Abstract
Aims. Idiopathic myocardial fibrosis (IMF) was observed to be the most prevalent autopsy finding in the victims of sudden cardiac death (SCD) under the age of 40 years in the FinGesture cohort. To elucidate further the mechanisms of IMF, we examined the collagen composition from the myocardial samples taken from the victims of IMF-associated SCD.
Methods. Eighteen cases with IMF as a cause of death, confirmed by autopsy, were selected for the analysis. Controls (n = 27) included were cases in whom no cardiac or non-cardiac disease could be found as a cause of unexpected death at autopsy. In addition to conventional histological examination, immunohistochemical staining of procollagens I and III (PINP and PIINP), mature collagen III (IIINTP), and the cross-linked collagen I degradation product (ICTP) were performed.
Results. Increased accumulation of PINP was observed in the fibrotic tissue of the IMF cases in comparison with control samples. In contrast, type III collagen was not as frequently expressed in the fibrotic areas.
Conclusion. Myocardial accumulation of PINP in the victims of IMF-associated SCD indicates increased type I collagen synthesis. Future studies on the role of circulating type I collagen biomarkers are needed to study further the implications of the described association.
Key words::
Key message
The accumulation of procollagen I was increased in the fibrotic tissue of the idiopathic myocardial fibrosis cases.
Introduction
The Finnish Genetic Study for Arrhythmic Events (FinGesture) is a prospective study assessing the characteristics and genetic background of consecutive series of autopsy-verified out-of-hospital victims of sudden cardiac death (SCD) in a specific geographical area in northern Finland (Citation1). Our recent sub-study of the FinGesture cohort reported that idiopathic myocardial fibrosis (IMF) was the largest subgroup, accounting for 28% of non-ischemic SCDs in victims under the age of 40 years in Northern Finland (Citation1). Cardiac fibrosis may provide electrical heterogeneity and a substrate for severe arrhythmias resulting in SCD. The purpose of this study was to determine the collagen composition of the fibrosis from myocardium samples taken from victims of IMF-associated SCD.
An increase of collagen production has been observed in many cardiac diseases, which results in development of fibrosis throughout the myocardium. Of all 29 different collagen types that have been described, collagen types I (80%) and III (11%) are most abundant in the heart (Citation2,Citation3). Lesser amounts of types IV and V are detected in the basement membrane of the myocytes, and in the perivascular, and pericellular space (Citation4).
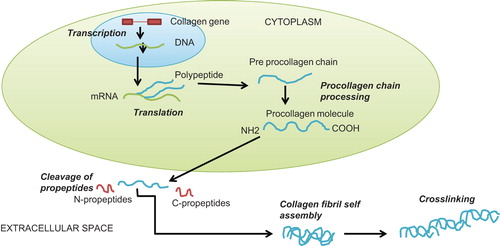
In recent years the study of bloodstream biomarkers related to cardiac remodelling in heart failure has been intense. Fibrotic biomarkers that can be used for the detection of collagen synthesis are the propeptides or other breakdown products of collagens (). The propeptides are aminoterminal propeptides (PINP and PIIINP), which characterize the newly synthesized type I and III collagens. Some peptides (carboxyterminal telopeptides (ICTP) and aminoterminal telopeptides (IIINTP)) are not removed until the collagen fibril is degraded. IIINTP is a marker of cross-linked mature collagen III, whereas ICTP detects a degradation product of cross-linked type I collagen (Citation5,Citation6). Differences in these biomarker levels have been shown in longitudinal follow-up studies and case-control studies in dilated cardiomyopathy (DCM) and severe congestive heart failure (CHF) (Citation7–12). However, while serologic collagen studies have been popular, the histological studies of the collagen content of myocardial fibrosis have not been performed with the same enthusiasm, mainly because the feasibility of direct tissue examinations is limited.
Methods
Study population
The FinGesture study population was derived from 2661 consecutive victims of SCD in the Province of Oulu, Northern Finland, among whom post-mortem examinations were performed at the Department of Forensic Medicine of the University of Oulu between 1998 and 2007 (Citation13). Victims with non-cardiac causes of sudden death were excluded.
