Abstract
Introduction. The benefit of aspirin in primary prevention of myocardial infarction and the associated gastro-intestinal bleeding risks have not been well established in the elderly population with diabetes.
Methods. Using Quebec administrative databases, we conducted two nested case-control analyses within a cohort of individuals aged ≥ 66 years newly treated with an oral antidiabetes drug between 1998 and 2003. The 28,067 individuals had no cardiovascular disease recorded in the database in the year prior cohort entry. They had not used prescribed aspirin, antiplatelet, or anticoagulant drugs, and were not hospitalized for gastro-intestinal bleeding in the year prior cohort entry. The odds of myocardial infarction and gastro-intestinal bleedings were compared between individuals who were current, past, or non-users of aspirin.
Results. There were 1101 (3.9%) cases of myocardial infarction. Compared to non-users, neither aspirin users (OR 0.89; 95% CI 0.71–1.13) nor aspirin past users (0.81; 0.62–1.06) showed a statistically significant lower risk of myocardial infarction. There were 373 (1.3%) cases of gastro-intestinal bleeding. Current users of aspirin had about a 2-fold greater risk of gastro-intestinal bleeding compared to non-users (2.19; 1.53–3.13).
Conclusions. Our results suggest that individual assessment of bleeding risk and cardiovascular risk is mandatory among elderly people with diabetes before introducing aspirin therapy.
Introduction
Although clinical guidelines (Citation1,Citation2) recommend aspirin for cardiovascular prevention among individuals with type 2 diabetes, there is little evidence that this therapy confers benefits with regard to cardiovascular events or mortality in this population (Citation3,Citation4). Recent meta-analyses have also questioned the benefits of aspirin in primary prevention of cardiovascular disease (CVD) (Citation5–8). The question then arises whether aspirin should be used systematically to reduce cardiovascular risk in people with type 2 diabetes.
This question is even more pertinent for elderly individuals, as they are the age-group most affected by diabetes and CVD (Citation9,Citation10). There is little information about aspirin protective effect in this age-group, since most of the benefits of aspirin in high-risk populations derive from studies focusing on the middle-aged (Citation11). It has also been established that the gastro-intestinal (GI) bleeding risk associated with aspirin therapy increases with age, especially among individuals aged ≥ 70 years (Citation12). Therefore, the risk/benefit ratio of aspirin therapy among elderly individuals with type 2 diabetes is not known.
The objective of this study was to evaluate the effect of aspirin on myocardial infarction (MI) and GI bleeding among elderly individuals who start an oral antidiabetes drug treatment and have no previous history of CVD.
Patients and methods
Overview
We built a population-based cohort that was analyzed using a nested case-control approach. The cohort consisted of older individuals treated with oral antidiabetes drugs that did not present clinical CVD. Using administrative databases, we explored the association between two distinct outcomes (MI and GI bleeding) and exposure to aspirin. This study was approved by the institutional ethics review board of the Centre hospitalier affilié universitaire de Québec.
Source of data
The Régie de l’assurance maladie du Québec (RAMQ) databases supplied information on demographics (age, sex, region of residence), physician services (date and diagnosis), prescription drugs dispensed (drug identification, dispensing date, and number of days’ supply) and death. Thus, for each prescription of aspirin, the number of dispensed doses and number of days’ supply were known. The Quebec registry of hospitalizations (Maintenance et exploitation des données pour l’étude de la clientèle hospitalière (Med-Écho)) supplied hospitalization data (dates, primary diagnosis, and up to 15 secondary diagnoses). By using a unique encrypted health number, we were able to link the RAMQ databases and Med-Écho at the individual level. The RAMQ health insurance plan covers all permanent residents of the province of Quebec, Canada, for both medical services and hospitalizations. Its public drug plan covers almost all non- institutionalized individuals aged ≥ 65 years. The drug plan database is known to be accurate for prescription claims (Citation13).
