Abstract
Chronic hepatitis B virus (HBV) infection is common in HIV-positive individuals, mainly among those with sexually risky behaviors. Although HBV vaccination is mandatory in all HIV-infected persons with negative HBV markers, lower rates of protection due to abnormal immune responses are achieved. HIV accelerates the course of liver disease caused by chronic HBV infection, leading rapidly to end-stage hepatic illness and increasing the risk of hepatocellular carcinoma. Treatment of HIV including nucleos(t)ide analogues active against HBV highly improves outcomes, especially when tenofovir is part of the antiviral regimen. The use of lamivudine as the only active anti-HBV agent in HIV-HBV co-infected patients should be limited to individuals with low serum HBV-DNA levels. Otherwise, selection of drug resistance may eliminate any clinical benefit, produce cross-resistance to other antivirals, and favor the emergence of HBV vaccine escape mutants.
Key messages
Assessment of HBV status is warranted in all HIV-positive persons, and HBV vaccination should be given to all susceptible individuals.
Treatment of HIV including anti-HBV active agents should be given to all HIV-HBV co-infected individuals, regardless of CD4 counts. Although tenofovir is the drug of choice, lamivudine as the only active anti-HBV agent might be considered in certain scenarios.
Periodic assessment of liver fibrosis using non-invasive tools (i.e. elastometry) is warranted in all HIV-HBV co-infected patients. Likewise, periodic screening for hepatocellular carcinoma should be performed in cirrhotics and persons with elevated serum HBV-DNA.
Introduction
Nearly 2 billion people have been infected with the hepatitis B virus (HBV). Around 350 million remain chronically infected and depict positive HBV surface antigen (HBsAg) persistently in their blood (Citation1,Citation2). Progressive liver disease, including development of liver cancer, occurs lifelong in 20% to 30% of chronic HBsAg carriers in the absence of antiviral treatment (Citation2–4). As for human immunodeficiency virus (HIV), HBV is primarily transmitted by sexual, parenteral, and perinatal exposure, which explains why HIV-HBV co-infection is relatively common (Citation5–8). Current estimates of chronic hepatitis B among HIV-infected patients range from 5% to 15%. Thus, 3 million out of 35 million people living with HIV worldwide have chronic hepatitis B (Citation5,Citation9). In some regions of Sub-Saharan Africa and Southeast Asia, persistent HBsAg+ can be found in up to 15%–20% of the HIV population (Citation10), whereas in Western Europe fewer than 10% of HIV-infected individuals have chronic hepatitis B, although this rate is more than 100-fold higher than in the general population (Citation5). In the United States half of HIV-positive persons have been exposed to HBV and therefore exhibit markers of spontaneously self-limited HBV infection (HBV core antibodies [anti-HBc] with/without HBV surface antibodies [anti-HBs]) or have current serum HBsAg+ (Citation11).
The prevalence of HBsAg+ after HIV diagnosis has remained relatively unchanged since 2000, which means that recommendations for HBV vaccination in high-risk populations need to be refreshed and implemented. In developing countries, HBV is mainly transmitted in the perinatal period from HBsAg+ mothers, in early childhood from infected peers, or in early youth by heterosexual contact (Citation10). In Western countries HBV vaccination campaigns have halted HBV infection of newborns and infants, meaning that most infections currently seen are among immigrants or adults with high-risk practices, such as men who have sex with men (MSM) (Citation2).
The distribution of HBV genotypes, 10 in total and named with letters from A to J, differs in distinct geographical regions (Citation1). HBV genotype A predominates in Northern Europe and North America, whereas genotypes B and C are common in Asia, and genotype D is mostly found around the Mediterranean basin and in Eastern Europe. The HBV variant may influence the course of chronic hepatitis B, HBV genotypes C and D being associated with faster progression to cirrhosis and liver cancer, whereas HBV genotypes A and B may be more susceptible to interferon therapy (Citation12). In European HIV-positive patients with chronic hepatitis B, genotype A is overall the most prevalent, although HBV genotype D predominates among injecting drug users (IDUs) in Southern Europe (Citation13).
Diagnosis
All HIV-infected persons must be tested for HBV markers of current (HBsAg+) or past infection (anti-HBc+ and anti-HBs+). In individuals lacking serological markers, HBV vaccination should be recommended (Citation6). In the absence of HBV immunity, testing must be refreshed in subjects with unexplained liver enzymes elevations, visits to or birth in endemic areas, household contacts with HBsAg+ persons, IDUs, those with multiple sexual contacts, history of sexually transmitted diseases, MSM, prison inmates, pregnant women, and chronic hepatitis C individuals (Citation3,Citation8).
As fulminant viral hepatitis is more common in patients with underlying chronic liver disease, hepatitis A virus (HAV) vaccination is recommended in all patients with chronic hepatitis B and negative HAV-Ab (Citation4). Given overlapping transmission routes, the presence of concomitant HCV and/or hepatitis delta virus (HDV) infection should also be excluded in all HIV-HBV co-infected patients (Citation3,Citation4,Citation8).
In all patients with chronic hepatitis B, alanine aminotransferase (ALT) values, HBV genotype, HBV e antigen (HBeAg), and serum HBV-DNA levels need to be assessed at baseline in order to consider therapeutic decisions and select the best treatment option. If treatment is started with any antiviral, liver enzymes, HBeAg, HBsAg, and HBV-DNA should be monitored every 3 to 12 months. The extent of liver fibrosis should be checked at baseline and ideally every 6 months using non-invasive tests, such as transient elastometry, which is currently one of the most reliable and feasible tools to discriminate between advanced and minimal/absent liver fibrosis (Citation8,Citation14). gives a list of parameters that must be recorded at baseline and thereafter periodically in HIV-infected patients with chronic hepatitis B.