Because post-mortem studies are mandatory in Finland whenever SCD is not due to a known disease, the deceased has not been treated by a physician during his/her last illness, or when death has been otherwise unexpected (Act on the Inquest into the Cause of Death, 459/1973, 7th paragraph: Finnish Law) (Citation14), selection bias of forensic studies in victims with unexpected SCD is minimal. Information about the SCD victims was obtained from a combination of the available medical records, post-mortem examination reports, the medication used at the time of SCD, and a standardized questionnaire to the closest family members of the victims of SCD. From this population 18 cases with IMF as a cause of death were selected for the analysis. IMF was defined as macroscopic and/or microscopic evidence of interstitial myocardial fibrosis without signs of cardiac dilatation or hypertrophy suggestive of either dilated or hypertrophic cardiomyopathy (HCM). Myofibrillar disarray in histological studies suggesting HCM or DCM also led to exclusion. Family history of sudden cardiac death was defined by occurrence of SCD in the first-degree relatives (parents, siblings, or children) of the deceased subject. All dissections were complete autopsies; no partial dissections were performed. A systematic number of biopsies was taken for the histologic analysis: 1) left ventricle anterior wall, 2) septum, 3) left ventricle lateral wall, 4) left ventricle posterior wall, and 5) right ventricle. All the section samples were taken from the central parts of the walls.
Control subjects were collected from the Province of Oulu, Northern Finland, among whom post-mortem examinations were performed at the Department of Forensic Medicine of the University of Oulu. Controls included were cases in whom the death was defined as suicide after toxicologic examination, or caused by a traffic accident, and no cardiac or non-cardiac disease could be found as a cause of unexpected death at autopsy. Twenty-seven such cases with the same age range as the IMF cases were identified between 2008 and 2009, which served as a control group.
The study complied with the Declaration of Helsinki, and the Ethics Committee of the University of Oulu approved the study. The National Supervisory Authority for Welfare and Health (Valvira) approved the review of post-mortem data by the investigators.
Histology, immunohistochemistry, and quantitation of collagen
Formalin-fixed paraffin-embedded heart tissues were obtained from the archives of the Institute of Diagnostics, Department of Forensic Medicine, University of Oulu, Finland. The samples taken at autopsy had been stained by hematoxylin-eosin (HE). In order to visualize better the distribution of fibrotic tissue, selected samples (n = 18) with variable distribution of possible collagen observed in the HE-stained slides were deparaffinized and then stained with Masson's trichrome method. Tissue sections (5 μm) were deparaffinized and rehydrated before epitope retrieval. Immunohistochemical staining was carried out by Autostainer Plus (Dako, Glostrup, Denmark) using the EnVision (Peroxidase/DAB) detection system (Dako). Polyclonal antibodies recognizing the aminoterminal propeptide of type I collagen (PINP) were used at 1:10,000 dilution. These methods have been described in detail by Bode et al. 2000 (Citation6). Also, polyclonal antibodies recognizing the carboxyterminal telopeptide of collagen type I (ICTP), the aminoterminal propeptide of type III collagen (PIIINP), and the aminoterminal telopeptide of type III collagen (IIINTP) were used at 1:2000, 1:4000, and 1:4000 dilution, respectively. Also, additional dilution series 1:500, 1:1000, and 1:1000 were tested for ICTP, PIIINP, and IIINTP, respectively. Slides were counterstained with hematoxylin. Collagen area was measured by computerized image analysis in the slides stained by the Masson's trichrome method and in the PINP-stained slides, using UTHSCSA Image Tool Version 3.0 (UTHSCSA, University of Texas Health Science Center, San Antonio, TX, USA), and expressed in percentages of the section area. Since these measurements yielded virtually identical results, only PINP-stained sections were used for morphological measurements of the whole material. Samples taken from the anterior left ventricular wall were used in the measurements, except in the two cases showing maximal fibrosis in the posterior left ventricular wall or in the septum.