Study individuals
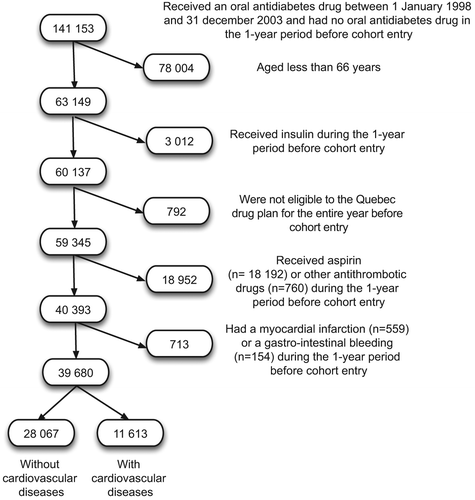
We first selected individuals aged 66 years and older, treated with oral antidiabetes drugs between 1 January 1998 and 31 December 2003. The cohort entry date was defined as the date of the first prescription for an oral antidiabetes drug. We excluded individuals who had received an oral antidiabetes drug, insulin, prescribed aspirin, or any other antithrombotic drug (clopidogrel, ticlopidine, dipyridamole/aspirin, nicoumalone, warfarin) in the year before cohort entry. In addition, we excluded those who had a GI bleeding episode or a history of CVD during that same period. We defined CVD as the presence of at least one of the following ICD-9 codes: 411–414 (ischemic heart disease), 420–438 (other heart diseases and cerobrovascular diseases), and 440 (atherosclerosis), in the year before cohort entry. We also considered anyone with a prescription claim for a nitrate to have CVD. GI bleeding events comprised primary diagnosis of ulceration, perforation, or bleeding in the upper gastro-intestinal tract (531.x–534.x, 535.01, 535.31, 578.0, 578.1, 578.9, 537.83) and primary diagnosis of gross rectal bleeding, lower gastro-intestinal perforation, ulceration, or diverticulitis with hemorrhage (562.02, 562.03, 562.12, 562.13, 569.3, 569.41, 569.82, 569.83, 569.85). For each outcome, we followed up eligible individuals until they: 1) experienced an event, 2) had termination of health coverage (death or emigration), 3) had a prescription of an antithrombotic drug other than aspirin, or 4) until the date 31 December 2004, whichever came first.
Case and control definitions
To determine the association between aspirin use and MI, we performed a case-control study. Cases were individuals who had an MI during the follow-up period, the index date being the date of the MI. MI was identified using the hospital discharge diagnosis of acute MI (ICD-9 code 410, all diagnostic fields). This diagnostic code has been shown to be valid for elderly individuals in the Med-Écho database (Citation14). To improve predictive value, we considered the diagnosis of MI as valid only if the related hospital stay was 3 days or more, unless the individual was transferred to or from another hospital, had a percutaneous coronary intervention performed, or died (Citation15).
We randomly selected five controls for each case using incidence density sampling. We matched cases and controls for age at entry into the cohort, year of entry, and sex. The index date for controls was the date when the matched case had the MI.
For our assessment of the association between aspirin use and GI bleeding, we also used a case-control study. GI bleeding was defined as previously described (see ‘Study individuals’). We used incidence density sampling to select randomly five controls for each case. Cases and controls were matched for age, year of cohort entry, and sex. The index date for a control was the date when the matched case underwent a GI bleeding event associated with hospitalization.
Assessment of aspirin use
Aspirin usage was classified in three categories: 1) current, 2) past, or 3) non-use. Individuals were deemed current users if their index date (date of the case's outcome) was included in the interval between the date of the last aspirin claim and the end date of its days’ supply, to which we added a grace period of 10 days. Since the residual effect of aspirin lasts around 10 days (Citation12), the 10-day grace period allows for the recognition of aspirin effect even if the person has actually stopped the therapy. (Results were not sensitive to the variation in the duration of the grace period (results not shown).) Other individuals who used aspirin in the observation period were considered past users, whereas those who did not were deemed non-users.
We also assessed duration of exposure to aspirin using the number of 30-day periods of use. As there is no information available on the in-hospital use of drugs, we assumed that, if aspirin was taken before hospitalization, it was also taken during the hospital stay.