Table I. Main parameters to be assessed in HIV-infected patients with chronic hepatitis B.
In patients with advanced liver fibrosis, periodic screening of liver cancer, including serum alpha-fetoprotein and ultrasonography every 6 months, is warranted. HIV individuals could be at greater risk for hepatocellular carcinoma (HCC) even in the absence of cirrhosis. This consideration particularly applies to African patients over 20 years of age, Asians over 40 years, persons with a family history of HCC, and subjects with high HBV-DNA levels (> 2 million IU/mL). Screening for esophageal varices should be performed periodically in cirrhotics, and prophylaxis should be considered with propranolol or other procedures accordingly (Citation4).
Clinical outcome
More than 95% of adults exposed to HBV for the first time attain HBsAg seroconversion within the first 6 months and develop anti-HBc with/without anti-HBs. These subjects have resolved but not cured HBV infection, as integrated, episomal HBV-DNA remains within the hepatocytes and may reactivate if severe immune suppression occurs for any reason (i.e. administration of chemotherapy) and/or as result of selection of envelope HBV escape mutants (Citation15–17).
Patients with detectable HBsAg in serum for longer than 6 months are considered as having chronic hepatitis B. During the first years, HBeAg is generally positive, which is associated with elevated serum HBV-DNA levels (> 20,000 IU/mL). HBeAg seroconversion occurs at a rate of 8% to 12% per year, followed by normalization in liver enzymes, and decline or even suppression in HBV-DNA levels (inactive HBsAg carrier state) (Citation18). This event occurs more frequently with older age, high ALT and in HBV genotype B. However, in 10% to 30% of HBeAg-negative patients ALT is elevated and serum HBV-DNA levels are above 2000 IU/mL. These individuals usually harbor and replicate HBV core or pre-core mutants (Citation19,Citation20). Spontaneous HBsAg clearance is a rare event and occurs at a rate of 0.5% per year in chronic hepatitis B (resolved hepatitis B). It is generally preceded by low/undetectable HBV-DNA and followed by development of anti-HBs (Citation18).
The natural history of chronic hepatitis B is often more complex than described above. Patients can exhibit a wide range of hepatic damage with diverse degrees of inflammation and fibrosis (Citation21). Final events are cirrhosis, end-stage liver disease (ESLD), and HCC. Factors associated with fatal outcomes in chronic hepatitis B are male gender, black or Asian race, family history of liver cancer, high HBV-DNA levels, and HBeAg+ (Citation22,Citation23). As compared to HBV-monoinfected individuals, HIV-HBV co-infected patients have lower chances for spontaneous HBeAg and HBsAg clearance, and serum HBV-DNA levels are more elevated, which may contribute to a faster progression to ESLD and HCC, characteristically seen in HIV-HBV co-infected patients (Citation6,Citation7,Citation23–27).
Following the advent and broader use of highly active antiretroviral therapy (HAART), opportunistic complications have declined dramatically in HIV patients. However, liver-related complications are on the rise in subjects co-infected with hepatitis viruses, mainly B and C (Citation28–30). Treatment of both HIV and HBV may prevent or slow down the development of hepatic complications in co-infected patients (Citation30,Citation31). Accordingly, most HIV treatment guidelines recommend earlier HIV and HBV therapy in HIV-HBV co-infection regardless of CD4 counts (Citation8,Citation32,Citation33). This goal can be easily accomplished using oral drugs with dual antiviral activity (i.e. lamivudine [LAM], emtricitabine [FTC], or tenofovir [TDF]) (Citation34). The enhanced risk of liver toxicity of antiviral agents, particularly among cirrhotic HIV-HBV co-infected patients (Citation35–37), should not preclude prescription of HIV plus HBV therapy, although antivirals with the safest liver profile should be preferred (Citation6). A strong warning should be issued against stopping HAART with anti-HBV drugs for any reason, since abrupt resumption of HBV replication may lead to serious liver enzyme flares and even fulminant hepatic failure (Citation6).
Treatment
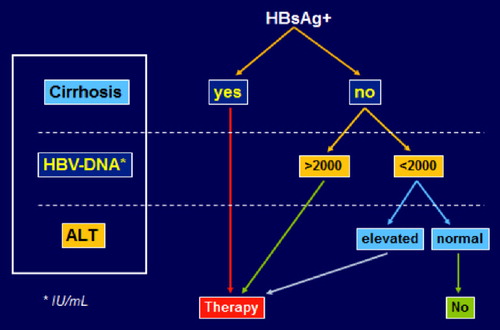
In HIV patients with chronic hepatitis B, dual antiviral therapy is indicated without controversy in all individuals with cirrhosis, serum HBV-DNA > 2000 IU/mL, and/or elevated liver enzymes () (Citation6,Citation8). Most experts even recommend dual therapy irrespectively of CD4 counts, given the accelerated progression of liver disease in HIV patients. The best option would be triple combination of antiretrovirals, including two reverse transcriptase inhibitors with anti-HBV activity, namely TDF plus LAM or FTC (Citation6,Citation8,Citation32). The co-formulation of TDF/FTC (Truvada®) makes this option the preferred one. The third drug can be any antiretroviral agent with a safe liver profile.