Statistical analysis
All analyses were performed with the Statistical Package for Social Studies version 13.0 (SPSS, Inc., Chicago, IL, USA). P < 0.05 was considered statistically significant. Two-sided t test and chi-square analyses were used for comparisons between study groups.
Results
Patient characteristics
The demographical data, detailed patient characteristics, and description of the fibrosis are presented in . The age, gender, and body mass index (BMI) distribution was not different between the cases and controls (age 46.8 (SD 17.3) versus 45.1 (16.9) years (P = 0.903); male gender 55.0% versus 70.4% (P = 0.218); BMI 25.8 (SD 4.3) versus 25.1 (SD 4.8) (P = 0.546)) (). Different formations of fibrosis were observed in various locations of the myocardium (). One of the victims (patient 4) had lamin A/C gene (LMNA) mutation R541C (Citation15). In additional genetic testing the same LMNA mutation or any other LMNA mutations was not observed in other cases of IMF. Two of the SCD victims had a family history of SCD.
Table I. Detailed patient characteristics.
Table II. Characteristics of idiopathic myocardial fibrosis (IMF) cases and controls.
Immunohistochemistry and quantification of collagen
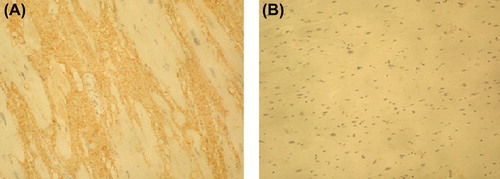
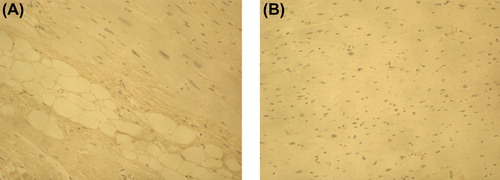
Increased accumulation of PINP was observed in the fibrotic tissue of the IMF cases in comparison with control samples in all cases when the 1:10,000 antibody dilution was used (). ICTP that reflects collagen type I degradation did not differ between the fibrosis cases and control myocardium (). Immunohistochemical staining of PIIINP did not show any differences between the victims of SCD and controls with 1:4000 antibody dilution. However, by using the antibody dilution of 1:2000, small amounts of PIIINP could be detected in two IMF cases with most evident fibrosis on macroscopic evaluation, including the lamin A/C mutation case (); in other IMF cases the staining remained negative. The amount of IIINTP did not differ between the SCD cases and control myocardium (). The amount of fibrosis was quantified with ImageTool measurement from the cases and controls stained with PINP; the quantity of fibrosis was also found to be significantly (P = 0.007) increased in the IMF cases (27.2% ± 6.5%) when compared with control samples (8.6% ± 2.2%) ().
Figure 2. Immunohistochemical staining of PINP in myocardium of idiopathic fibrosis case (A) and in control case (B). Magnification × 200. The amount of PINP (stained brown) was increased in the fibrotic tissue of the IMF case in comparison with control sample.
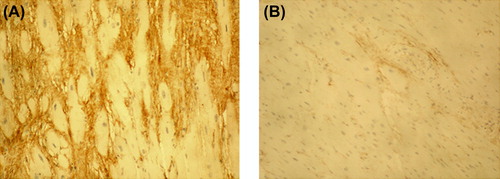
Figure 3. Immunohistochemical staining of ICTP in myocardium of idiopathic fibrosis case (A) and in control case (B). Magnification × 200. ICTP reflecting collagen type I degradation did not differ between the IMF case and control myocardium.
Discussion
The results of this study indicate that myocardial type I collagen synthesis is increased in victims of IMF-associated SCD. No significant difference was observed in the myocardium of IMF SCD cases and controls in the amount of IIINTP in the initial analysis. The PIIINP staining with the more concentrated antibody dilution showed small amounts of type III collagen in two IMF cases with most evident fibrosis, including the lamin A/C mutation case, which might be a clinically different entity than the other IMF cases. Detailed histology data from medico-legal autopsy of this R541C mutation carrier have been described before (Citation15). The same mutation or any other LMNA mutations was not observed in other cases of IMF, showing that the excess in the accumulation of collagen type I in the myocardium is not only related to this specific mutation but may result from various disorders. Thus, there seems to be excess accumulation of collagen type I in the myocardium of SCD victims with IMF, but there is some variation in the amount of collagen type III.