Potentially confounding variables
For all analyses, age, sex, and calendar year were accounted for by the study design. Other potential confounders included: individual-, drug-, and health service-related characteristics. The following individual-related characteristics were added in each model: 1) residency area (rural/urban), 2) socio-economic status (no/partial/maximum guaranteed income supplement) at cohort entry, and 3) a marker of co-morbidity (the number of different drugs dispensed (Citation16) in the observation period). We considered drug-related characteristics including type of oral antidiabetes drug regimen dispensed at cohort entry. Insulin use was defined as the presence of a prescription claim for insulin in the observation period. Finally, the health service-related characteristics considered were the number of medical visits and hospitalizations (yes/no) during the observation period.
In the case-control analyses on MI, we also took into account the proportion of days before the index date during which individuals used at least one 1) ACE inhibitor or ARB, 2) statin, 3) antihypertensive drug other than ACE inhibitor/ARB, and 4) lipid-lowering drug other than statin. Because individuals might have developed CVD during follow-up and because some types of CVD could be associated with different MI risk, we adjusted for the occurrence and type of CVD (ischemic heart diseases (411–414); other forms of heart diseases (420–429); hemorrhagic cerebrovascular diseases (430–432); and ischemic and ill-defined cerebrovascular diseases (433–438)) experienced during the observation period.
In the case-control analyses on GI bleeding, current/past/ non-use of and duration of exposure to: 1) acetaminophen, 2) oral corticosteroids, 3) cytoprotective drugs, 4) COX-1 (traditional) non-steroidal anti-inflammatory drugs (NSAIDs), and 5) COX-2 (non-traditional) NSAIDs during the observation period were included in the analyses.
Statistical analysis
Frequency distributions were used to describe matching characteristics of cases and controls. The respective risks of MI and of GI bleeding associated with the use of aspirin were estimated using multivariate conditional logistic regressions. We assessed multicollinearity using the procedure described by Belsley et al. (Citation17). Analyses were carried out with SAS, version 9.2.
Results
In total 28,067 individuals were included in the cohort (). There were 1101 cases of MI during the observation period for an incidence rate of 11.03 per year per 1000 persons (95% CI 10.38–11.58). Characteristics of cases and controls are presented in . The risk of MI for users of aspirin (0.89; 0.71–1.13) and past users of aspirin (0.81; 0.62–1.06) was not statistically different than the risk for non-users (). Moreover, increased duration of exposure to aspirin was not associated with a change in MI risk. All types of CVD, except hemorrhagic cerebrovascular disease, were associated with a statistically significant increase in MI risk. Other characteristics that were associated with increased MI risk included the use of sulfonylurea (compared to metformin) and a high number of medications used.
Table I. Distribution of matching variables of cases and controls.
Table II. Adjusted odds ratios (AOR) of myocardial infarction (MI).
There were 373 cases of GI bleeding for an incidence rate of 3.71 per 1000 persons per year (95% CI 3.33–4.08). Characteristics of cases and controls are presented in . Current use of aspirin, when compared to non-use, was associated with a higher risk of GI bleeding (2.19; 1.53–3.13). The risk for past use, when compared to non-use, was increased by 47%, although it was not statistically significant (1.47; 0.91–2.38) (). Other characteristics associated with an increased bleeding risk included: low income, having a high number of physician encounters, current use of acetaminophen, current use of COX-1 NSAIDs, and current use of cytoprotective agents. However, a longer duration of cytoprotective agents was associated with a decreased bleeding risk.
Table III. Adjusted odds ratios (AOR) of gastro-intestinal (GI) bleeding.