Figure 1. Treatment indication for chronic hepatitis B in HIV-infected individuals. Note: In patients with significant liver fibrosis (Metavir F2-F3), HBV treatment may be considered even when serum HBV-DNA is < 2000 IU/mL and liver enzymes are not elevated.
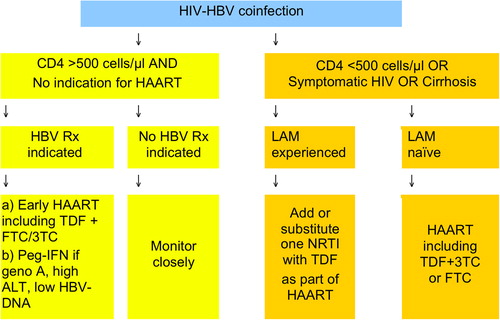
Only in very specific situations may peginterferon alpha be considered as therapy for chronic hepatitis B in HIV-co- infected patients. This is the case for subjects with normal CD4 counts unwilling to start HAART, or those with HBeAg+, low HBV-DNA, and elevated ALT (). Interferon is contraindicated in patients with decompensated liver disease (Citation4).
Figure 2. Treatment options for chronic hepatitis B in HIV-positive individuals (adapted from European AIDS Clinical Society Guidelines (Citation8)).
Other treatment options, such as adefovir (ADV) or telbivudine (LdT) therapy, do not fit in the HIV setting due to their lack and/or residual activity against HIV along with a relatively weak activity against HBV. Treatment with entecavir (ETV) may be considered when TDF cannot be used, generally in patients with kidney disease or due to toxicity. As ETV displays weak activity against HIV and may select for resistance mutations, it should be administered only in the context of a fully suppressive HIV treatment (Citation38).
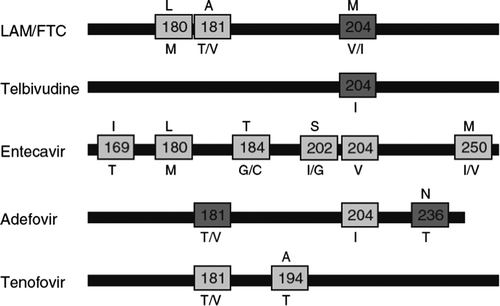
Oral anti-HBV drugs may select changes at the HBV polymerase leading to loss of susceptibility to the corresponding drug and cross-resistance to other antivirals. As compared with HIV or HCV, HBV selects drug-resistant mutants more slowly, which largely depends on the longer half-life of infected hepatocytes carrying viral cccDNA (Citation39). Among drugs used to treat hepatitis B, LAM exhibits the lowest resistance barrier, with emergence of drug-resistant mutants in 25% and 65% of patients treated for 1 and 5 years, respectively (Citation4). LdT selects drug-resistant mutant viruses at a rate of 20% within 2 years. This rate is around 29% for ADV at 5 years. The greatest barrier to resistance is exhibited by ETV and especially by TDF. ETV resistance being very rare in naïve individuals (< 2% at 5 years), it may develop in nearly half of patients with prior LAM failure after 5 years of ETV treatment (Citation40).
The change M204I/V is usually responsible for LAM, FTC, and LdT resistance, while more changes (L180M+ M204V+ T250) are generally needed to produce ETV resistance. Cross-resistance is almost universal between LAM, FTC, and LdT, and less so ETV. There is some cross-resistance to ADV in the presence of A181S+ M204I in patients that have failed LAM therapy (Citation41,Citation42). No mutations have been uniformly associated with significant loss of susceptibility to TDF in vivo, although anecdotal reports have pointed out that A194T in the context of LAM resistance mutations might produce TDF resistance in HBV (Citation43). records the main changes in HBV polymerase associated with drug resistance.
Resistance to LAM in HBV is more frequent and develops faster in HIV-HBV co-infected patients than in HBV- monoinfected individuals, largely due to higher serum HBV- DNA levels in the former (Citation44–46). Selection of LAM resistance in chronic hepatitis B is associated with poor clinical outcomes, including liver enzyme flares that occasionally can be life- threatening (Citation47,Citation48), and precluding the success of rescue antiviral interventions due to cross-resistance with other antivirals.
Due to overlapping polymerase and envelope genes in the HBV genome, LAM resistance mutations may result in HBsAg changes, causing diminished HBs antigen–antibody binding. This may translate into failure of diagnostic tests, vaccine escape, or both (Citation49–51). Finally, and to a lesser extent than in HIV, transmission of drug-resistant HBV strains has already been reported (Citation52–54). It seems restricted to LAM, although mutations conferring resistance to other anti-HBV agents have already been reported (Citation55,Citation56). Transmission of LAM-resistant HBV strains has been reported in up to 5%–8% of newly diagnosed HBV patients in some studies (Citation57,Citation58). This observation constitutes a further argument against the use of LAM as the only active anti-HBV drug in HIV-HBV co-infected patients (Citation45,Citation46).