It is possible that lack of histological examination in many previous studies has underestimated myocardial fibrosis as a potential factor leading to SCD. The texture of fibrosis plays an essential role in propagation of the electrical impulse, besides the amount of it. Fibrosis may be interstitial, compact, patchy, or diffuse (Citation16). This intermingling of fibrosis and myocardium creates an arrhythmogenic substrate. In the present study, different textures of fibrosis were observed in various locations of the myocardium in different victims of SCD.
Initial reports have suggested that myocarditis may play a role in IMF (Citation17). However, in this study the SCD victims had no clinical history of prior myocarditis, and there was no histological evidence of it (diagnosed according to the presence of inflammatory infiltrates of the myocardium with the degeneration and/or necrosis of adjacent myocytes).
Other biomarkers for extracellular matrix (ECM) remodelling have been also studied in recent years. For example, circulating matrix metalloproteinase 3 (MMP-3), which degrades matrix proteins, has been suggested to be a marker of enhanced myocardial ECM turnover in HCM patients (Citation18). MMP-9 levels are increased in hypertension (Citation19) and also predict adverse cardiovascular disease events (Citation20,Citation21). Tissue inhibitors of metalloproteinases (TIMPs) are indicatives of collagen turnover, which inhibit MMP action in the matrix, and TIMP-1 levels have been presented to correlate positively with left ventricular hypertrophy in several studies (Citation22–24). Procollagen peptides have been intensively studied in recent years specifically in DCM and CHF. Patients with DCM (Citation11) and CHF (Citation10,Citation12) have higher serum PIIINP levels than do healthy controls. On the other hand, the levels of PINP have been described to be increased in patients with HCM. Additionally, elevated serum levels of PICP (carboxy-terminal propeptide of procollagen type I), also reflecting increased collagen type I synthesis, have been observed in sarcomere mutation carriers without obvious HCM (Citation25). Another previous study reported that the serologic test of type I collagen turnover (the PINP/ICTP ratio) was associated with resting diastolic dysfunction in HCM patients (Citation26). Additionally, no significant difference was observed in serum PINP levels between controls and CHF patients (Citation9), nor with hypertensive patients with or without diastolic CHF (Citation10). These results from previous studies are in concordance with the present study; the accumulation of collagen type I seems to be associated with arrhythmogenic heart diseases such as SCD associated to IMF and HCM. It is possible that collagen type I is specifically overexpressed in the myocardium of subjects with various gene mutations coding for structural proteins, such as lamin and sarcomeric proteins. An increased amount of collagen type III is more typically found in acquired heart diseases such as CHF due to various causes.
The direct correlation between biomarkers and histological myocardial collagen deposits has only been explored in a few studies (Citation11,Citation27). The serum concentrations of extracellular matrix proteins were higher in patients with DCM than in control subjects, and they reflected the concomitant increase in their myocardial tissue analogues to some extent. Nevertheless, there are some reservations that a non-invasive serologic test could correctly estimate the degree of myocardial fibrosis. Direct examination of cardiac tissues represents the most reliable method for the evaluation of collagen metabolism and the quantification of myocardial fibrosis. However, the feasibility of direct tissue examinations is limited. Therefore studies on the correlations of biomarker levels and direct or indirect evidence of increased myocardial fibrosis by histology or gadolinium late enhancement cardiac MRI are needed.