Discussion
In this study, the use of aspirin was not associated with a statistically significant reduced risk of MI, while GI bleeding risk was increased. Our results are in contrast with the benefit observed among the non-diabetic, middle-aged population, where aspirin usage has been associated with a reduction in the risk of MI in primary prevention (0.18% versus 0.23% per year) (Citation18). However, in subgroup analyses of two randomized trials (Citation19,Citation20), no statistically significant benefits were found for individuals with type 2 diabetes treated with aspirin in primary prevention. Moreover, in two recent clinical trials (Citation21,Citation22), benefits with aspirin therapy were not observed for individuals with diabetes in primary prevention of CVD. However, an elevated dropout rate in the POPADAD trial (Citation21) may have contributed to the inability to observe statistically significant benefits. In the JPAD trial (Citation22), a very low event rate has decreased the statistical power of the study, which could explain the absence of statistically significant benefits. Interestingly, the subgroup of elderly participants receiving aspirin in the JPAD study did benefit from the therapy (hazard ratio 0.68; 0.46–0.99) (Citation22). However, recent meta-analyses could not demonstrate the benefits of aspirin (Citation5–8,Citation18). According to the latest Antithrombotic Trialists’ Collaboration meta-analysis, there was no statistically significant reduction in the risk of MI, stroke, or death from a vascular cause, in primary prevention for individuals with diabetes (RR 0.88; 95% CI 0.67–1.15) (Citation18). Nevertheless, the authors considered the results consistent with the benefit observed in people without diabetes (0.87; 0.79–0.96) (Citation18). Yet, there remains no robust evidence that aspirin confers benefits for individuals with diabetes in primary prevention. Our results add to the controversial issue of possible aspirin resistance among individuals with diabetes (Citation11,Citation23), which could in part explain why aspirin does not prove beneficial in primary prevention of cardiovascular events in this population. The mechanisms of possible aspirin resistance are not well understood. Rocca et al. have studied whether an accelerated renewal of platelet cyclo-oxygenase (COX) activity may explain the lower response to aspirin antiplatelet effect in individuals with diabetes (Citation23). They did not observe differences between individuals with diabetes and those without diabetes, although there was important interindividual variability. According to these authors, in addition to body mass index, the rate of recovery of COX activity is likely to be influenced by determinants that differ according to whether or not individuals suffer from diabetes.
With regard to the risk of GI bleeding, we observed that current use of aspirin was associated with twice the risk of non-use. Studies on this topic consistently report aspirin therapy as being associated with an approximately 2-fold increased risk of major extracranial bleeding among patients at high cardiovascular risk (Citation11,Citation24). Nonetheless, a recent observational study reported that aspirin increased bleeding risk, but not in patients with diabetes (Citation25). Our result may be explained by the inclusion of an older population, who may experience a greater bleeding risk. Interestingly, in our study, we did not observe an increased GI bleeding risk with longer aspirin use. Depletion of susceptible individuals may explain the association: only those who tolerated aspirin used it for longer periods of time (Citation26). We also observed an increased risk of GI bleeding among acetaminophen and COX-1 NSAIDs users, which are known risk factors for GI bleeding (Citation27). However, individuals currently using cytoprotective agents might have been at higher GI bleeding risk, thereby causing a spurious association between the cytoprotective agents and GI bleeding (indication bias).
One strength of our study is the use of a large population-based cohort, which enables the evaluation of actual medication use. Our cohort seems to compare to other cohorts that have been used to evaluate the impacts of medication use on health. For example, Simpson et al. have noted that the use of sulfonylurea is associated with a greater risk of death caused by an acute ischemic event while the use of metformin was not (Citation28). This finding is concordant with the increased risk of MI associated with the use of sulfonylurea that we have observed in our cohort. We therefore believe the results may be reproducible in other jurisdictions with similar clinical practices. Despite this strength, our study has some limitations. Even though we matched cases and controls for a variety of factors and incorporated several potential confounders in the analyses, there may be residual confounding unaccounted for. Administrative databases do not capture clinical and lifestyle data, such as smoking status, weight, physical activity, serum cholesterol, blood pressure, and level of glycemic control. Since we did not perform sex-specific analysis, it remains a possibility that results may vary according to sex. Also, since the data only permitted one-year anterior history evaluation, some individuals may have had CVD prior to their inclusion in the cohort. However, we did adjust for ongoing CVD other than MI during follow-up, which reduces the potential confounding effect of CVD. Next, we did not assess the dose-response relationship. Although there is no evidence that low doses (50–100 mg/d) of aspirin are less efficient than high doses (650–1500 mg/d) regarding cardioprotective purposes, it has been well established that side effects, notably bleeding, are dose-dependent (Citation11). Lastly, since aspirin is also available without prescription, we may have misclassified exposure. However, misclassification is likely to be minimal. Indeed in a recent survey conducted on a sample of elderly patients in Quebec, only 2.3% of them used over-the-counter aspirin as a cardioprotective agent (Citation29). Since aspirin is reimbursed under the Quebec drug insurance plan, there is a financial incentive for elderly patients to fill their aspirin prescription rather than to buy it over the counter.