Rethinking therapeutic strategies for HBV in response to economic constraints is always challenging, as patient safety has to be preserved (Citation59). The cost of LAM (US$1–2/day), now available as a generic drug, is much cheaper than for TDF (US$10–12/day) or ETV (US$15/day). Besides, while LAM is usually very well tolerated (Citation60), TDF may be associated with kidney tubular abnormalities (Citation61,Citation62), which infrequently may lead to Fanconi syndrome (Citation63) or even more rarely to overt renal insufficiency (Citation64). In HIV patients on prolonged TDF therapy, cases of bone demineralization with increased risk of osteopenia and bone fractures have been described (Citation65).
Besides the unaffordable cost of medications, another important limitation for the judicious use of antiviral drugs in resource-limited settings is the high cost of HBV-DNA testing and of tools for liver fibrosis assessment, including liver biopsy. In this scenario HBeAg negativity could be a good surrogate marker for low HBV-DNA levels, and here LAM as the only active anti-HBV drug could be a good first-line option () (Citation59,Citation66,Citation67). If HBV-DNA testing is available, LAM could be initially prescribed as far as HBV-DNA suppression is potent enough to prevent selection of drug-resistant mutants. Patients with HBV-DNA levels below 2000 IU/mL at week 24 on LAM therapy may be expected to attain complete viral suppression under this regimen within the following 24 months. If not, adding TDF at that time may be necessary.
Figure 4. Strategies for safer lamivudine use in the treatment of chronic hepatitis B in resource-limited settings (adapted from Soriano and McMahon (Citation59)). (A) add-on strategy; and (B) switch on strategy.
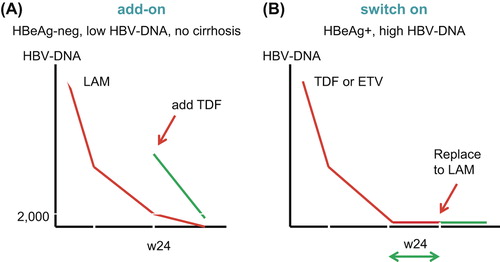
In patients with high HBV-DNA (i.e. > 106 IU/mL), or with positive HBeAg, a more potent regimen containing TDF should initially be used (Citation59,Citation68). Thereafter, when undetectable HBV-DNA has been achieved, simplification with a cheaper regimen with LAM could be enough to maintain viral suppression (). Even so, LAM would never be a good option in cirrhotic patients, as eventual virological failures may produce potentially life-threatening liver decompensation (Citation68). As previously mentioned, primary LAM resistance, as found in persons who acquired HBV mutants at first infection, seems to be very rare (Citation52–56). Only if this phenomenon gains frequency in certain areas should LAM use in the scenarios described above be discouraged.
HBV vaccination
Universal infant HBV vaccination was recommended by the World Health Organization in 1992 (Citation69). All HIV-infected adults without protective HBV surface antibody (HBsAb) titers should be vaccinated. The response rate and durability of the vaccine protection is poorer in HIV-positive compared to HIV-negative persons (Citation70,Citation71) and is influenced by both CD4 counts and plasma HIV-RNA (Citation72). Accordingly, in patients with low CD4 counts (< 200 cells/μL) and uncontrolled HIV replication, the success of HBV immunization is low. In these individuals, prior antiretroviral therapy for at least 6 months may increase HBV vaccine response rates.
Although most HIV guidelines still recommend a conventional HBV vaccination schedule, the lack of achievement of protective anti-HBs titers (> 10 mIU/mL) frequently warrant re-vaccination using double doses and/or additional recall injections (months 0, 1, 6, and 12) (Citation72,Citation73). Of note, some protection from HBV vaccine exists even when anti-HBs titers drop below 10 mIU/mL (Citation74).
Isolated anti-HBc are commonly seen in HIV-infected individuals. It may reflect three circumstances, as follows: 1) false-positive reactions, 2) clearance of HBsAg with inability to mount an adequate anti-HBs response or to maintain it over time, and 3) interference with HCV, with low HBsAg titers and low-level HBV-DNA (Citation72). At this time it remains uncertain whether HIV-infected persons with isolated anti-HBc should be vaccinated against HBV. Testing must be repeated first. A single vaccine recall injection may distinguish the three possibilities previously mentioned (false positivity, prior immunity with undetectable anti-HBs, low-level HBV infection). If anti-HBs become positive at 1 month with high titers, an anamnestic response should be suspected, and no further vaccine injections are necessary. On the other hand, if anti-HBs remain negative after the single HBV vaccine dose, serum HBV-DNA should be tested using a sensitive technique. If low-level HBV-DNA is found, the patient should be considered as HBV-infected and therefore does not need any HBV vaccine prophylaxis. In contrast, a negative serum HBV-DNA along with undetectable anti-HBs would suggest that the patient is not infected by HBV nor has been previously exposed; and the three-shot vaccine series should be completed (Citation72).
Hepatitis delta
Hepatitis D virus (HDV) infection only occurs in subjects with hepatitis B, as HDV requires HBsAg to complete its replication cycle at the hepatocyte. Approximately 5% of patients with chronic hepatitis B worldwide have HDV co-infection, with large geographical disparities, and peaking to 8% or above in some areas of the Mediterranean basin, Eastern Europe, and Latin America (Citation75). The prevalence of hepatitis delta among HIV-HBV co-infected patients is around 15% in Western Europe (Citation76). It is steadily going down as the population of IDUs has declined dramatically and HBV vaccination is broadly ensured in the HIV population (Citation77).
