were treated as out-patients.
Table III. Significant hematoma and other bleeding complications.
Predictors of significant pocket hematoma
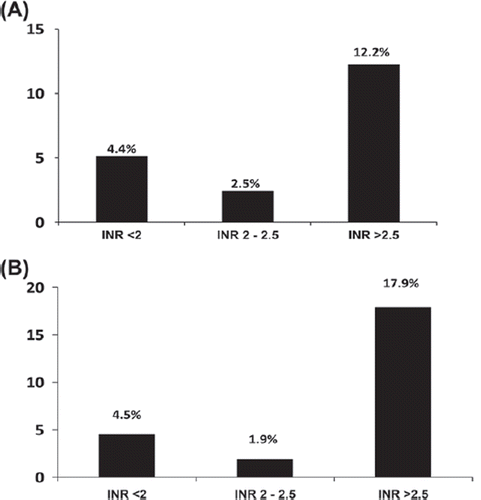
In patients on long-term OAC, post-procedural INR (P = 0.04) and implantable cardioverter defibrillator (ICD) device (P = 0.04) correlated with significant pocket hematoma in the univariate analyses, and clopidogrel use showed a trend to correlation (P = 0.09). In the multivariable logistic regression analysis, the only independent predictor of significant pocket hematoma was post-procedural INR (HR 2.6, 95% CI 1.0–6.6, P = 0.045) (). In patients on long-term aspirin, the only independent predictor of significant pocket hematoma in the multivariable logistic regression analysis was the duration of the procedure (HR 1.01, 95% CI 1.00–1.03, P = 0.05). Of note, HAS BLED score, access vein, or use of hemostatic agents did not predict incidence of significant pocket hematoma among patients treated with OAC or aspirin.
Discussion
The main finding of our study is that the risk of clinically relevant pocket hematoma and other bleeding complications in patients undergoing CRMD implantation during therapeutic OAC without heparin bridging is low and similar to that during ongoing aspirin therapy. The risk of significant pocket hematoma was only weakly associated with higher post-procedural INR, but not with the estimated bleeding risk assessed by the HAS BLED score. Most importantly, the only stroke occurred in a patient with a low INR level due to warfarin pause, supporting the view that therapeutic OAC should be maintained during the whole perioperative period.
Antithrombotic management during CRMD implantation
Pocket hematoma formation is the most common complication of CRMD implantation. Its incidence in patients on long-term OAC has been 5%–8% (Citation5,Citation13–15). Management of antithrombotic therapy during CRMD implantation in patients on long-term OAC is controversial. The options are either to stop temporarily OAC and start low-molecular-weight heparin a few days before the procedure (‘heparin bridging’), to interrupt OAC with no heparin bridging, or to perform the procedure under therapeutic OAC (Citation16). The goal of heparin bridging is to reduce the frequency of bleeding complications without predisposing the patient to thromboembolic events. According to multiple retrospective observational studies, the risk of bleeding complications was higher with heparin bridging than with uninterrupted OAC (Citation1,Citation4–6,Citation17,Citation18). Importantly, one recent randomized trial was terminated prematurely because of a marked reduction of clinically significant pocket hematoma with the strategy of uninterrupted OAC versus heparin bridging in patients undergoing CRMD implantation (Citation7). Moreover, two prospective randomized trials showed comparable rates of bleeding complications between the strategy of uninterrupted and that of interrupted OAC in patients undergoing CRMD implantation (Citation8,Citation19).