Diabetes-related cardiovascular diseases impose a huge burden on health care. Given the increasing number of elderly suffering from diabetes, effective cardioprotective agents may help reduce this burden. Nonetheless, when considering its benefits and side effects, our results suggest that aspirin may not have a favorable benefit/risk profile in primary prevention in elderly individuals with type 2 diabetes. Because all potential confounding variables could not be included in the analyses, there remains a possibility that aspirin could prove beneficial for MI primary prevention among elderly with type 2 diabetes. However, potential benefits are likely to be small. Therefore, it may be wise to give priority to other preventive strategies to reduce the MI risk, especially among elderly individuals who have higher bleeding risks. The cardioprotective role of aspirin needs to be better examined among the elderly population with type 2 diabetes. Data from large-scale intervention trials currently in progress should help position aspirin therapy in primary prevention of CVD among individuals with type 2 diabetes.
Acknowledgements
The authors thank Joanne Vidal for editing the manuscript and Éric Demers for statistical assistance.
Dr Sirois was supported by a Fonds de la recherche en santé du Québec (FRSQ) scholarship. Dr Poirier is a senior clinical research scholar from FRSQ.
Declaration of interest: The authors report no conflicts of interest.
References
- American Diabetes Association. Standards of medical care in diabetes--2011. Diabetes Care. 2011;34:S11–61.
- Pignone M, Alberts M, Colwell J, Cushman M, Inzucchi SE, Mukherjee D, et al. Aspirin for primary prevention of cardiovascular events in people with diabetes: a position statement of the American Diabetes Association, a scientific statement of the American Heart Association, and an expert consensus document of the American College of Cardiology Foundation. Circulation. 2010;121:2694–701.
- Sirois C, Poirier P, Moisan J, Grégoire J-P. The benefit of aspirin therapy in type 2 diabetes: what is the evidence?Int J Cardiol. 2008; 129:172–9.
- Nicolucci A, De Berardis G, Sacco M, Tognoni G. AHA/ADA vs. ESC/EASD recommendations on aspirin as a primary prevention strategy in people with diabetes: how the same data generate divergent conclusions. Eur Heart J. 2007;28:1925–7.
- Pignone M, Williams C. Aspirin for primary prevention of cardiovascular disease in diabetes mellitus. Nat Rev Endocrinol. 2010; 6:619–28.
- Zhang C, Sun A, Zhang P, Wu C, Zhang S, Fu M, et al. Aspirin for primary prevention of cardiovascular events in diabetes: a meta-analysis. Diabetes Res Clin Pract. 2010;87:211–18.
- Butalia S, Leung AA, Ghali WA, Rabi DM. Aspirin effect on the incidence of major adverse cardiovascular events in patients with diabetes mellitus: a systematic review and meta-analysis. Cardiovasc Diabetol. 2011;10:25.
- Stavakis S, Stoner J, Azar M, Wayangankar S, Thadani U. Low dose aspirin for primary prevention of cardiovascular events in patients with diabetes: a meta-analysis. Am J Med Sci. 2011;341:1–9.
- Millar WJ, Young TK. Tracking diabetes: prevalence, incidence and risk factors. Health Rep. 2003;14:35–47.
- Ouhoummane N, Abdous B, Louchini R, Rochette L, Poirier P. Trends in postacute myocardial infarction management and mortality in patients with diabetes. A population-based study from 1995 to 2001. Can J Cardiol. 2010;26:523–31.
- Patrono C, Rocca B. Aspirin: promise and resistance in the new millennium. Arterioscler Thromb Vasc Biol. 2008;28:s25–32.
- Patrono C, Garcia Rodriguez LA, Landolfi R, Baigent C. Low-dose aspirin for the prevention of atherothrombosis. N Engl J Med. 2005; 353:2373–83.