HDV co-infection causes chronic hepatitis B to progress faster to ESLD and HCC (Citation78,Citation79). The only approved treatment for HDV infection is peginterferon, although fewer than 20% of patients attain and sustain HDV-RNA suppression (Citation80,Citation81). Improvement in liver histology has been noticed in patients that achieve sustained viral response after HDV therapy with interferon (Citation82). The addition of ribavirin, LAM, or ADV to peginterferon has not provided any further benefit to interferon in terms of response rate (Citation83–85). Ongoing studies are exploring the potential benefit of adding the newest more potent oral anti-HBV drugs such as TDF or ETV. Information on TDF is particularly appealing since prior reports have already suggested some efficacy of the drug in HIV patients with delta hepatitis, particularly in those infected with HBV genotype A (Citation26,Citation86,Citation87). However, a recent study has not shown any benefit of TDF plus peginterferon over peginterferon alone in HDV-monoinfected patients (Citation88).
Doses and length of peginterferon therapy in hepatitis delta are not well established, but the drug should not be given for less than 1 year, considering extension based on initial biochemical/ virological responses and tolerance. In patients experiencing a significant serum HDV-RNA drop after 6–12 months of treatment, peginterferon should be maintained until the achievement of complete HDV-RNA suppression. Since HDV relapses are common upon premature drug discontinuation, when possible treatment should be extended until HBsAg loss is attained (Citation89). This circumstance, however, is rarely achieved. In patients with hepatitis delta and ESLD or HCC, liver transplantation should be strongly considered, especially in the absence of active HCV co-infection, as liver transplant may cure HBV and HDV infections (Citation90).
Funding
This work was supported in part by grants from Fundación Investigación y Educación en SIDA (FIES); European Community's Seventh Framework Programs NEAT (European AIDS Treatment Network; LSHM-CT-2006-037570) and CHAIN (Collaborative HIV and Anti-HIV Drug Resistance Network; FP7/2007-2013-223131); Red de Investigación en SIDA (ISCIII-RETIC-RD12/0017/0031).
Declaration of interest: The authors report no conflicts of interest.
References
- Te H, Jensen D. Epidemiology of hepatitis B and C viruses: a global overview. Clin Liver Dis. 2010;14:1–21.
- Lavanchy D. Hepatitis B virus epidemiology, disease burden, treatment, and current and emerging prevention and control measures. J Viral Hepat. 2004;11:97–107.
- Lok A, McMahon B. Chronic hepatitis B: update2009. Hepatology.2009;50:661–96.
- European Association For The Study Of The Liver. EASL Clinical Practice Guidelines: Management of chronic hepatitis B. J Hepatol. 2012;57:167–85.
- Konopnicki D, Mocroft A, de Wit S, Antunes F, Ledergerber B, Katlama C, et al. Hepatitis B and HIV: prevalence, AIDS progression, response to HAART and increased mortality in the EuroSIDA cohort. AIDS. 2005;19:2117–25.
- Soriano V, Puoti M, Peters M, Benhamou Y, Sulkowski M, Zoulim F, et al. Care of HIV patients with chronic hepatitis B: updated recommendations from the HIV-Hepatitis B virus international panel. AIDS. 2008;22:1399–410.
- Thio C. Hepatitis B and Human immunodeficiency virus coinfection. Hepatology. 2009;49(Suppl):138–45.
- European AIDS Clinical Society Guidelines. Version 7.0. October 2013. Available at: http://www.europeanaidsclinicalsociety.org (accessed 6 March 2014).
- Kellerman S, Hanson D, McNaghten A, Fleming P. Prevalence of chronic hepatitis B and incidence of acute hepatitis B infection in HIV-infected participants. J Infect Dis. 2003;188:571–7.
- Modi A, Feld J. Viral hepatitis and HIV in Africa. AIDS Rev. 2007;9: 25–39.
- Chun H, Fieberg A, Hullsied K, Lifson A, Crum-Cianflone NF, Weintrob A, et al. Epidemiology of hepatitis B virus infection in a US cohort of HIV-infected individuals during the past 20 years. Clin Infect Dis. 2010;50:426–36.
- Wong V, Sung J. Diagnosis and personalized management of hepatitis B including significance of genotypes. Curr Opin Infect Dis. 2012; 25:570–7.
- Soriano V, Mocroft A, Peters L, Rockstroh J, Antunes F, Kirkby N, et al. Predictors of hepatitis B virus genotype and viraemia in HIV-infected patients with chronic hepatitis B in Europe. J Antimicrob Chemother. 2010;65:548–55.
- Soriano V, Sheldon J, Ramos B, Núñez M. Confronting chronic hepatitis B virus infection in HIV: new diagnostic tools and more weapons. AIDS. 2006;20:451–3.
- Yeo W, Johnson P. Diagnosis, prevention and management of hepatitis B virus reactivation during anticancer therapy. Hepatology. 2006;43: 209–20.
- Milazzo L, Ebranati E, Cattaneo D, Gabanelli E, Lai A, Zehender G, et al. Recurrence of another hepatitis B virus escape mutant comes back in a patient infected with HIV and low CD4 + count. J Med Virol. 2014;86:97–101.
- Pei R, Grund S, Verheyen J, Esser S, Chen X, Lu M. Spontaneous reactivation of hepatitis B virus replication in an HIV coinfected patient with isolated anti-Hepatitis B core antibodies. Virol J. 2014; 11:9.
- McMahon B, Holck P, Bulkow L, Snowball M. Serologic and clinical outcomes of 1536 Alaska natives chronically infected with hepatitis B virus. Ann Intern Med. 2001;135:759–68.