Study limitations
To our knowledge, this is the first study to clarify the collagen content of the myocardium in victims of IMF-associated SCD. Nevertheless, this study has some limitations. The number of victims of SCD with IMF is relatively small, limiting the generalizability of the results. However, a large sample size would be difficult to obtain within a reasonable time frame. Secondly, no other extensive genotyping was performed, e.g. for mutations of HCM gene, in addition to lamin A/C gene. The on-going exome sequencing of the DNAs of the IMF cases will probably yield additional information in this respect. Furthermore, a healed myocarditis as a cause of IMF cannot be completely excluded despite the lack of histologic signs of active inflammation. Also, the exact reasons for myocardial fibrosis are largely unknown, and the etiology of IMF is likely to be heterogeneous, as reflected in the pattern of fibrosis, ages, and degree of fibrosis. Thus, the diagnosis of IMF was made only if other causes of death (including non-cardiac) were excluded. Moreover, cases and controls in a 1:1 ratio are highly unlikely to be successfully matched given such a rare outcome as IMF. Finally, we have to take into account that causality of IMF to SCD cannot be inferred from a cross-sectional retrospective study. Despite these limitations we feel that the results of this study are suitable to arouse further research interest into this area, especially on studies of biological surrogate serum biomarkers of arrhythmogenic cardiac fibrosis, such as type I collagen.
Conclusion
The results of this study indicate increased myocardial type I collagen synthesis in the victims of IMF-associated SCD. In contrast, the amount of type III collagen was not as frequently altered in the fibrotic areas. Future studies on the role of circulating type I collagen biomarkers are needed to study further the implications of the described association.
Declaration of interest: The study was funded by grant from Finnish Foundation for Cardiovascular Research, Helsinki, Finland. The authors report no conflicts of interest.
References
- Hookana E, Junttila MJ, Puurunen VP, Tikkanen JT, Kaikkonen KS, Kortelainen ML, et al. Causes of nonischemic sudden cardiac death in the current era. Heart Rhythm. 2011;8:1570–5.
- Weber KT, Janicki JS, Shroff SG, Pick R, Chen RM, Bashey RI. Collagen remodeling of the pressure-overloaded, hypertrophied nonhuman primate myocardium. Circ Res. 1988;62:757–65.
- Medugorac I, Jacob R. Characterisation of left ventricular collagen in the rat. Cardiovasc Res. 1983;17:15–21.
- Eghbali M, Weber KT. Collagen and the myocardium: fibrillar structure, biosynthesis and degradation in relation to hypertrophy and its regression. Mol Cell Biochem. 1990;96:1–14.
- Risteli J, Risteli L. Analysing connective tissue metabolites in human serum. Biochemical, physiological and methodological aspects. J Hepatol. 1995;22:77–81.
- Bode M, Soini Y, Melkko J, Satta J, Risteli L, Risteli J. Increased amounts of type III pN-collagen in human abdominal aortic aneurysms: evidence for impaired type III collagen fibrillogenesis. J Vasc Surg. 2000;32:1201–7.
- Biolo A, Rohde LE, Goldraich LA, Mascarenhas M, Palombini DV, Clausell N. Serum procollagen type III is associated with elevated right-sided filling pressures in stable outpatients with congestive heart failure. Biomarkers. 2009;14:438–42.
- González A, López B, Querejeta R, Zubillaga E, Echeverría T, Díez J. Filling pressures and collagen metabolism in hypertensive patients with heart failure and normal ejection fraction. Hypertension. 2010; 55:1418–24.
- Alla F, Kearney-Schwartz A, Radauceanu A, Das Dores S, Dousset B, Zannad F. Early changes in serum markers of cardiac extra-cellular matrix turnover in patients with uncomplicated hypertension and type II diabetes. Eur J Heart Fail. 2006;8:147–53.
- Martos R, Baugh J, Ledwidge M, O’Loughlin C, Conlon C, Patle A, et al. Diastolic heart failure: evidence of increased myocardial collagen turnover linked to diastolic dysfunction. Circulation. 2007;115: 888–95.
- Klappacher G, Franzen P, Haab D, Mehrabi M, Binder M, Plesch K, et al. Measuring extracellular matrix turnover in the serum of patients with idiopathic or ischemic dilated cardiomyopathy and impact on diagnosis and prognosis. Am J Cardiol. 1995;75:913–18.
- Barasch E, Gottdiener JS, Aurigemma G, Kitzman DW, Han J, Kop WJ, et al. Association between elevated fibrosis markers and heart failure in the elderly: the cardiovascular health study. Circ Heart Fail. 2009;2:303–10.