- Tamblyn R, Lavoie G, Petrella L, Monette J. The use of prescription claims databases in pharmacoepidemiological research: the accuracy and comprehensiveness of the prescription claims database in Québec. J Clin Epidemiol. 1995;48:999–1009.
- Levy AR, Tamblyn RM, Fitchett D, McLeod P, Hanley JA. Coding accuracy of hospital discharge data for elderly survivors of myocardial infarction. Can J Cardiol. 1999;15:1277–82.
- Kiyota Y, Schneeweiss S, Glynn RJ, Cannuscio CC, Avorn J, Solomon DH. Accuracy of Medicare claims-based diagnosis of acute myocardial infarction: estimating positive predictive value on the basis of review of hospital records. Am Heart J. 2004;148:99–104.
- Schneeweiss S, Seeger JD, Maclure M, Wang PS, Avorn J, Glynn RJ. Performance of comorbidity scores to control for confounding in epidemiologic studies using claims data. Am J Epidemiol. 2001;154: 854–64.
- Belsley D, Kuh E, Welsch R. Regression diagnostics: identifying influential data and sources of collinearity. New York: Wiley; 1980.
- , Antithrombotic Trialists’ (ATT) CollaborationBaigent C, Blackwell L, Collins R, Emberson J, Godwin J, Peto R, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet. 2009;373:1849–60.
- Steering Committee of the Physicians’ Health Study Research Group. Final report on the aspirin component of the ongoing Physicians’ Health Study. N Engl J Med. 1989;321:129–35.
- Sacco M, Pellegrini F, Roncaglioni MC, Avanzini F, Tognoni G, Nicolucci A, PPP Collaborative Group. Primary prevention of cardiovascular events with low-dose aspirin and vitamin E in type 2 diabetic patients: results of the Primary Prevention Project (PPP) trial. Diabetes Care. 2003;26:3264–72.
- Belch J, MacCuish A, Campbell I, Cobbe S, Taylor R, Prescott R, et al. The prevention of progression of arterial disease and diabetes (POPADAD) trial: factorial randomised placebo controlled trial of aspirin and antioxidants in patients with diabetes and asymptomatic peripheral arterial disease. BMJ. 2008;337:a1840.
- Ogawa H, Nakayama M, Morimoto T, Uemura S, Kanauchi M, Doi N, et al. Low-dose aspirin for primary prevention of atherosclerotic events in patients with type 2 diabetes: a randomized controlled trial. JAMA. 2008;300:2134–41.
- Rocca B, Santilli F, Pitocco D, Mucci L, Petrucci G, Vitacolonna E, et al. The recovery of platelet cyclooxygenase activity explains interindividual variability in responsiveness to low-dose aspirin in patients with and without diabetes. J Thromb Haemost. 2012;10:1220–30.
- Garcia Rodriguez LA, Hernandez-Diaz S, de Abajo FJ. Association between aspirin and upper gastrointestinal complications: systematic review of epidemiologic studies. Br J Clin Pharmacol. 2001; 52:563–71.
- De Berardis G, Lucisano G, D’Ettore A, Pellegrini F, Lepore V, Tognoni G, et al. Association of aspirin use with major bleeding in patients with and without diabetes. JAMA. 2012;307;2286–94.
- Moride Y, Abenhaim L. Evidence of the depletion of susceptibles effect in non-experimental pharmacoepidemiologic research. J Clin Epidemiol. 1994;47:731–7.
- Rahme E, Barkun A, Nedjar H, Gaugris S, Watson D. Hospitalizations for upper and lower GI events associated with traditional NSAIDs and acetaminophen among the elderly in Quebec, Canada. Am J Gastroenterol. 2008;103:872–82.
- Simpson SH, Majumdar SR, Tsuyuki RT, Eurich DT, Johnson JA. Dose-response relation between sulfonylurea drugs and mortality in type 2 diabetes mellitus: a population-based cohort study. CMAJ. 2006;174:169–74.
- Guénette L, Sirois C. Pharmacy record registration of acetyl salicylic acid (ASA) prescriptions in Quebec. J Pharm Pharmaceut Sci. 2012; 15:252–5.