Risk factors of hematoma formation during CRMD implantation
Our data show that the level of peri-procedural OAC has minor effect on hematoma formation, although post-procedural INR was weakly associated with significant pocket hematoma. The clinical value of this finding is limited, since it cannot be used to predict pocket hematoma before the procedure. Similarly, HAS BLED score, introduced to classify the risk of bleeding during long-term OAC, did not predict the occurrence of significant pocket hematoma in our cohort. Implantation of ICD was weakly associated with increased risk of significant pocket hematoma, but as in some prior studies (Citation20,Citation21) the type of the implanted device was not an independent predictor of a significant pocket hematoma. A potential bias in this finding is that most of the ICDs were implanted by an experienced operator, which may carry a lower risk of hematoma than the procedures performed by trainees (Citation13,Citation15,Citation21). In our study, however, operation by a trainee was only weakly associated with significant pocket hematoma and only in patients on long-term aspirin. Longer duration of the procedure was an independent predictor of significant pocket hematoma in patients using aspirin in line with a previous study (Citation5). In a recent registry, procedures including lead revisions and device upgrades were associated with more frequent hematoma formation (Citation22). Consistent with our results, previous studies have shown no advantage of the cephalic vein approach in terms of bleeding complications (Citation1,Citation13,Citation20,Citation23). There were more patients with atrial fibrillation in the OAC group, explaining the lower number of pacemaker leads in this group, which may reduce the bleeding risk (Citation17), but this was counterbalanced by the fact that the number of patients who received cardiac resynchronization therapy devices was higher in the OAC group. Finally, the use of clopidogrel and dual antiplatelet therapy has been reported to increase the incidence of hematoma formation (Citation5,Citation23). In our study, the use of clopidogrel was very rare and only weakly associated with significant pocket hematoma in patients on long-term OAC.
Limitations of the study
The statistical power of the current analysis is limited by the infrequent occurrence of significant complications. The results may have been affected by the fact that the patients were treated according to our routine clinical practice by cardiologists and trainees, and that various techniques were employed for venous access (e.g. subclavian or axillary vein puncture, and cephalic vein cut-down). It should also be emphasized that in this study most of the INR values in patients on long-term OAC were at subtherapeutic or low therapeutic level. Therefore, caution is needed when extrapolating these results to patients with higher INR values. Moreover, baseline characteristics were heterogeneous among the three groups, reflecting the healthier state of the controls. These differences may contribute to the lower rate of pocket hematoma in the control group, but show the level of bleeding complications in low-risk patients referred for CRMD implantation.
Conclusion
In patients undergoing CRMD implantation during OAC therapy, the incidence of significant pocket hematomas was low and comparable to that during ongoing aspirin therapy. Peri-procedural INR had little effect on the incidence of significant pocket hematoma formation. Hence, patients at moderate-to-high risk of thromboembolism should undergo CRMD implantation without interruption of OAC.
Declaration of interest: This study was supported by grants from the Finnish Foundation for Cardiovascular Research, Helsinki, Finland. The authors declare that they have no conflict of interests.
References
- Marquie C, De Geeter G, Klug D, Kouakam C, Brigadeau F, Jabourek O, et al. Post-operative use of heparin increases morbidity of pacemaker implantation. Europace. 2006;8:283–7.
- Giudici MC, Paul DL, Bontu P, Barold SS. Pacemaker and implantable cardioverter defibrillator implantation without reversal of warfarin therapy. Pacing Clin Electrophysiol. 2004;27:358–60.
- Douketis JD, Spyropoulos AC, Spencer FA, Mayr M, Jaffer AK, Eckman MH, et al. Perioperative management of antithrombotic therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141:e326S–50S.
- Ahmed I, Gertner E, Nelson WB, House CM, Dahiya R, Anderson CP, et al. Continuing warfarin therapy is superior to interrupting warfarin with or without bridging anticoagulation therapy in patients undergoing pacemaker and defibrillator implantation. Heart Rhythm. 2010;7:745–9.
- Tompkins C, Cheng A, Dalal D, Brinker JA, Leng CT, Marine JE, et al. Dual antiplatelet therapy and heparin “bridging” significantly increase the risk of bleeding complications after pacemaker or implantable cardioverter-defibrillator device implantation. J Am Coll Cardiol. 2010;55:2376–82.
- Robinson M, Healey JS, Eikelboom J, Schulman S, Morillo CA, Nair GM, et al. Postoperative low-molecular-weight heparin bridging is associated with an increase in wound hematoma following surgery for pacemakers and implantable defibrillators. Pacing Clin Electrophysiol. 2009;32: 378–82.
- Birnie DH, Healey JS, Wells GA, Verma A, Tang AS, Krahn AD, et al. Pacemaker or defibrillator surgery without interruption of anticoagulation. N Engl J Med. 2013;368:2084–93.
- Airaksinen KE, Korkeila P, Lund J, Ylitalo A, Karjalainen P, Virtanen V, et al. Safety of pacemaker and implantable cardioverter-defibrillator implantation during uninterrupted warfarin treatment - The FinPAC study. Int J Cardiol. 2013;168:3679–82.