- Kaikkonen KS, Kortelainen ML, Linna E, Huikuri HV. Family history and the risk of sudden cardiac death as a manifestation of an acute coronary event. Circulation. 2006;114:1462–7.
- Lahti RA. From findings to statistics: an assessment of Finnish medical cause-of-death information in relation to underlying-cause coding. Helsinki, Finland: Helsinki University Printing House; 2005.
- Hookana E, Junttila MJ, Särkioja T, Sormunen R, Niemelä M, Raatikainen MJ, et al. Cardiac arrest and left ventricular fibrosis in a Finnish family with the lamin A/C mutation. J Cardiovasc Electrophysiol. 2008;19:743–7.
- Kawara T, Derksen R, de Groot JR, Coronel R, Tasseron S, Linnenbank AC, et al. Activation delay after premature stimulation in chronically diseased human myocardium relates to the architecture of interstitial fibrosis. Circulation. 2001;104:3069–75.
- Lecomte D, Fornes P, Fouret P, Nicolas G. Isolated myocardial fibrosis as a cause of sudden cardiac death and its possible relation to myocarditis. J Forensic Sci. 1993;38:617–21.
- Zachariah JP, Colan SD, Lang P, Triedman JK, Alexander ME, Walsh EP, et al. Circulating matrix metalloproteinases in adolescents with hypertrophic cardiomyopathy and ventricular arrhythmia. Circ Heart Fail. 2012;5:462–6.
- Sundström J, Evans JC, Benjamin EJ, Levy D, Larson MG, Sawyer DB, et al. Relations of plasma matrix metalloproteinase-9 to clinical cardiovascular risk factors and echocardiographic left ventricular measures: the Framingham Heart Study. Circulation. 2004;109:2850–6.
- Blankenberg S, Rupprecht HJ, Poirier O, Bickel C, Smieja M, Hafner G, et al.; for the AtheroGene Investigators. Plasma concentrations and genetic variation of matrix metalloproteinase 9 and prognosis of patients with cardiovascular disease. Circulation. 2003;107:1579–85.
- Timms PM, Mannan N, Hitman GA, Noonan K, Mills PG, Syndercombe-Court D, et al. Circulating MMP9, vitamin D and variation in the TIMP-1 response with VDR genotype: mechanisms for inflammatory damage in chronic disorders?QJM. 2002;95:787–96.
- Sundström J, Evans JC, Benjamin EJ, Levy D, Larson MG, Sawyer DB, et al. Relations of plasma total TIMP-1 levels to cardiovascular risk factors and echocardiographic measures: the Framingham heart study. Eur Heart J. 2004;25:1509–16.
- Timms PM, Wright A, Maxwell P, Campbell S, Dawnay AB, Srikanthan V. Plasma tissue inhibitor of metalloproteinase-1 levels are elevated in essential hypertension and related to left ventricular hypertrophy. Am J Hypertens. 2002;15:269–72.
- Laviades C, Varo N, Fernández J, Mayor G, Gil MJ, Monreal I, et al. Abnormalities of the extracellular degradation of collagen type I in essential hypertension. Circulation. 1998;98:535–40.
- Ho CY, López B, Coelho-Filho OR, Lakdawala NK, Cirino AL, Jarolim P, et al. Myocardial fibrosis as an early manifestation of hypertrophic cardiomyopathy. N Engl J Med. 2010;363:552–63.
- Shim CY, Ha JW, Choi EY, Lee HJ, Moon SH, Kim JM, et al. Relationship between serum biochemical markers of myocardial fibrosis and diastolic function at rest and with exercise in hypertrophic cardiomyopathy. Korean Circ J. 2009;39:519–24.
- Polyakova V, Loeffler I, Hein S, Miyagawa S, Piotrowska I, Dammer S, et al. Fibrosis in endstage human heart failure: severe changes in collagen metabolism and MMP/TIMP profiles. Int J Cardiol. 2011;151: 18–33.