- Chan H, Leung N, Hussain M, Wong M, Lok A. Hepatitis B e antigen-negative chronic hepatitis B in Hong Kong. Hepatology. 2000;31: 763–8.
- Grandjacques C, Pradat P, Stuyver L, Chevallier M, Chevallier P, Pichoud C, et al. Rapid detection of genotypes and mutations in the pre-core promoter and the pre-core region of hepatitis B virus genome: correlation with viral persistence and disease severity. J Hepatol. 2000;33:430–9.
- McMahon B. The natural history of chronic hepatitis B virus infection. Hepatology. 2009;49(Suppl):45–55.
- Iloeje U, Yang H, Su J, Jen C, You S, Chen C, et al. Predicting cirrhosis risk based on the level of circulating hepatitis B virus viral load. Gastroenterology. 2006; 130:678–86.
- Chen C, Yang H, Su J, Jen C, You S, Lu S, et al. Risk of hepatocellular carcinoma across biological gradient of serum hepatitis B virus DNA level. JAMA. 2006;295:65–73.
- Puoti C, Torti C, Bruno R, Filice G, Carosi G. Natural history of chronic hepatitis B in co-infected patients. J Hepatol. 2006;44:S65–70.
- Walter S, Thein H, Amin J, Gidding HF, Ward K, Law M, et al. Trends in mortality after diagnosis of hepatitis B or C infection: 1992–2006. J Hepatol.2011;54:879–86.
- Martín-Carbonero L, Teixeira T, Poveda E, Plaza Z, Vispo E, Gonzalez-Lahoz J, et al. Clinical and virological outcomes in HIV-infected patients with chronic hepatitis B on long-term nucleos(t)ide analogues. AIDS. 2011;25:73–9.
- Clifford G, Rickenbach M, Polesel J, Dal Maso L, Steffen I, Ledergerber B, et al. Influence of HIV-related immunodeficiency on the risk of hepatocellular carcinoma. AIDS. 2008;22:2135–41.
- Thio C, Seaberg E, Skolasky R, Phair J, Visscher B, Muñoz A, et al. HIV-1, hepatitis B virus, and risk of liver-related mortality in the Multicenter Cohort Study (MACS). Lancet. 2002;360:1921–6.
- Weber R, Sabin C, Friis-Moller N, Reiss P, El-Sadr W, Kirk O, et al. Liver related deaths in persons with the HIV: the D:A:D study. Arch Intern Med. 2006;166:1632–41.
- Nikolopoulos G, Paraskevis D, Hatzitheodorou E, Moschidis Z, Sypsa V, Zavitsanos X, et al. Impact of hepatitis B virus infection on the progression of AIDS and mortality in HIV-infected individuals: a cohort study and meta-analysis. Clin Infect Dis. 2009;48:1763–71.
- Hoffmann C, Seaberg E, Young S, Witt M, D’Acunto K, Phair J, et al. Hepatitis B and long-term HIV outcomes in coinfected HAART recipients. AIDS. 2009;23:1881–9.
- DHHS. Recommendations for use of antiretroviral drugs in HIV-1-infected adults and adolescents. Available at: http://aidsinfo.nih.gov/contentfiles/lvguidelines/.pdf.
- Thompson M, Aberg J, Hoy J, Telenti A, Benson C, Cahn P, et al. Antiretroviral treatment of adult HIV infection: 2012 recommendations of the International Antiviral Society-USA panel. JAMA. 2012;308: 387–402.
- Soriano V, Tuma P, Vispo E, Labarga P, Fernández JV, Medrano J, et al. Hepatitis B in HIV patients: what is the current treatment and what are the challenges?J HIV Ther. 2009;14:13–18.
- Sulkowski M, Thomas D, Chaisson R, More R.Hepatotoxicity associated with antiretroviral therapy in adults infected with HIV and the role of hepatitis C or B virus infection. JAMA. 2000;283:74–80.
- Aceti A, Pasquazzi C, Zechini B, De Bac C; LIVERHAART Group. Hepatotoxicity development during antiretroviral therapy containing protease inhibitors in patients with HIV: the role of hepatitis B and C virus infection. J Acquir Immune Defic Syndr. 2002;29:41–8.
- Soriano V, Puoti M, Garcia-Gascó P, Rockstroh J, Benhamou Y, Barreiro P, et al. Antiretroviral drugs and liver injury. AIDS. 2008; 22:1–13.
- McMahon B, Jilek B, Brennan T, Shen L, Zhou Y, Wind-Rotolo M, et al. The HBV drug entecavir - effects on HIV-1 replication and resistance. N Engl J Med. 2007;356:2614–21.
- Soriano V, Perelson A, Zoulim F. Why are there different dynamics in the selection of drug resistance in HIV and hepatitis B and C viruses?J Antimicrob Chemother. 2008;62:1–4.
- Known H, Lock M. Hepatitis B therapy. Gastroenterol Hepatol. 2011;8:275–84.
- Karatayli E, Karayalcin S, Karaaslan H, Kayhan H, Turkyilmaz A, Sahin F, et al. A novel mutation pattern emerging during lamivudine treatment shows cross-resistance to adefovir dipivoxil treatment. Antivir Ther. 2007;12:761–8.
- Tenney D. Genotypic determinants and phenotypic properties of antiviral-resistant HBV variants: insight from entecavir resistance studies. Antivir Ther. 2010;15:529–35.