- Karjalainen P, Vikman S, Niemelä M, Porela P, Ylitalo A, Vaittinen M, et al. Safety of percutaneous coronary intervention during uninterrupted oral anticoagulant treatment. Eur Heart J. 2008;29:1001–10.
- Annala AP, Karjalainen PP, Biancari F, Niemelä M, Ylitalo A, Vikman S, et al. Long-term safety of drug-eluting stents in patients on warfarin treatment. Ann Med. 2012;44:271–8.
- Korkeila P, Ylitalo A, Koistinen J, Airaksinen K. Progression of venous pathology after pacemaker and cardioverter-defibrillator implantation: a prospective serial venographic study. Ann Med. 2009;41:216–23.
- Crossley GH, Poole JE, Rozner MA, Asirvatham SJ, Cheng A, Chung MK, et al. The Heart Rhythm Society (HRS)/American Society of Anesthesiologists (ASA) Expert Consensus Statement on the perioperative management of patients with implantable defibrillators, pacemakers and arrhythmia monitors: facilities and patient management this document was developed as a joint project with the American Society of Anesthesiologists (ASA), and in collaboration with the American Heart Association (AHA), and the Society of Thoracic Surgeons (STS). Heart Rhythm. 2011;8:1114–54.
- Wiegand UK, LeJeune D, Boguschewski F, Bonnemeier H, Eberhardt F, Schunkert H, et al. Pocket hematoma after pacemaker or implantable cardioverter defibrillator surgery: influence of patient morbidity, operation strategy, and perioperative antiplatelet/anticoagulation therapy. Chest. 2004;126:1177–86.
- Cheng M, Hua W, Chen K, Pu J, Ren X, Zhao X, et al. Perioperative anticoagulation for patients with mechanic heart valve(s) undertaking pacemaker implantation. Europace. 2009;11:1183–7.
- Tolosana JM, Berne P, Mont L, Heras M, Berruezo A, Monteagudo J, et al. Preparation for pacemaker or implantable cardiac defibrillator implants in patients with high risk of thrombo-embolic events: oral anticoagulation or bridging with intravenous heparin?A prospective randomized trial. Eur Heart J. 2009;30:1880–4.
- Airaksinen K, Schlitt A, Rubboli A, Karjalainen P, Lip G. How to manage antithrombotic treatment during percutaneous coronary interventions in patients receiving long-term oral anticoagulation: to “bridge” or not to “bridge”?EuroIntervention. 2010;6:520–6.
- Tischenko A, Gula LJ, Yee R, Klein GJ, Skanes AC, Krahn AD. Implantation of cardiac rhythm devices without interruption of oral anticoagulation compared with perioperative bridging with low- molecular weight heparin. Am Heart J. 2009;158:252–6.
- Jamula E, Douketis JD, Schulman S. Perioperative anticoagulation in patients having implantation of a cardiac pacemaker or defibrillator: a systematic review and practical management guide. J Thromb Haemost. 2008;6:1615–21.
- Cheng A, Nazarian S, Brinker JA, Tompkins C, Spragg DD, Leng CT, et al. Continuation of warfarin during pacemaker or implantable cardioverter-defibrillator implantation: a randomized clinical trial. Heart Rhythm. 2011;8:536–40.
- Przybylski A, Derejko P, Kwaśniewski W, Urbańczyk-Swić D, Zakrzewska J, Orszulak W, et al. Bleeding complications after pacemaker or cardioverter-defibrillator implantation in patients receiving dual antiplatelet therapy: results of a prospective, two-centre registry. Neth Heart J. 2010;18:230–5.
- Pakarinen S, Oikarinen L, Toivonen L. Short-term implantation-related complications of cardiac rhythm management device therapy: a retrospective single-centre 1-year survey. Europace. 2010;12:103–8.
- Poole JE, Gleva MJ, Mela T, Chung MK, Uslan DZ, Borge R, et al. Complication rates associated with pacemaker or implantable cardioverter-defibrillator generator replacements and upgrade procedures: results from the REPLACE registry. Circulation. 2010; 122:1553–61.
- Kutinsky IB, Jarandilla R, Jewett M, Haines DE. Risk of hematoma complications after device implant in the clopidogrel era. Circ Arrhythm Electrophysiol. 2010;3:312–18.