- Sheldon J, Camino N, Rodes B, Bartholomeusz A, Kuiper M, Tacke F, et al. Selection of hepatitis B virus polymerase mutations in HIV coinfected patients treated with tenofovir. Antivir Ther. 2005;10: 727–34.
- Benhamou Y, Bochet M, Thibault V, Di Martino V, Caumes E, Bricaire F, et al. Long-term incidence of hepatitis B virus resistance to lamivudine in HIV-infected patients. Hepatology. 1999;30:1302–6.
- Hoffmann C, Charalambous S, Martin D, Innes C, Churchyard G, Chaisson R, et al. Hepatitis B virus infection and response to antiretroviral therapy (ART) in a South African ART program. Clin Infect Dis. 2008;47:1479–85.
- Soriano V, Rivas P, Nuñez M. Risks and benefits of using antiretroviral therapy in HIV-infected patients with chronic hepatitis B in developing regions. Clin Infect Dis. 2008;47:1486–9.
- Matthews G, Bartholomeusz A, Locarnini S. Characteristics of drug resistant HBV in an international collaborative study of HIV-HBV-infected individuals on extended lamivudine therapy. AIDS. 2006;20: 863–70.
- Gouskos T, Wightman F, Chang J, Earnest-Silveira L, Sasadeusz J, Lewin S, et al. Severe hepatitis and prolonged hepatitis B virus-specific CD8 T-cell response after selection of hepatitis B virus YMDD variant in an HIV/hepatitis B virus co-infected patient. AIDS. 2004;18:1734–7.
- Hsu C, Yeh C, Chang M, Liaw Y. Identification of a hepatitis B virus S gene mutant in lamivudine-treated patients experiencing HBsAg seroclearance. Gastroenterology. 2007;132:543–50.
- Sheldon J, Soriano V. Hepatitis B virus escape mutants induced by antiviral therapy. J Antimicrob Agents. 2008;61:766–8.
- Martín-Carbonero L, Soriano V. New paradigms for treating hepatitis B in HIV/HBV co-infected patients. J Antimicrob Chemother. 2010; 65:379–82.
- Thibault V, Aubron-Olivier C, Agut H, Katlama C. Primary infection with a lamivudine-resistant hepatitis B virus. AIDS. 2002;16:131–3.
- Tuma P, Pineda J, Labarga P, Vidal F, Rodriguez C, Poveda E, et al. HBV primary drug resistance in newly diagnosed HIV-HBV-coinfected individuals in Spain. Antivir Ther. 2011;16:585–89.
- Fujisaki S, Yokomaku Y, Shiino T, Koibuchi T, Hattori J, Ibe S, et al. Outbreak of infections by hepatitis B virus genotype A and transmission of genetic drug resistance in patients coinfected with HIV-1 in Japan. J Clin Microbiol. 2011; 49:1017–24.
- Vutien P, Trinh H, García R, Nguyen HA, Levitt BS, Nguyen K, et al. Mutations in HBV DNA polymerase associated with nucleos(t)ide resistance are rare in treatment-naive patients. Clin Gastroenterol Hepatol. 2014; 12:1363–70.
- Baxa D, Thekdi A, Golembieski A, Nguyen HA, Levitt BS, Nguyen K, et al. Evaluation of anti-HBV drug resistant mutations among patients with acute symptomatic hepatitis B in the United States. J Hepatol. 2013;58:212–16.
- Xu Z, Liu Y, Xu T, Chen L, Si L, Wang Y, et al. Acute hepatitis B infection associated with drug resistant hepatitis B virus. J Clin Virol. 2010; 48:270–4.
- Fung S, Mazulli T, El-Kashab M; and Canadian Association for the Study of the Liver. Lamivudine-resistant mutation among treatment-naïve hepatitis B patients is common and may be associated with treatment failure. American Association for the Study of Liver Diseases. 31 Oct–4 Nov 2008; San Francisco, CA, USA [Abstract 888].
- Soriano V, McMahon B. Rethinking therapeutic strategies for hepatitis B. Antivir Res. 2013;100:435–8.
- Lok A, Lai C, Leung N, Yao G, Cui Z, Schiff E, et al. Long-term safety of lamivudine treatment in patients with chronic hepatitis B. Gastroenterology. 2003;125:1714–22.
- Labarga P, Barreiro P, Martin-Carbonero L, Rodriguez-Novoa S, Medrano J, Rivas P, et al. Kidney tubular abnormalities in the absence of impaired glomerular function in HIV patients treated with tenofovir. AIDS. 2009;23:689–96.
- Del Palacio M, Romero S, Casado JL. Proximal tubular renal dysfunction or damage in HIV-infected patients. AIDS Rev. 2012;14:179–87.
- Rifkin B, Perazella M. Tenofovir-associated nephrotoxicity: Fanconi syndrome and renal failure. Am J Med. 2004;117:282–4.
- Karras A, Lafaurie M, Furco A, Bourgarit A, Droz D, Sereni D, et al. Tenofovir-related nephrotoxicity in HIV-infected patients: three cases of renal failure, Fanconi syndrome, and nephrogenic diabetes insipidus. Clin Infect Dis. 2003;36:1070–3.
- Bedimo R, Maalouf N, Zhang S, Drechsler H, Tebas P. Osteoporotic fracture risk associated with cumulative exposure to tenofovir and other antiretroviral agents. AIDS. 2012;26:825–31.
- Chen L, Zhang Q, Yu DM, Wan M, Zhang X.Early changes of HBV quasispecies during lamivudine treatment and the correlation with antiviral efficacy. J Hepatol. 2009;50:895–905.
- Chang M, Chien R, Yeh C, Liaw Y. Virus and transaminase levels determine the emergence of drug resistance during long-term lamivudine therapy in chronic hepatitis B. J Hepatol. 2005;43:72–7.
- Koklu S, Tuna Y, Gulsen M, Demir M, Köksal AŞ, Koçkar MC, et al. Long-term efficacy and safety of lamivudine, entecavir, and tenofovir for treatment of hepatitis B virus-related cirrhosis. Clin Gastroenterol Hepatol. 2013;11:88–94.
- Kane M. Global programme for control of hepatitis B infection. Vaccine. 1995;13(Suppl):47–9.
- Schirmera P, Wintersb M, Holodniy M. HIV-HBV vaccine escape mutant infection with loss of HBV surface antibody and persistent HBV viremia on tenofovir/emtricitabine without antiviral resistance. J Clin Virol. 2011;52:261–4.
- Gandhi R, Wurcel A, Lee H, McGovern B, Shopis J, Geary M, et al. Response to hepatitis B vaccine in HIV-1-positive subjects who test positive for isolated antibody to hepatitis B core antigen: implications for hepatitis B vaccine strategies. J Infect Dis. 2005;191:1435–41.
- Rivas P, Herrero MD, Puente S, Ramírez-Olivencia G, Soriano V. Immunizations in HIV-infected adults. AIDS Rev. 2007;9:173–87.
- Launay O, van der Vliet D, Rosenberg AR, Michel ML, Piroth L, Rey D, et al. Safety and immunogenicity of 4 intramuscular double doses and 4 intradermal low doses vs standard hepatitis B vaccine regimen in adults with HIV-1: a randomized controlled trial. JAMA. 2011;305:1432–40.
- World Health Organization. Hepatitis B vaccines: WHO position paper. Wkly Epidemiol Rec. 2009;84:405–19.
- Wedemeyer H, Heidrich B, Manns M. Hepatitis D virus infection – not a vanishing disease in Europe! Hepatology. 2007;45:1331–2.
- Soriano V, Grint D, d’Arminio-Monforte A, Horban A, Leen C, Poveda E, et al. Hepatitis delta in HIV-infected individuals in Europe. AIDS. 2011;25:1987–92.
- Calle-Serrano B, Manns MP, Wedemeyer H. Hepatitis delta and HIV infection. Semin Liver Dis. 2012;32:120–9.
- Kew M. Hepatitis viruses (other than hepatitis B and C viruses) as causes of hepatocellular carcinoma: an update. J Viral Hepat. 2013; 20:149–57.
- Farci P, Niro G. Clinical features of hepatitis D. Semin Liver Dis. 2012;32:228–36.
- Erhardt A, Gerlich W, Starke C, Wend U, Donner A, Sagir A, et al. Treatment of chronic hepatitis D with pegylated interferon-a 2b. Liver Int. 2006;26:805–10.
- Yurdaydin C. Treatment of chronic delta hepatitis. Semin Liver Dis. 2012;32:237–44.
- Farci P, Roskams T, Chessa L, Peddis G, Mazzoleni A, Scioscia R, et al. Long-term benefit of interferon a therapy of chronic hepatitis D: regression of advanced hepatic fibrosis. Gastroenterology. 2004; 126:1740–9.
- Niro G, Ciancio A, Gaeta G, Smedile A, Marrone A, Olivero A, et al. Pegylated interferon alpha 2b as monotherapy or in combination with ribavirin in chronic hepatitis D. Hepatology. 2006;44:713–20.
- Yurdaydin C, Bozkaya H, Onder F, Sentürk H, Karaaslan H, Akdoğan M, et al. Treatment of chronic d hepatitis with lamivudine vs lamivudine + interferon vs interferon. J Viral Hepat. 2008;15: 314–21.
- Wedemeyer H, Yurdaydìn C, Dalekos G, Erhardt A, Çakaloğlu Y, Değertekin H, et al.;HIDIT Study Group. Peginterferon plus adefovir versus either drug alone for hepatitis delta. N Engl J Med. 2011;364: 322–31.
- Sheldon J, Ramos B, Toro C, Ríos P, Martínez-Alarcón J, Bottecchia M, et al. Does treatment of hepatitis B virus (HBV) infection reduce hepatitis delta virus (HDV) replication in HIV-HBV-HDV-coinfected patients?Antivir Ther. 2008;13:97–102.
- Vispo E, Sierra-Enguita R, Barreiro P, Labarga P, Fernandez-Montero JV, de Mendoza C, et al. Efficacy of prolonged tenofovir therapy on hepatitis delta in HIV-infected patients. J Infect Dis. (In press).
- Wedemeyer H, Port K, Heidrich S; and HIDIT-2 Study Group. 96 weeks of pegylated interferon alpha-2a plus tenofovir or placebo for the treatment of hepatitis delta: the HIDIT-2 study. AASLD, Washington DC, November 2013 [abstract 31].
- Farci P. Treatment of chronic hepatitis D: new advances, old challenges. Hepatology. 2006;44:536–9.
- Roche B, Samuel D. Liver transplantation in delta virus infection. Semin Liver Dis. 2012;32:245–55.